Abstract
The nicotinic acetylcholine receptor (nAChR) β3 subunit is thought to serve an accessory role in nAChR subtypes expressed in dopaminergic regions implicated in drug dependence and reward. When β3 subunits are expressed in excess, they have a dominant-negative effect on function of selected nAChR subtypes. In this study, we show, in Xenopus oocytes expressing α2, α3 or α4 plus either β2 or β4 subunits, that in the presumed presence of similar amounts of each nAChR subunit, co-expression with wild-type β3 subunits generally (except for α3*-nAChR) lowers amplitudes of agonist-evoked, inward peak currents by 20–50% without having dramatic effects (≤ 2-fold) on agonist potencies. By contrast, co-expression with mutant β3V9'S subunits generally (except for α4β2*-nAChR) increases agonist potencies, consistent with an expected gain-of-function effect. This most dramatically demonstrates formation of complexes containing three kinds of subunit. Moreover, for oocytes expressing nAChR containing any α subunit plus β4 and β3V9'S subunits, there is spontaneous channel opening sensitive to blockade by the open channel blocker, atropine. Collectively, the results indicate that β3 subunits integrate into all of the studied receptor assemblies and suggest that natural co-expression with β3 subunits can influence levels of expression and agonist sensitivities of several nAChR subtypes.
Keywords: ligand-gated ion channel, nicotinic acetylcholine receptor(s), receptor structure-function
Nicotinic acetylcholine receptors (nAChR) are pentameric ligand-gated ion channels expressed throughout the nervous system. Those other than the muscle-type (embryonic α1β1γδ- or adult α1β1γε-) nAChR are thought to be composed of different permutations of nine α subunits (α2–α10) and three β subunits (β2–β4) in humans (Lukas et al. 1999). Some of these subunits form homopentameric receptors when expressed in heterologous expression systems (α7, α8 or α9), whereas other subunits assemble into heteropentameric structures with various combinations of α and β subunits (Chavez-Noriega et al. 1997). nAChR β2 or β4 subunits form functional receptors in binary complexes with α2, α3, α4 or α6 subunits, and functionally-relevant agonist-binding pockets are thought to form at interfaces between these α and β subunits (Luetje and Patrick 1991). In some cases, trinary complexes are formed including β3 or α5 subunits thought to play structural roles as they occupy an accessory subunit position adjacent to the other α/β subunit pairs (Groot Kormelink and Luyten 1997; Nelson et al. 2001). For example, β2*-nAChR [where the asterisk indicates that additional subunits are known or possible assembly partners with the specified subunit(s)] found in the nervous system include α4β2-, α6β2-, α6β2β3- or α6α4β2β3-nAChR (Picciotto et al. 2001; Luetje 2004; Salminen et al. 2004).
Results from nAChR subunit knockout mouse studies suggest that α6β2β3- or α6α4β2β3-nAChR found in the striatum require incorporation of the β3 subunit to ensure efficient assembly (Cui et al. 2003; Gotti et al. 2005). α3β4β3-nAChR are thought to be expressed in the medial habenula (Sheffield et al. 2000; Grady et al. 2009), largely because this is one of the few brain areas expressing these subunits. Although its breadth of expression is narrow, interest is high in the nAChR β3 subunit in part because it is expressed in brain regions implicated in pleasure, reward and possibly nicotine dependence (Cui et al. 2003).
Little is known about functional roles played by β3 subunits, which are not thought to provide sites for agonist binding given their occupancy of the accessory subunit position (Groot-Kormelink et al. 1998; Boorman et al. 2003; Broadbent et al. 2006), although an exception may be in functional receptors formed from β3 and α7 subunits (Palma et al. 1999). Although evidence points to functional activity of naturally expressed β3*-nAChR, recent findings suggest that nAChR β3 subunits exert dominant-negative effects on function of selected, binary nAChR assemblies when heterologously expressed in Xenopus oocytes in excess over other subunits (Broadbent et al. 2006). When wild-type β3 subunits are heterologously co-expressed at presumed 20-fold higher levels than their partner subunits, functional responses of α2β2-, α2β4-, α3β2-, α3β4-, α4β2- and α4β4-nAChR are abolished, but function can be rescued if mutant β3 subunits are substituted that have a gain-of-function V273S amino acid substitution at the 9’ residue in their second transmembrane domain (TM2 9’ mutation; β3V273S = β3V9'S; Broadbent et al. 2006). Incorporation of the nAChR β3V9'S subunit also potentiated function of α6β2-and α6β4-nAChR (Broadbent et al. 2006), and we report elsewhere our studies examining α6β3*-nAChR (Dash et al. 2011a). However, to assess effects of wild-type or V9'S mutant β3 subunits on other nAChR subtypes, especially under conditions where subunit ratios are more likely to be physiological, we characterized effects under conditions where oocytes were injected with equal amounts of human nAChR subunit cRNAs.
Our results confirm that human nAChR β3 or β3V9'S subunits are incorporated into functional α2β2-, α2β4-, α3β2-, α3β4-, α4β2- or α4β4-nAChR. Although there are exceptions, and some effects seem to be agonist-specific, in general, the incorporation of β3 subunits reduces magnitudes of agonist-induced currents mediated by β2*- or β4*-nAChR and can modestly affect agonist potency. Mutant β3V9'S subunit incorporation almost always increases both agonist sensitivity and response levels. Channels containing β4 and mutant β3V9'S subunits also are fractionally spontaneously open as indicated by the abilities of nicotinic antagonists to produce outward current responses.
Materials and methods
Chemicals
All chemicals for electrophysiology were obtained from Sigma Chemical Co. (St Louis, MO, USA). Fresh stock solutions of acetylcholine (ACh), nicotine, atropine or mecamylamine were made daily in Ringer's solution and were diluted as needed.
Subcloning, mutagenesis and in vitro transcription of nicotinic receptor subunits
Human nAChR α2, α3, α4, β2, β3 and β4 subunits were subcloned into the oocyte expression vector pGEMHE. Mutation in the β3 subunit was introduced in the pGEMHE background using the QuickChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). Oligonucleotides used in mutagenesis are 5′-cattatccacatcggtcttgtcttctctgacagttttcc-3′ (β3V9'S-Forward) and 5′-ggaaaactgtcagagaagacaagaccgatgtggataatg-3′ (β3V9'S-Reverse). Identities of all wild-type or mutant subunits were confirmed by sequencing referenced to nucleotide/protein sequences available in GenBank.
All pGEMHE plasmids were linearized immediately downstream of the 3′-polyadenylation sequence. NheI was used to linearize nAChR α2, α3, α4, β3, β3V9'S and β4 subunit-containing plasmids, and SbfI was used for linearizing β2 subunit-containing plasmids. Capped mRNA was transcribed from linearized plasmids in a reaction mixture (25 μL) containing 1× transcription buffer, 1.6 mM rNTPs (Promega, Madison, WI, USA), 0.5 mM 7m-CAP (New England Biolabs (NEB), Ipswitch, MA, USA), 1 μL RNasin plus (NEB) and 1 μL T7 RNA polymerase (NEB) following standard protocols. Integrity and quality of the cRNA was checked by electrophoresis and UV-spectroscopy.
Oocyte preparation and cRNA injection
Female Xenopus laevis (Xenopus I, Ann Arbor, MI, USA) were anesthetized using 0.2% tricaine methanesulfonate (MS-222). The ovarian lobes were surgically removed from the frogs and placed in an incubation solution that consisted of (in mM) 82.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 1 Na2HPO4, 0.6 theophylline, 2.5 sodium pyruvate, 5 HEPES supplemented with 50 mg/mL gentamycin, 50 U/mL penicillin, and 50 μg/mL streptomycin; pH 7.5. The frogs were allowed to recover from surgery before being returned to the incubation tank. Ovarian lobes were cut into small pieces and digested with 0.08 Wunsch U/mL liberase blendzyme 3 (Roche Applied Science, Indianapolis, IN, USA) with constant stirring at 23 ± 2 °C for 1.5–2 h. The dispersed oocytes were thoroughly rinsed with incubation solution. Stage VI oocytes were selected and incubated at 16 °C before injection. Micropipettes used for injection were pulled from borosilicate glass (Drummond Scientific, Broom-all, PA, USA) using a Sutter P87 horizontal puller, and the tips were broken with forceps to ~40 μm in diameter. cRNA was drawn up into the micropipette and injected into oocytes using a Nanoject microinjection system (Drummond Scientific) at a total volume of ~60 nL. Generally, to express nAChR in oocytes, about 1 ng (for α3*- or α4*-nAChR) or 4 ng (for α2*-nAChR) of cRNA corresponding to each subunit was injected; that is, at ratios of 1 : 1 or 1 : 1 : 1 for binary or trinary receptors, respectively. Although there is no guarantee that this approach will result in expression of equal amounts of each subunit protein, it is more likely to do so than an approach where widely different ratios of subunit-encoding cRNAs are injected into oocytes. The latter kind of approach has been proven to favor, for example expression of α4β2-nAChR having 3 : 2 or 2 : 3 ratios of α4:β2 subunits in closed assemblies and relevant, low or high respective sensitivity to nicotinic agonists (Zwart and Vijverberg 1998). In fact, we also exploited this approach, using 10 : 1 ratios for injection of cRNA encoding α4:β2 subunits to bias toward expression of low sensitivity, (α4)3(β2)2-nAChR and using 10 : 1 : 1 ratios for injection of cRNA encoding α4:β2:β3 or β3V9'S subunits to bias toward substitution of β3 or β3V9'S subunits for the extra α4 subunit in those complexes.
Oocyte electrophysiology
Two to 7 days after injection, oocytes were placed in a small-volume chamber and continuously perfused with oocyte Ringer solution, which consisted of (in mM) 92.5 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES; pH 7.5. The chamber was grounded through an agarose bridge. The oocytes were voltage-clamped at –70 mV (unless otherwise noted) to measure agonist-induced currents using GeneClamp 500B and pClamp 10.2 software (Axon Instruments, Sunnyvale, CA, USA). The current signal was low-pass filtered at 10 Hz with the built-in low-pass Bessel filter in the Axoclamp 900A and digitized at 20 Hz with Axon Digidata1440A and pClamp10. Electrodes contained 3 M KCl and had a resistance of 1–2 MΩ. Drugs (agonists and antagonists) were prepared daily in bath solution. Drug was applied using a Valvelink 8.2 perfusion system (Automate Scientific, Berkeley, CA, USA). One-micromolar atropine was always co-applied for acetylcholine (ACh)-based recordings to eliminate muscarinic receptor responses. All drug applications lasted for 10 s. All electrophysiological measurements were conducted or checked in at least two batches of oocytes. All experimental procedures were conducted in accordance with the guidelines of the National Institutes of Health for the proper use of laboratory animals and approved by the Institutional Animal Care and Use Committee at Barrow Neurological Institute.
Experimental controls
Injection of cRNA corresponding to one subunit alone or pairwise combinations of β3 or β3V9'S subunits with either an α subunit or β2 or β4 subunits (3–12 ng total of cRNA) did not result in the expression of functional nAChR. Current responses to 100 μM ACh or nicotine were less than 5–20nA (data not shown).
Data analyses
Raw data were collected and processed in part using pClamp 10.2 (Molecular Devices, Sunnyvale, CA, USA) and a spreadsheet (Excel; Microsoft, Bellevue, WA, USA), using peak current amplitudes as measures of functional nAChR expression and results pooled across experiments (mean ± SEM for data from at least three oocytes). In some cases, mean peak current amplitudes in response to a single concentration of an agonist were compared across different subunit combinations. However, assessment of true Imax values for different nAChR subunit combinations required assessment based on more complete concentration–response relationships, in which mean peak current amplitudes at specified ligand concentrations were fit to the Hill equation or its variants using Prism 4 (GraphPad Software, San Diego, CA, USA). F-Tests (p < 0.05 to define statistical significance) were carried out to compare the best fit values of log molar EC50 values across specific nAChR subunit combinations.
There are limitations in the ability to compare levels of functional nAChR expression, even though we injected similar amounts of RNA for all constructs. This is because expression levels assessed as peak current amplitudes are affected by batch-to-batch variation in oocytes, time between cRNA injection and recording, and subunit combination-specific parameters, such as open probability (influenced by gating rate constants, rates and extents of desensitization), single channel conductance, assembly efficiency, and efficiency of receptor trafficking to the cell surface (Groot-Kormelink et al. 2001). In some cases, oocytes collapse a few days after injection with nAChR subunits that produce spontaneously-opening channels, precluding comparisons of functional levels to those achieved in oocytes expressing receptors from other subunits combinations. We made no attempt to measure or control for subunit combination-specific effects, but whenever preliminary studies revealed possible differences in peak current amplitudes, findings were further confirmed across different subunit combinations using the same batch of oocytes and the same time between cRNA injection and recording. Peak current amplitudes shown from representative traces in some figures presented below differ sometimes from mean peak current amplitudes across all studies for a given combination of subunits given because of these factors. However, when we make statements about results comparing ligand potencies and peak current amplitudes across subunit combinations, we do so for studies done under the same or very similar conditions, and the observations are clear, statistically significant, and in agreement whether for pooled data or for results from smaller sets of studies [one-way analyses of variance (anova) followed by Tukey's multiple comparison tests].
Note also our recognition that nAChR formed with or without wild-type β3 subunits could have lower amplitude responses that would be masked if those receptors were expressed in the presence of a smaller number of nAChR containing gain of function β3V9'S subunits having much more robust responses. However, this possible confound was ameliorated given our findings that mutant β3V9'S subunit incorporation had only modest effects on agonist-evoked current amplitudes.
For estimation of desensitization time constants, responses to the most efficacious concentration of an agonist over a 10-s application were evaluated. Generally, the decay from the peak to the steady-state inward current was fit to a mono- or bi-phasic expression (Clampfit 10.2; Axon Instruments; the equation for a bi- exponential fit is: I(t)=Aexp(–t/τ1) + Bexp(-t/τ2) + C, where I(t) is the current amplitude at any given time, A is the amplitude of the component with time constant τ1, B is the amplitude of the component with time constant τ2 and C is the value of a constant). The rate of desensitization in representative traces shown in some figures differs sometimes from that assesses across all studies for a given combination of subunits, but the latter, pooled data are presented in the tables.
Results
nAChR β3 subunits incorporate into α2β2- or α2β4-nAChR
Human nAChR were assembled in Xenopus oocytes after injection of a mixture of cRNAs in equal quantities encoding α2 and β2 subunits, α2, β2 and β3 subunits, or α2, β2 and β3V9'S subunits, and were functionally activated by ACh or nicotine (Fig. 1 and Figure S1). Oocytes injected with nAChR α2, β2 and β3 subunit cRNAs exhibited peak current responses (Imax; 25 nA) to ACh that were comparable (p > 0.05) to those in oocytes injected with just α2 and β2 subunits, although β3 subunit inclusion produced oocytes with reduced (~33%; p < 0.001) Imax for exposure to nicotine (Table 1). However, oocytes injected with the cRNA mixture of α2, β2 and β3V9'S constructs expressed higher (~50% for ACh and >2-fold for nicotine) nAChR function in response to either agonist compared with oocytes injected with α2 and β2 or with α2, β2 and β3 cRNA (Table 1). Concentration-response relationships yielded EC50 values (concentrations for half-maximal activation) indicating higher potencies (p < 0.0001) for both ACh (~50-fold) and nicotine (~10-fold) acting at α2β2β3V9'S- than acting at α2β2- or α2β2β3-nAChR, which have comparable agonist sensitivities (Table 1). Nevertheless, absolute levels of α2β2-nAChR function (~25–30 nA Imax) were lowest for all the combinations tested, even though 4-times higher levels of cRNA were injected for these constructs than for α3 or α4 subunit combinations. Adverse effects of β3 subunit inclusion were minor, and levels of functional potentiation in the presence of β3V9'S subunits were modest, although agonist sensitivity increases demonstrated a gain-of-function effect (Table 1). There was no indication that lower sensitivity α2β2-nAChR responses were present in oocytes expressing α2, β2 and β3V9'S subunits (i.e. concentration–response curves had similar slopes and were monophasic rather than biphasic, inconsistent with the presence of more than one class of functional α2β2-, α2β2β3-, and α2β2β3V9'S-nAChR, especially given the comparable amplitudes of responses across these complexes).
Fig. 1.
Functional properties of α2β2*- or α2β4*-nAChR. Results averaged across experiments were used to produce concentration-response curves (ordinate – mean normalized current ± SEM; abscissa – ligand concentration in log μM) for responses to ACh [(a) and (c)] or nicotine [(b) and (d)] as indicated for oocytes expressing nAChR α2 and either β2 [(a) and (b)] or β4 [(c) and (d)] subunits alone (■) or with either β3 (○) or β3V9'S (●) subunits. Incorporation of wild-type nAChR β3 subunits into α2β2- or α2β4-nAChR did not change (p > 0.05) the potency either for ACh or nicotine. Leftward shifts in agonist concentration–response curves are evident for α2β2β3V9'S- or α2β4β3V9'S-nAChRs (p < 0.0001; ~66- and ~4-fold, respectively, for ACh, and ~9- and 4-fold, respectively, for nicotine relative to in the absence of β3 subunits). Parameters for drug action are summarized in Table 1.
Table 1.
Parameters for drug action at human α2*-nAChR
| Potency |
Peak Response |
|||||||
|---|---|---|---|---|---|---|---|---|
| Agonist | nAChR subunit combinations | n | EC50 (μM) (95% CI) | nH ± SE nH | n | Imax (nA) (mean ± SE) | τ (ms) | Imax conc. (μM) |
| ACh | α2β2 | 5 | 59 (45–77) | 1.02 ± 0.13 | 5 | 25 ± 3 | 5400 ± 400 | 1000 |
| α2β2β3 | 3 | 48 (38–60) | 1.11 ± 0.12 | 3 | 25 ± 3 | 4500 ± 800 | 1000 | |
| α2β2β3V9'S | 9 | 0.89 (0.69–1.16)↑▲ | 0.65 ± 0.05 | 12 | 38 ± 4 ↑▲ | 6200 ± 900 | 100↑▲ | |
| α2β4 | 5 | 18 (17–19) | 1.33 ± 0.06 | 4 | 3800 ± 800 | 1600 ± 300 | 316 | |
| α2β4β3 | 4 | 20 (19–20)↓ | 1.39 ± 0.03 | 3 | 2500 ± 200 | 1500 ± 200 | 316 | |
| α2β4β3V9'S | 4 | 4.8 (4.2–5.5)↑▲ | 0.99 ± 0.06 | 4 | 6300 ± 600↑▲ | 2800 ± 600 | 316 | |
| Nicotine | α2β2 | 5 | 10 (8–13) | 0.99 ± 0.13 | 5 | 30 ± 2 | 7900 ± 2500 | 100 |
| α2β2β3 | 4 | 12 (9–15) | 1.08 ± 0.14 | 5 | 20 ± 3↓ | 3200 ± 700 | 100 | |
| α2β2β3V9'S | 4 | 1.1 (0.9–1.3)↑▲ | 1.08 ± 0.11 | 4 | 78 ± 13↑▲ | 1700 ± 300↓ | 10↑▲ | |
| α2β4 | 4 | 8.6 (7.5–9.8) | 1.34 ± 0.11 | 4 | 3500 ± 900 | – | 100 | |
| α2β4β3 | 4 | 7.3 (6.5–8.1) | 1.28 ± 0.08 | 4 | 2200 ± 700 | 2400 ± 700 | 100 | |
| α2β4β3V9'S | 4 | 2.0 (1.6–2.5)↑▲ | 1.06 ± 0.12 | 3 | 5500 ± 900↑▲ | 3900 ± 300 | 100 | |
Potencies (micromolar EC50 values and 95% confidence intervals), Hill coefficents (nH ± SE), mean ± SE peak current responses (Imax in nanoamps), current desensitization time constants (τ ± SE in milliseconds), and the concentration where Imax is achieved (μM) are provided for the indicated agonist (ACh or nicotine) acting at nAChR composed of the indicated subunits and from the indicated number of independent experiments based on studies as shown in Fig. 1 and Figure S1. ↑ or ↓ indicate a significant (p < 0.05) increase or decrease, respectively, in the relevant parameter at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit; and ▲ indicates a significant increase, in the relevant parameter of the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits. ‘–’ indicates cases where negligible desensitization was evident, precluding accuracy in fits of the data to mono- or bi-phasic current decay models.
Similarly, functional nAChR expression was achieved in oocytes injected with a mixture of cRNAs encoding human nAChR α2 and β4 subunits, α2, β4 and β3 subunits, or α2, β4 and β3V9'S subunits (Fig. 1 and Figure S1). Oocytes injected with α2, β4 and β3 subunit cRNAs gave lowest levels of responses to both ACh and nicotine (~2.2–2.4 μA; about two-thirds of responses of α2β4-nAChR), and responses were highest (5.5–6.3 μA) in oocytes injected with α2, β4 and β3V9'S subunit cRNAs (Table 1). Concentration–response relationships yielded EC50 values for agonist action that were comparable for α2β4- and α2β4β3-nAChR (p = 0.037), but agonist potencies are ~4-fold higher for α2β4β3V9'S-nAChR (Table 1). The only adverse effect of β3 subunit incorporation was a slight lowering of functional receptor levels, but mutant β3V9'S subunit incorporation potentiated function ~2-fold and increased agonist potency significantly, again showing a gain-of-function effect. Especially given the modest potentiation of receptor function, there was little evidence for functional expression of lower agonist sensitivity α2β4-nAChR in oocytes expressing α2, β4 and mutant β3 V9'S subunits (Fig. 1 and Figure S1).
Each form of α2β4*-nAChR had about 100-times more responsiveness to agonists than the corresponding α2β2*-nAChR (Table 1). There were no significant changes in rates of desensitization during the 10-s period of agonist exposure as a function of inclusion of wild type β3 or mutant β3V9'S subunits into α2*-nAChR, perhaps except for nicotine acting at α4β2*-nAChR (Table 1; Figure S1).
Although wild-type β3 subunit co-expression with α2 and either β2 or β4 subunits had no effect on ACh or nicotine potencies, an indication that β3 subunits incorporated into functional α2β2*- or α2β4*-nAChR assemblies came from the slight decrease in apparent levels of functional nAChR expression (not seen for ACh actions at α2β2*-nAChR) upon coexpression with β3 subunits. However, incorporation of mutant β3V9'S subunits was more clearly evident, as agonist potencies increased for all four α2*-nAChR subtypes as did levels of apparent functional nAChR expression. Interestingly, the degree of the gain-of-function effect on agonist potency related to β3V9'S subunit incorporation is higher for α2β2*- than for α2β4*-nAChR and more profound for ACh than for nicotine action at α2β2β3V9'S-nAChR. More work is needed to determine bases for these differences. The former difference in gain-of-function degree due to mutant subunit incorporation could be explained simply due to differences attributed to β2 or β4 subunit inclusion in complexes.
nAChR β3 subunits incorporate into α3β2- or α3β4-nAChR
Oocytes expressing a mixture of human nAChR α3, β2 and β3 subunits exhibited 8-fold lower (p < 0.001) Imax values for responses to ACh and about 12-fold lower (p < 0.001) Imax for responses to nicotine compared with oocytes expressing α3 and β2 subunits (Table 2). However, oocytes expressing a mixture of α3, β2 and mutant β3V9'S subunits expressed higher (p < 0.05) Imax for both ACh and nicotine compared with oocytes injected with α3, β2 and β3 subunit cRNAs (Table 2). Concentration–response relationships reveal that agonist potency increases about 2-fold (p < 0.0001) when β3 subunits are co-expressed with α3 and β2 subunits, relative to when α3 and β2 subunits are expressed alone, and then an additional > 350-fold (for ACh) or > 70-fold (for nicotine) when α3 and β2 subunits are co-expressed with β3V9'S subunits (Table 2). The increase in apparent agonist potency when β3 subunits are expressed with α3 and β2 subunits is accompanied by a decrease in Hill coefficients, perhaps suggesting that there is an admixture of α3β2- and α3β2β3-nAChR contributing to function and that the shift in agonist potency as indicated in Table 1 may underestimate the potency effect. However, concentration–response curves for α3β2β3V9'S-nAChR function show no evidence (shallow Hill slope, biphasic fit) for a low agonist sensitivity phase.
Table 2.
Parameters of drug action at human α3*-nAChR
| Potency |
Peak Response |
|||||||
|---|---|---|---|---|---|---|---|---|
| Agonist | nAChR subunit combinations | n | EC50 (μM) (95% CI) | nH ± SE nH | n | Imax (nA) (mean ± SE) | τ (ms) | Imax conc. (μM) |
| ACh | α3β2 | 7 | 200 (170–230) | 1.34 ± 0.12 | 6 | 2600 ± 200 | 2600 ± 300 | 3160 |
| α3β2β3 | 6 | 110 (83–140)↑ | 0.70 ± 0.07 | 3 | 310 ± 80↓ | 2000 ± 500 | 3160 | |
| α3β2β3V9'S | 6 | 0.28 (0.24–0.31)↑▲ | 0.97 ± 0.05 | 7 | 970 ± 250↓▲ | 3300 ± 400 | 10↑▲ | |
| α3β4 | 6 | 75 (68–82) | 1.39 ± 0.08 | 5 | 6300 ± 500 | 2000 ± 400 | 1000 | |
| α3β4β3 | 5 | 81 (73–89) | 1.20 ± 0.06 | 5 | 5900 ± 500 | 3500 ± 300 | 3160↓ | |
| α3β4β3V9'S | 5 | 2.7 (2.4–3.1)↑▲ | 1.13 ±0.08 | 4 | 6800 ± 1100 | – | 316↑▲ | |
| Nicotine | α3β2 | 8 | 38 (34–43) | 1.61 ± 0.13 | 8 | 3400 ± 200 | 3000 ± 100 | 316 |
| α3β2β3 | 5 | 18 (12–27)↑ | 0.58 ± 0.07 | 4 | 270 ± 70↓ | 6800 ± 500↑ | 316 | |
| α3β2β3V9'S | 4 | 0.25 (0.18–0.35)↑▲ | 0.79 ± 0.10 | 4 | 2400 ± 200↓▲ | 3110 ± 30▼ | 10↑▲ | |
| α3β4 | 10 | 39 (36–43) | 1.37 ± 0.08 | 3 | 7800 ± 500 | – | 1000 | |
| α3β4β3 | 5 | 66 (59–74)↓ | 1.38 ± 0.09 | 4 | 7800 ± 200 | 3900 ± 300 | 1000 | |
| α3β4β3V9'S | 7 | 3.3 (2.8–3.9)↑▲ | 1.06 ± 0.09 | 4 | 7200 ± 400 | – | 100↑▲ | |
Potencies (micromolar EC50 values and 95% confidence intervals), Hill coefficents (nH ± SE), mean ± SE peak current responses (Imax in nanoamps), current desensitization time constants (τ ± SE in milliseconds), and the concentration where Imax is achieved (μM) are provided for the indicated agonist (ACh or nicotine) acting at nAChR composed of the indicated subunits and from the indicated number of independent experiments based on studies as shown in Fig. 2 and Figure S2. ↑ or ↓ indicate a significant (p < 0.05) increase or decrease, respectively, in the relevant parameter at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit; and ▼ or ▲ indicates a significant increase or decrease, respectively, in the relevant parameter of the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits. ‘–’ indicates cases where negligible desensitization was evident, precluding accuracy in fits of the data to mono- or bi-phasic current decay models.
Consistent with prior observations (Groot-Kormelink et al. 1998; Boorman et al. 2000, 2003), functional nAChR assemble in oocytes injected with a mixture of cRNAs encoding α3 and β4 subunits, α3, β4 and β3 subunits, or α3, β4 and β3V9'S subunits (Fig. 2 and Figure S2). However, in our hands, when injected cRNA quantities are the same for each subunit, there are no significant differences in mean peak current amplitudes across the different nAChR constructs (Table 2). Wild-type β3 subunit incorporation into α3β4*-nAChR does not alter ACh sensitivity, although it lowers nicotine sensitivity by about 2-fold (p < 0.001). However, incorporation of β3V9'S subunits into assemblies containing α3 and β4 subunits increases potency 33-fold for ACh and ~13-fold for nicotine (Table 2). Concentration–response profiles and Hill coefficients are consistent with the formation of a single form of functional nAChR regardless of subunit makeup (Table 2, Fig. 2 and Figure S2).
Fig. 2.
Functional properties of α3β2*- or α3β4*-nAChR. Results averaged across experiments were used to produce concentration-response curves (ordinate – mean normalized current ± SEM; abscissa – ligand concentration in log μM) for responses to ACh [(a) and (c)] or nicotine [(b) and (d)] as indicated for oocytes expressing nAChR α3 and either β2 [(a) and (b)] or β4 [(c) and (d)] subunits alone (■) or with either β3 (○) or β3V9'S (●) subunits. Incorporation of wild-type β3 subunits into α3β2-nAChR shifted agonist concentration–response curves towards the left with a relative increase (p < 0.05) in sensitivity of 1.8- or 2.2-fold for ACh or nicotine, respectively, but incorporation of wild-type β3 subunits into α3β4-nAChR did not change (p > 0.05) the potency for either ACh or nicotine (although sensitivity increased 1.1- or 1.7-fold for ACh or nicotine, respectively). Leftward shifts in agonist concentration-response curves are evident for α3β2β3V9'S or α3β4β3V9'S nAChR (p < 0.0001; ~700- or ~27-fold, respectively, for ACh, and ~150- or ~12-fold, respectively, for nicotine relative to in the absence of β3 subunits). Parameters for drug action are summarized in Table 2.
α3β4*-nAChR forms had 3-25-fold more responsiveness to agonists than the corresponding α3β2*-nAChR (Table 1). There was no solid evidence for changes in rates of desensitization as a function of β3 subunit incorporation into α3*-nAChR, except perhaps for nicotine acting at α3β2*-nAChR (Table 1; Figure S2).
Effects of nAChR β3 subunits on function of α4β2- or α4β4-nAChR
(i) Effects of nAChR β3 subunits on α4β2-nAChR
There is formation of functional, human nAChR in oocytes injected with a mixture of cRNAs encoding α4 and β2 subunits, α4, β2 and β3 subunits, or α4, β2 and β3V9'S subunits (Fig. 3 and Figure S3). Introduction of β3 subunits produces a modest (35%; p > 0.05) reduction in Imax values for responses to ACh or a significant (45%; p < 0.01) diminution in Imax values for responses to nicotine relative to values obtained in studies of oocytes expressing just α4 and β2 subunits (Table 3). There is restoration in oocytes expressing α4, β2 and β3V9'S subunits of functional responsiveness to ACh (p < 0.05) and partially so of responsiveness to nicotine (p > 0.05; Table 3). Concentration–response relationships indicate that EC50 values are more than 3-fold lower (p < 0.0001) for nicotine acting at α4β2β3V9'S- than at α4β2- or α4β2β3-nAChR (Table 3). Curiously, the EC50 value is higher (i.e. there is lower potency; p < 0.0001) for ACh acting at α4β2β3V9'S- than at α4β2-nAChR, and the ACh EC50 value is intermediate for actions at α4β2β3-nAChR (Table 3). These results give some indication that wild-type β3 or mutant β3V9'S subunits can incorporate into α4β2-nAChR, but either effects are not dramatic, or there are limits on proportions of functional receptors containing either type of β3 subunit.
Fig. 3.
Functional properties of α4β2*- or α4β4*-nAChR. Results averaged across experiments were used to produce concentration–response curves (ordinate – mean normalized current ± SEM; abscissa – ligand concentration in log μM) for responses to ACh [(a), (c) and (d)] or nicotine [(b) and (e)] as indicated for oocytes expressing nAChR α4 and either β2 [(a), (b) and (c)] or β4 [(d), (e)] subunits alone (■) or with either β3 (○) or β3V9'S (●) subunits. Incorporation of wild-type β3 subunits into α4β2-nAChR shifted the agonist concentration-response curve toward the right with a relative decrease (p < 0.0001) in sensitivity of 2.4-fold for ACh but did not have any effect (p = 0.99) on the sensitivity for nicotine However, incorporation of β3V9'S subunits into α4β2-nAChR shifted the agonist concentration-response curve toward the right for a relative 2.8-fold decrease in ACh sensitivity or to the left with a relative 3.7-fold increase in nicotine sensitivity (p < 0.0001). Co-expression of α4, β2 and wild-type β3 subunits at 10 : 1 : 1 ratios (c) had a non-significant effect on ACh sensitivity, but co-expression instead with β3V9'S subunits caused a leftward shift in the ACh concentration-response curve correlating with a 1.5-fold increase in ACh sensitivity (p < 0.002). Incorporation of wild-type β3 subunits into α4β4-nAChR shifted nicotine concentration-response curve toward the right reflecting a relative, 2.6-fold decrease (p < 0.05) in nicotine sensitivity but had no effect on ACh sensitivity (p > 0.05). Leftward shifts in agonist concentration–response curves are evident for α4β4β3V9'S nAChRs (p < 0.0001; ~16- or ~15-fold increases in sensitivity for ACh or nicotine, respectively). Parameters for drug action are summarized in Table 3.
Table 3.
Parameters for drug agonist action at human α4*-nAChR
| Potency |
Peak Response |
|||||||
|---|---|---|---|---|---|---|---|---|
| Agonist | nAChR subunit combinations | n | EC50 (μM) (95% CI) | nH ± SE nH | n | Imax (nA) (mean ± SE) | τ (ms) | Imax conc. (μM) |
| ACh | α4β2 (1:1) | 7 | 13 (10–16) | 0.89 ± 0.09 | 3 | 4400 ± 200 | 2000 ± 200 (τ1) 770 ± 90 (τ2) | 316 |
| α4β2β3 (1:1:1) | 11 | 30 (26–34)↓ | 1.08 ± 0.07 | 7 | 2900 ± 800 | 2700 ± 100 | 1000↓ | |
| α4β2β3V9'S (1:1:1) | 12 | 36 (28–45)↓ | 0.62 ± 0.04 | 10 | 4700 ± 600▲ | 3800 ± 500 | 1000↓▼ | |
| α4β2 (10 : 1) | 6 | 60 (51–71) | 1.03 ± 0.08 | 4 | 2000 ± 200 | 7300 ± 900 | 1000 | |
| α4β2β3 (10 : 1 : 1) | 5 | 53 (42–67) | 1.06 ± 0.12 | 3 | 710 ±120↓ | 8700 ± 700 | 1000 | |
| α4β2β3V9'S (10 : 1 : 1) | 6 | 39 (32–48)↑▲ | 0.86 ± 0.07 | 4 | 520 ± 90↓ | 7200 ± 600 | 316↑▲ | |
| α4β4 | 3 | 8.7 (7.6–10.0) | 1.07 ± 0.07 | 4 | 7300 ± 700 | – | 316 | |
| α4β4β3 | 7 | 9.7 (7.4–13.2) | 1.02 ± 0.13 | 3 | 5700 ± 400 | 2500 ± 500 | 316 | |
| α4β4β3V9'S | 6 | 0.56 (0.46–1.97)↑▲ | 0.64 ± 0.05 | 3 | 7100 ± 400 | – | 31.6↑▲ | |
| Nicotine | α4β2 (1:1) | 7 | 8.4 (7.5–9.5) | 1.35 ± 0.13 | 3 | 3600 ± 800 | 3500 ± 200 | 100 |
| α4β2β3 (1:1:1) | 10 | 8.4 (7.3–9.6) | 1.24 ± 0.11 | 9 | 1900 ± 400↓ | 3600 ± 200 | 100 | |
| α4β2β3V9'S (1:1:1) | 9 | 2.3 (1.9–2.8)↑▲ | 0.77 ± 0.05 | 7 | 2200 ± 300↓ | 4200 ± 500 | 100 | |
| α4β4 | 6 | 4.0 (3.7–4.4) | 1.50 ± 0.09 | 3 | 7500 ± 1600 | – | 31.6 | |
| α4β4β3 | 6 | 10.5 (9.5–11.7)↓ | 1.67 ± 0.15 | 3 | 5800 ± 1200 | 4700 ± 2400 | 100↓ | |
| α4β4β3V9'S | 10 | 0.26 (0.22–0.31)↑▲ | 1.22 ± 0.13 | 4 | 7500 ± 600 | – | 10↑▲ | |
Potencies (micromolar EC50 values and 95% confidence intervals), Hill coefficents (nH ± SE), mean ± SE peak current responses (Imax in nanoamps), current desensitization time constants (τ ± SE in milliseconds), and the concentration where Imax is achieved (μM) are provided for the indicated agonist (ACh or nicotine) acting at nAChR composed of the indicated subunits and from the indicated number of independent experiments based on studies as shown in Figs 3 and 4, and Figure S3. ↑ or ↓ indicate a significant (p < 0.05) increase or decrease, respectively, in the relevant parameter at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit; and ▲ or ▼ indicate a significant increase or decrease, respectively, in the relevant parameter of the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits. The desensitizing current phase of α4β2-nAChR in response to activation by 316 μM ACh is best described by bi-exponential expression and its time constants are τ1 (for slow phase) and τ2 (fast phase). ‘–’ indicates cases where negligible desensitization was evident, precluding accuracy in fits of the data to mono- or bi-phasic current decay models.
(ii) Effects of co-expression with nAChR β3 subunits on putative, low sensitivity α4β2-nAChR
The unusual effects of wild-type or mutant, nAChR β3 subunit incorporation into α4β2-nAChR complexes could reflect additional influences on low sensitivity and high sensitivity α4β2-nAChR isoforms postulated to have 3 : 2 or 2 : 3 ratios, respectively, of α4:β2 subunits (Zwart and Vijverberg 1998; Nelson et al. 2003; Tapia et al. 2007; Kuryatov et al. 2008). Thus, we conducted some experiments to manipulate subunit stoichiometries in functional α4β2-nAChR expressed in oocytes and to assess effects of β3 subunit co-expression (Fig. 3 and Figure S3).
As expected, oocytes injected with a 10 : 1 ratio of α4:β2 subunit cRNAs expressed α4β2-nAChR presumed to have the stoichiometry of 3 : 2 α4:β2 subunits and functional responses with lower ACh sensitivity (60 μM EC50) than receptors in oocytes injected with 1 : 1 subunit cRNA ratios and likely representing a mixture of (α4)3(β2)2- and (α4)2(β2)3-nAChR (12.5 μM EC50; Table 3). Levels of functional responses also were ~50% lower in oocytes expressing low sensitivity receptors. Oocytes injected with a 10 : 1 : 1 ratio of α4:β2:β3 subunit cRNAs had lower mean peak current responses to ACh (p < 0.001) than oocytes injected with 10 : 1 α4:β2 cRNAs, but ACh potency was similar (Table 3). Oocytes injected with 10 : 1 : 1 α4:β2:β3V9'S subunit cRNAs had slightly lower, yet measureable, functional responsiveness, although there was a modest increase in ACh potency. As for studies of receptors formed after 1 : 1 α4:β2 subunit cRNA injections, after 10 : 1 α4:β2 subunit cRNA injections, effects of wild-type or mutant β3 subunits on agonist potency were not dramatic, although reductions in peak current amplitudes suggest that β3 subunits lower levels of functional expression.
(iii) Effects of nAChR β3 subunits on α4β4-nAChR
Receptors assembled after injection in equal quantities of a mixture of cRNAs encoding α4 and β4 subunits, α4, β4 and β3 subunits, or α4, β4 and β3V9'S subunits gave functional responses to ACh and nicotine (Fig. 3 and Figure S4). There were comparable, ~22% reductions in Imax values for agonists when β3 subunits were added to α4 and β4 subunits. Functional levels in the presence of β3V9'S subunits were more like those for oocytes expressing α4 and β4 subunits alone, reversing the slight losses due to wild-type β3 subunit co-expression (Table 3). Concentration–response relationships revealed that wild-type β3 subunit co-expression had no effect on ACh sensitivity but decreased nicotine sensitivity ~2-fold for actions at α4β4β3- compared with α4β4-nAChR. By contrast, there was an about 15-fold increase in sensitivity to both agonists for α4β4β3V9'S-compared with α4β2-nAChR. These studies suggest that there is, again, incorporation of wild-type β3 or mutant β3V9'S subunits into complexes with α4 and β4 subunits. α4β4*-nAChR forms had 2- to 3-fold more responsiveness to agonists than the corresponding α4β2*-nAChR (Table 3).
Desensitization of α4β2-nAChR in the presence of ACh was best fit to a biphasic current decay, but there were no clear effects of β3 subunit incorporation on desensitization half lives
Receptors containing β3V9'S mutants open spontaneously
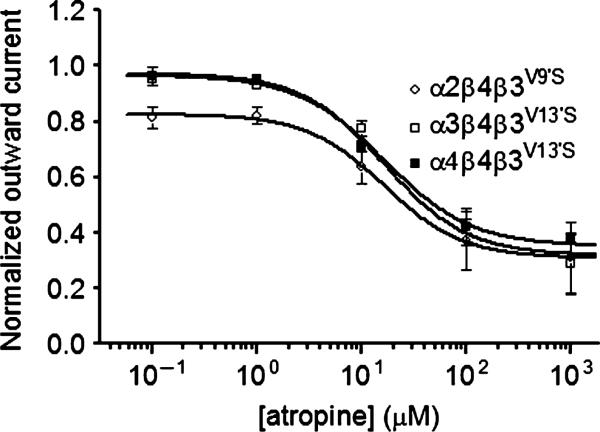
Atropine, which is a classic muscarinic acetylcholine receptor antagonist, was used at 1 μM to block possible muscarinic responses whenever ACh was applied to oocytes injected with nAChR subunit cRNAs. However, because atropine at higher concentrations also can interact with different nAChR subtypes (Zwart and Vijverberg 1997; Zwart et al. 1999; Parker et al. 2003; Baker et al. 2004), and initially as a simple control, we assessed effects of atropine at all receptor combinations studied. Although it had no effect when applied to oocytes expressing wild-type nAChR subunits in any combination, atropine reversibly produced outward currents when applied to oocytes expressing receptors containing mutant β3V9'S subunits. Atropine responses of α2β2β3V9'S-, α3β2β3V9'S- or α4β2β3V9'S-nAChR were not robust or strongly concentration-dependent, but were statistically significantly different from zero at the highest concentration of 1 mM atropine tested (data not shown). However, atropine concentration–response curves yielded apparent IC50 values of 16.91, 28.13 and 12.04 μM for atropine induction of outward currents mediated by α2β4β3V9'S-, α3β4β3V9'S-, and α4β4β3V9'S-nAChR, respectively (Table 4). Similarly, mecamylamine reversibly produced outward currents at receptors containing β4 and β3V9'S mutant subunits in a concentration-dependent manner (data not shown). Current response traces for α3β4β3V9'S- and α4β4β3V9'S-nAChR were unremarkable, indicating rapid return to baseline levels of current flow after removal of atropine, but in oocytes expressing α2, β4 and β3V9'S subunits, upon washout of atropine at lower concentrations, there was a reflex inward current that later decayed to baseline levels (Fig. 4 and Figure S5).
Table 4.
Parameters for atropine block of human α/β/β3V9'S nAChR spontaneous channel opening
| Peak response |
|||||||
|---|---|---|---|---|---|---|---|
| Drug | nAChR subunit combinations | n | IC50 (μM) (95% CI) | nH ± SE nH | n | Imax (nA) (mean ± SE) | Imax conc. (μM) |
| Atropine | α2β4β3V9'S | 3 | 17 (8–37) | –1.16 ± 0.36 | 3 | 620 ± 70 | 1000 |
| α3β4β3V9'S | 6 | 28 (9–87) | –0.99 ± 0.35 | 6 | 1140 ± 40 | 1000 | |
| α4β4β3V9'S | 5 | 12 (9–17) | –1.26 ± 0.34 | 5 | 640 ± 280 | 1000 | |
Inhibitory potency (micromolar IC50 values and 95% confidence intervals), Hill coefficents (nH ± SE), mean ± SE peak current responses (Imax in nanoamps) and the concentration where Imax is achieved (μM) are provided for the atropine acting at nAChR composed of the indicated subunits and from the indicated number of independent experiments based on studies as shown in Fig. 4 and Figure S5.
Fig. 4.
Atropine-sensitive blockade of spontaneous channel opening of α2β4β3V9'S-, α3β4β3V9'S-, and α4β4β3V9'S-nAChR. Atropine concentration (abscissa; log μM)–response (mean ± SEM normalized peak outward current) curves for data pooled from experiments for α2β4β3V9'S- (○), α3β4β3V9'S- (□), or α4β4β3V9'S- (■) nAChR. Parameters for atropine action are summarized in Table 4.
We attribute these effects to antagonism of spontaneous channel opening for nAChR containing mutant β3V9'S subunits. Spontaneous channel opening is commonly seen for receptors of the ligand-gated ion channel family containing gain-of-function mutations (V9'S) in subunit second transmembrane domains (Chang and Weiss 1998; Dash et al. 2011a,b). Judging from amplitudes of outward currents relative to inward currents evoked by nicotinic agonists for the same receptor complexes, about 9% (620/(6300 + 620) (see Tables 1–4) of α2β4β3V9'S-nAChR are spontaneously open, and ~14% or 8% of α3β4β3V9'S- or α4β4β3V9'S-nAChR are spontaneously open.
Discussion
The principal findings in this study are that co-expression with human nAChR wild-type β3 subunits, or especially with β3V9'S mutant subunits, influences function of nAChR expressed in oocytes and composed of α2, α3 or α4 plus β2 or β4 subunits. These effects occur when numbers of subunits are likely to be comparable in oocytes injected with equal amounts of subunit cRNAs. Generally, β3 subunit co-expression typically attenuates peak current responses to nicotinic agonists without substantially affecting agonist potencies. Co-expression with mutant β3V9'S subunits almost always has a gain-of-function effect manifest as increased agonist sensitivity, providing the strongest evidence for formation of trinary complexes (containing three kinds of subunits), although effects of mutant β3V9'S subunits on levels of agonist-elicited peak currents (i.e. levels of receptor function) are dependent on assembly partner subunits. There was atropine- or mecamylamine-sensitive blockade of spontaneous opening of β4β3V9'S*-nAChR.
Recently, Broadbent et al. (2006) showed almost complete loss of function when nAChR are formed in oocytes injected with cRNAs encoding different pairs of α and β subunits along with a 20-fold excess of β3 subunit cRNA, a strategy likely done in principle to ensure that the majority of receptors contain β3 subunits. However, the lack of or low levels of function in the prior studies meant that abilities to use concentration–response curves to characterize effects of β3 subunit incorporation were compromised, and physiological relevance could be questioned because nAChR subunit levels in neurons are unlikely to differ by 20-fold. This problem is more prominent in studies of β2*-nAChR that tend to yield lower Imax values than β4*-nAChR. Moreover, given evidence that β3 subunits integrate efficiently into nAChR complexes, often promoting the formation of non-functional, dead-end intermediates (Kuryatov et al. 2008), it is perhaps not surprising that these effects would be exacerbated in the presence of a large excess of β3 subunits. In fact, the current studies actually are in good agreement with the more limited scope of work done by Broadbent et al. (2006) when investigating, as did we, oocytes injected with subunit cRNAs at 1 : 1 or 1 : 1 : 1 ratios, showing smaller but substantial decreases in functional responses when less extreme transfection ratios were used instead and wild-type β3 subunits were co-expressed in nAChR assemblies.
In discussion of the results, we presume that our empirical measures of peak current responses are measures of the level of functional nAChR expression. With only a few exceptions, Hill coefficients are consistent with expression of a single class of functional nAChR, and changes in peak current levels, although mixed, suggest that wild-type β3 or mutant β3V9'S subunits have indeed successfully incorporated into functional nAChR. However, we must remain silent about efficiencies of subunit assembly in intracellular pools or even on the oocyte surface into complexes that have no function. On the other hand, our conclusions about incorporation of wild-type or mutant β3 subunits into closed nAChR assemblies are predominantly based on evidence that V9'S mutations in the nAChR β3 subunit serve as reporter mutations and on the typical and unmistakable increase in agonist potency inducing channel opening in oocytes injected with mutant β3V9'S subunit cRNA.
Consistent with our generalizations about the data, co-expression with wild-type β3 subunits moderately decrease apparent levels of functional α2β2*- or α2β4*-nAChR expression without having large effects on agonist potency, but co-expression with mutant β3V9'S subunits more than restores levels of functional receptor expression while also substantially increasing agonist potency. Natural incorporation of β3 subunits into α2*-nAChR thus would be expected to have modest effects. A similar prediction derives from the lack of effects of wild-type β3 subunits on α4β4*-nAChR functional levels or agonist potencies, although the large increase in agonist potencies for α4β4β3V9'S-nAChR indicates that β3 subunits can and likely do incorporate into α4β4*-nAChR. Evidence for spontaneous opening of α2β4β3V9'S- and α4β4β3V9'S-nAChR also indicate that β3 subunits integrate into those complexes.
α3β4*-nAChR naturally expressed in autonomic ganglia or in related, human tumor cells lines such as SH-SY5Y or IMR-32 seem to include α5 subunits as accessory subunits, so it perhaps would not be surprising for β3 subunits to substitute for α5 subunits. There is little-to-no effect of wild-type β3 subunit co-expression in oocytes with α3 and β4 subunits in our hands, except for a slight decrease in nicotine potency. A reduction in nicotine potency seen for α3β4β3-nAChR relative to α3β4-nAChR also was observed by Groot-Kormelink et al. (1998) and Boorman et al. (2000, 2003). Nevertheless, and although there was no apparent effect on levels of functional nAChR expression, a profound increase in agonist sensitivity (and in spontaneous channel opening) was evident in the presence of co-expressed β3V9'S subunits, clearly indicating incorporation of those subunits into α3β4*-nAChR.
The most striking reduction (~10-fold) in levels of functional nAChR expression in the presence of wild-type β3 subunits is for α3β2*-nAChR, and response amplitudes are lower even for α3β2β3V9'S- than for α3β2-nAChR. Nevertheless, agonist potencies are increased ~2-fold for α3β2β3-nAChR relative to those for α3β2-nAChR, and concentration–response curves show Hill slope-based indications of expression of a mixture of receptor subtypes, probably α3β2- and α3β2β3-nAChR. In addition, agonist potency is dramatically increased for α3β2β3V9'S-nAChR relative to agonist potency at α3β2-nAChR, showing the strongest effect of mutant β3V9'S subunits on any nAChR subtype. These findings provide clear indications that natural incorporation of wild-type β3 subunits would affect α3β2*-nAChR function.
The findings that co-expression of wild-type β3 subunits with α3 and β2 subunits produced functional nAChR with ~2-fold higher sensitivities to ACh or nicotine, even though levels of presumed functional nAChR expression were reduced, might reflect an adverse effect of β3 subunits on assembly of functional nAChR. However, the change in agonist potency suggests that wild-type β3 subunits incorporate into functional α3β2*-nAChR. It is possible that the presence of β3 subunits indirectly affects assembly of α3β2(non-β3)-nAChR, favoring those with an alternate ratio of α:β2 subunits and slightly higher agonist sensitivity. The much more substantial increase in agonist potencies in the presence of β3V9'S subunits suggests more strongly that mutant subunit incorporation occurs, perhaps more preferentially than for wild-type β3 subunits. The unexpectedly more profound increase in ACh relative to nicotine sensitivity at α3β2β3V9'S-nAChR is more difficult to explain, even given the caveats about absolute levels of peak current mentioned in Methods, but there also is an unexpected, lower effect of mutant β3V9'S subunit incorporation on magnitudes of ACh- as opposed to nicotine-induced responses. Similarly, the modest effects of wild-type β3 subunit co-expression on functional levels and agonists sensitivities of α3β4*-nAChR could be explained if β3 subunits do not incorporate well into functional assemblies. Nevertheless, the large effects of mutant β3V9'S subunit co-expression on agonist sensitivities strongly suggest that mutant subunits are efficiently incorporated into functional complexes.
It has become clear, initially from studies using oocytes injected with disproportionate ratios of α4 and β2 subunit cRNAs, that α4β2-nAChR can exist as two isoforms differing in subunit stoichiometries and agonist sensitivities (Zwart and Vijverberg 1998; Nelson et al. 2003; Tapia et al. 2007; Kuryatov et al. 2008). Presumed (α4)2(β2)3-nAChR have comparatively high agonist sensitivity, but their overall levels of expression as functional nAChR are lower than for ‘low sensitivity’, (α4)3(β2)2-nAChR (Carbone et al. 2009). The differences in function between these two isoforms could be attributed to the presence of either the nAChR α4 or β2 subunit in the closed assembly's accessory subunit position.
As shown in the current studies, when subunits are presumably expressed in equal proportions and are expected to have an equal chance to incorporate into a closed receptor assembly, and certainly into the accessory subunit position, co-expression with wild-type β3 subunits produces an ~2-fold lower level of functional α4β2*-nAChR expression having slightly lower overall sensitivity to ACh but not nicotine. The lower level of functional expression could reflect diversion of complexes containing β3 subunits into a pool of functionally inactive, dead end intermediates Kuryatov et al. (2008). Another possibility is that β3 subunit incorporation is restricted to the accessory subunit position in such a way that the level of expression of (α4β2)2β3-nAChR is like that of high sensitivity (α4)2(β2)3-nAChR. However, ACh or nicotine, respectively, have ~2-fold lower or unaltered potency when acting at α4β2β3V9'S-nAChR than at α4β2-nAChR, and even substitution of mutant β3V9'S for wild-type β3 subunits does not markedly affect levels of function or agonist potencies. Moreover, even when oocytes were injected with 10 : 1 : 1 ratios of α4:β2:β3 or β3V9'S subunits to bias toward formation of low sensitivity α4β2-nAChR, levels of function were diminished even more, and as opposed to effects on all the other nAChR subtypes, there was a > 2-fold effect on agonist potency. Thus, we agree more with Kuryatov et al. (2008) that β3 subunit incorporation promotes formation of dead end, α4β2*-nAChR intermediates rather than having a profound, dominant-negative effect on function.
The presence of mutant β3V9'S subunits differentially altered magnitudes of Imax responses to ACh or to nicotine and potencies of those agonists for each form of β2*-nAChR. The agonist with higher potency elicited a smaller increase in peak current amplitude for β2β3V9'S*-nAChR, perhaps consistent with overall lower functional responsiveness of isoforms having higher agonist sensitivity. However, given that β3V9'S subunits seem to integrate into complexes, likely into the accessory subunit position, it is not possible to explain these findings based on formation of isoforms differing in α:β2 subunit ratios, which is associated with differences in agonist sensitivity. By contrast, differences between agonists in terms of functional potencies or magnitudes of current elicited are largely absent for β4*-nAChR in the presence of wild-type or mutant β3 subunits, suggesting that β3 subunits more neatly replace the third β4 (or α) subunit in those complexes to yield more homogenous populations of (α)2(β4)2(β3)1-nAChR.
There were no dramatic effects of wild-type β3 or mutant β3V9'S subunits on rates of desensitization of the different nAChR subtypes examined. However, it is clear that β4*-nAChR are more resistant to desensitization than are β2*-nAChR, and only for α4β2-nAChR there was evidence for two phases of desensitization in the presence of ACh, consistent with some prior observations (Paradiso and Steinbach 2003; Yu et al. 2009).
In conclusion, the results suggest that natural incorporation of β3 subunits could occur into each of the nAChR subtypes investigated. Such incorporation is predicted to have modest effects on sensitivity to natural ACh or pharmacologically-provided nicotine or other nicotinic pharmaceuticals. Co-expression with β3 subunits also would be predicted to generally have modest effects on levels of nAChR function through a milder than previously suggested dominant-negative mechanism, the exceptions being a larger effect on α3β2*-nAChR functional levels and an effect on α4β2-*-nAChR reflective instead of dead end intermediate formation. Further studies are needed to determine whether β3 subunit co-expression effects are on receptor assembly, trafficking, or biophysical properties and are due to influences of extracellular, cytoplasmic or other domains in the accessory subunit.
Supplementary Material
Acknowledgements
Research described in this article was supported through their External Research Program by Philip Morris USA Inc. and Philip Morris International, by the National Institutes of Health (R01 DA015389), and by endowment and capitalization funds from the Men's and Women's Boards of the Barrow Neurological Foundation. The contents of this report are solely the responsibility of the authors and do not necessarily represent the views of the aforementioned awarding agencies. We thank the Barrow Neurological Institute's Dr Paul Whiteaker for comments about the project and manuscript.
Abbreviations used
- ACh
acetylcholine
- Imax
peak current response
- nAChR
nicotinic acetylcholine receptor(s)
Footnotes
Portions of this work have been presented in abstract form: Dash B, Zhang J, Chang Y and Lukas RJ (2007). Pharmacological properties of nicotinic receptors containing a mutant β3 (val273ser) subunit expressed in Xenopus oocytes Soc Nsci Abst 33: 39.19.
The authors do not have any conflicting or competing interests.
Supporting information
Additional supporting information may be found in the online version of this article:
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Baker ER, Zwart R, Sher E, Millar NS. Pharmacological properties of α9 α10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol. Pharmacol. 2004;65:453–460. doi: 10.1124/mol.65.2.453. [DOI] [PubMed] [Google Scholar]
- Boorman JP, Groot-Kormelink PJ, Sivilotti LG. Stoichiometry of human recombinant neuronal nicotinic receptors containing the b3 subunit expressed in Xenopus oocytes. J. Physiol. 2000;529(Pt 3):565–577. doi: 10.1111/j.1469-7793.2000.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JP, Beato M, Groot-Kormelink PJ, Broadbent SD, Sivilotti LG. The effects of β3 subunit incorporation on the pharmacology and single channel properties of oocyte-expressed human α3β4 neuronal nicotinic receptors. J. Biol. Chem. 2003;278:44033–44040. doi: 10.1074/jbc.M211719200. [DOI] [PubMed] [Google Scholar]
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, Sivilotti LG. Incorporation of the beta3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol. Pharmacol. 2006;70:1350–1357. doi: 10.1124/mol.106.026682. [DOI] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. Pentameric concatenated (α4)(2)(β2)(3) and (α4)(3)(β2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br. J. Pharmacol. 2009;156:970–981. doi: 10.1111/j.1476-5381.2008.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Substitutions of the highly conserved M2 leucine create spontaneously opening rho1 gamma-aminobutyric acid receptors. Mol. Pharmacol. 1998;53:511–523. doi: 10.1124/mol.53.3.511. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h α2 β2, h α2 β4, h α3 β2, h α3 β4, h α4 β2, h α4 β4 and h α7 expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, et al. The β3 nicotinic receptor subunit: a component of α-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J. Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash B, Bhakta M, Chang Y, Lukas RJ. Identification of N-terminal, extracellular domain determinants in nicotinic acetylcholine receptor (nAChR) {α}6 subunits that influence effects of wild-type or mutant {β}3 subunits on function of {α}6{β}2*-or {α}6{β}4*-nAChR. J. Biol. Chem. 2011a;286:37976–37989. doi: 10.1074/jbc.M111.263673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash B, Chang Y, Lukas RJ. Reporter mutation studies show that nicotinic acetylcholine receptor (nAChR) {α}5 subunits and/or variants modulate function of {α}6*-nAChRs. J. Biol. Chem. 2011b;286:37905–37918. doi: 10.1074/jbc.M111.264044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Zanardi A, et al. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype α3β2(α5 or β3) enriched in retinocollicular afferents. Mol. Pharmacol. 2005;68:1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the α3β4* and α3β3β4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot Kormelink PJ, Luyten WH. Cloning and sequence of full-length cDNAs encoding the human neuronal nicotinic acetylcholine receptor (nAChR) subunits beta3 and beta4 and expression of seven nAChR subunits in the human neuroblastoma cell line SH-SY5Y and/or IMR-32. FEBS Lett. 1997;400:309–314. doi: 10.1016/s0014-5793(96)01383-x. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J. Biol. Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, Sivilotti LG. Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br. J. Pharmacol. 2001;134:789–796. doi: 10.1038/sj.bjp.0704313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in α4β2(*) nicotinic receptors. Mol. Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol. Pharmacol. 2004;65:1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both α- and β-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Nelson ME, Wang F, Kuryatov A, Choi CH, Gerzanich V, Lindstrom J. Functional properties of human nicotinic AChRs expressed by IMR-32 neuroblastoma cells resemble those of α3β4 AChRs expressed in permanently transfected HEK cells. J. Gen. Physiol. 2001;118:563–582. doi: 10.1085/jgp.118.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J. Biol. Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat α4β2 neuronal nicotinic receptors. J. Physiol. 2003;553:857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JC, Sarkar D, Quick MW, Lester RA. Interactions of atropine with heterologously expressed and native α3 subunit-containing nicotinic acetylcholine receptors. Br. J. Pharmacol. 2003;138:801–810. doi: 10.1038/sj.bjp.0705124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol. Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol. Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2 + permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol. Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Yu KD, Liu Q, Wu J, Lukas RJ. Kinetics of desensitization and recovery from desensitization for human α4β2-nicotinic acetylcholine receptors stably expressed in SH-EP1 cells. Acta Pharmacol. Sin. 2009;30:805–817. doi: 10.1038/aps.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and non-competitive effects. Mol. Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]
- Zwart R, Van Kleef RG, Vijverberg HP. Physostigmine and atropine potentiate and inhibit neuronal α4 β4 nicotinic receptors. Ann. N Y Acad. Sci. 1999;868:636–639. doi: 10.1111/j.1749-6632.1999.tb11339.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.