There are four groups of individuals in whom the diagnosis of toxoplasmosis is most critical: pregnant women who acquire their infection during gestation, fetuses and newborns who are congenitally infected, immunocompromised patients, and those with chorioretinitis (14, 20, 25). Although the diagnosis of patients in each of these four groups has been hampered by a number of problems, the most frequent challenge encountered by physicians the world over is how to determine if a pregnant woman acquired the acute infection during gestation. Women who acquired their infection prior to pregnancy are essentially not at risk for delivering an infected infant (unless the woman is immunosuppressed).
In the United States there is no systematic screening of pregnant women to detect seroconversion during gestation. Much of the literature is based on studies from France, where such screening is performed monthly to detect recently acquired infection. Thus, in the United States, a single serum sample from each woman is submitted for evaluation, and from this sample the physician hopes to learn if the patient has recently been infected, thereby placing the fetus at risk. The high prevalence and lifelong persistence of toxoplasma immunoglobulin G (IgG) antibodies among healthy individuals in many geographical areas preclude the use of any titer in any serologic test as reflecting recent infection. Another problem is the frequent lack of reliability when IgM, IgA, or IgE toxoplasma antibody test results are used in an attempt to discriminate between recent and more distant infection. In addition there are the vagaries associated with lack of quality control, specificity, and/or sensitivity of many commercial serologic test kits on the market. In this setting, physicians and other health care workers responsible for the care of pregnant women are confused when faced with conflicting results and disagreements about interpretations of results. This often leads to incorrect information being provided by the laboratories to the physicians as well as by the physicians to their patients.
In recent years, a major effort has been made toward improving our ability to diagnose recently acquired infection in the pregnant woman and congenital infection in the fetus and newborn. We now have a number of new methods that are proving to be of great value towards this end. Among these are the serum IgG avidity test, PCR with body fluids and tissues, and Western blots of serum from mother-baby pairs. At present, the first two methods are being widely used in Europe. Hopefully they will become more readily available in the United States as well. Although our focus here is on pregnant women and newborns, these methods are finding wide use in the other groups of patients alluded to above. Our purpose here is to review each of these methods, what is known about their proper interpretation, and their strengths and shortcomings.
AVIDITY
The IgG avidity test was developed to help discriminate between past and recently acquired infection. Results are based on the measurement of the avidity (functional affinity) of toxoplasma-specific IgG antibodies. Following an antigenic challenge, the antibodies produced usually have a low average affinity. During the course of the immune response, there is maturation of antibody affinity that increases progressively over weeks or months. Increase in IgG affinity results from an antigen-driven B-cell selection process, resulting in an increase in complementarity of the antigen-antibody-binding site. Tightness of the binding of the antibody to the antigen is established through chemical forces such as hydrogen binding or electrostatic and van der Waals interactions. In the toxoplasma IgG avidity enzyme-linked immunosorbent assay (ELISA), urea or another protein-denaturing agent is used to dissociate the antigen-antibody complex. The resulting titer reflects urea-resistant and total IgG and is determined using the ratios of optical densities of urea-treated and -untreated samples.
The method, originally developed by Hedman and his associates in Finland (9), is available in kit form in Europe. Commercial kits have not yet been licensed in the United States. Such licensure would more readily make available a test that would significantly lessen the likelihood of misdiagnosis. It would decrease the frequently required use of confirmatory or follow-up serologic tests, including PCR analysis of amniotic fluid; would decrease the need for spiramycin; and would remove the anxiety associated with further testing.
Depending on the method used, the avidity tests currently available are helpful primarily to rule out that a patient's infection occurred within the prior 4 to 5 months. This is most useful in pregnant women in their first months of gestation who have a positive test for both IgG and IgM toxoplasma antibodies. For example, a woman who has a high avidity test result in her first trimester did not acquire the acute infection in the preceding 3 months. Therefore, since her infection was acquired prior to gestation her fetus is essentially not at risk (the likelihood of congenital transmission as a result of an infection acquired in the weeks before or near the time of conception is extremely low, approaching zero [10, 20]). In the United States, the avidity method is particularly valuable in the common situation in which only a single serum sample, drawn during the first months of gestation, is available. In one recent study, high-avidity antibodies were demonstrated retrospectively in 17.5% of 40 women for whom spiramycin had been recommended because recently acquired infection could not be ruled out in the single serum samples available from each of them (12). Examples of the usefulness of the IgG avidity test are shown in Table 1.
TABLE 1.
Usefulness of the IgG avidity testa for pregnant women whose tests for IgM antibodies yielded positive results in the first trimester of pregnancyb
| Patient no. | Gestation wk | Dye test (IgG) titer | IgM titer | Avidity |
|---|---|---|---|---|
| 73 | 9 | 256 | Positive | High |
| 58 | 12 | 512 | Positive | High |
| 17 | 12 | 256 | Positive | High |
| 74 | 12 | 1,024 | Positive | High |
Vidas IgG avidity test (Bio Merieux, Lyon, France).
The combination of the high Dye test (IgG) titers and positive IgM test titers would strongly suggest to physicians that these women acquired their infection during gestation. The avidity test results reveal that each of the women was infected prior to the present pregnancy (see text).
Critical to proper interpretation of results in the IgG avidity test in the presence of IgM antibodies is the fact that a low-avidity result does not mean the patient had a recently acquired infection. Low-avidity results may persist for as long as 1 year. In addition, substantial numbers of patients will have borderline or equivocal results (12; J. G. Montoya, H. B. Huffman, and J. S. Remington, Abstr. 40th Annu. Meet. Infect. Dis. Soc. Am., abstr. 270, p. 92, 2002). (In one study, 40% of women who had IgG antibodies but no demonstrable IgM antibodies had borderline or low-avidity antibodies [J. G. Montoya et al., Abstr. 40th Annu. Meet. Infect. Dis. Soc. Am.].) An appropriate decision on how patients with low- or equivocal-avidity test results should be managed must be made using results of other serologic methods. For this purpose a panel of serologic tests is routinely performed at the Toxoplasma Serology Reference Laboratory of the Palo Alto Medical Foundation (http://www.pamf.org/serology/). The serologic panel consists of ELISA for IgM, IgA, and IgE, the Dye test (measures IgG antibodies), and the differential agglutination test (15). We consider the appropriate use of the avidity test to be as a confirmatory test along with a panel of other serologic tests as suggested by Ashburn and colleagues (1). Indeed, the avidity test should not be used alone as a definitive test for decision making (J. G. Montoya et al., Abstr. 40th Annu. Meet. Infect. Dis. Soc. Am.).
IgG AND IgM WESTERN BLOTS OF MOTHER-INFANT PAIRS
Serologic diagnosis of congenital toxoplasmosis in the newborn is most commonly made by demonstration of serum IgM or IgA Toxoplasma gondii antibodies in the infant. The serologic diagnosis cannot be made by demonstration of IgG antibodies in the newborn since maternal IgG antibodies are passively transferred in utero to the fetus. However, caution must be exercised in interpretation of IgM or IgA test results in the newborn since transmission of maternal IgM and/or IgA antibodies can occur during the birth process. Because of the relatively brief half-life of IgM and IgA, positive tests for these antibodies usually must be confirmed by repeat testing at 2 to 4 days of life in the case of IgM antibodies and at 10 days of life for IgA antibodies. In addition, some newborns with congenital toxoplasmosis may be negative for IgM or IgA antibodies or both early during the newborn period. To determine if IgG antibody in such infants is due to maternal transfer or to the infants' own immune response to the infection, monthly testing for IgG antibodies in the infant may be required for 6 months or longer depending on the original titer of IgG antibodies in the mother (maternal antibodies in the offspring decrease by approximately half each month of life) (20).
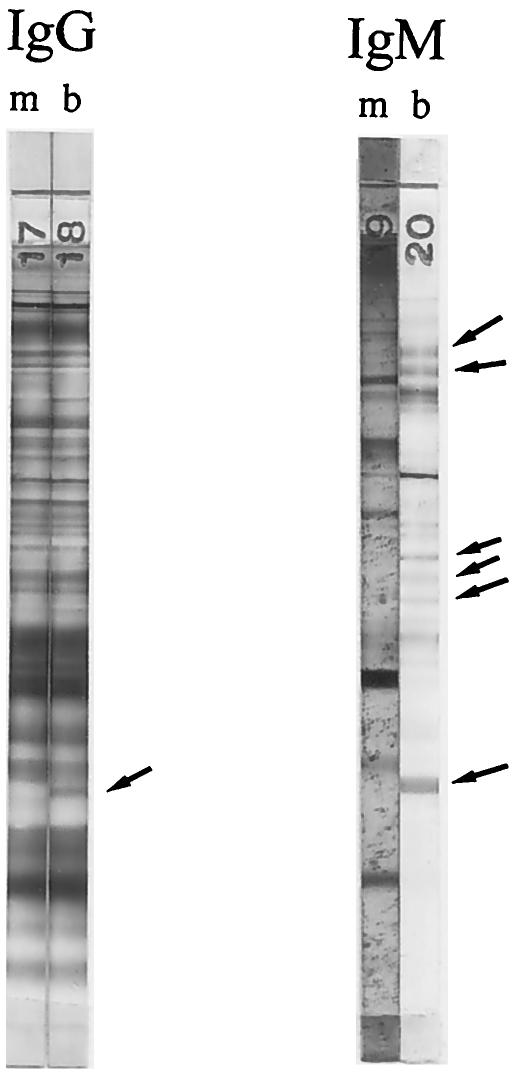
During attempts to develop new methods for diagnosis of the infection in the fetus and newborn, we demonstrated the usefulness of Western blots of paired maternal and baby sera for this purpose. In studies published in 1985, Remington et al. reported that bands were clearly demonstrable in IgG and/or IgM Western blots of serum from infected infants that were not present in blots of serum of their mothers (19) (Fig. 1). Had the IgG and/or IgM antibodies of the newborn been due to passive transfer of maternal antibodies before or at the time of parturition, one would not expect the bands in the blots of mother and baby to differ other than perhaps in intensity. That study confirmed and extended the observations published by Pinon and Gruson in 1982, who employed a unique enzyme-linked immunofiltration assay and demonstrated that antibodies formed by the fetus in response to the congenital infection may recognize different T. gondii antigens than do antibodies formed by the mother (17).
FIG. 1.
IgG and IgM Western blots of serum from a mother (m) and her newborn (b). Sera were drawn from mother and baby when the baby was 2 days of age. Arrows point to bands in the blot of the serum of the baby that were not present in the corresponding blot of the serum from the mother. Serologic test results for the mother were as follows (the baby’s results are in parentheses): for the Dye test, 8,000 (4,096); for the IgM ELISA, 2.9 (for the IgM ISAGA, 3), both positive; for the IgA ELISA, 1.8 (1.3), both positive; for the IgE ELISA: negative (positive); for the PCR on placental tissue, negative. Toxoplasma was isolated from the placental tissue.
Since the original description of immunoblotting for diagnosis of congenital toxoplasmosis by Remington et al. in 1985, almost 10 years elapsed before additional reports of its use appeared in the literature. Of note in this regard is that more than one-third of the published abstracts at the International Conference on Toxoplasmosis, held in Denmark in June 2003, were on the subject of the use of Western blots for diagnosis of toxoplasmosis. Recently, Tissot-Dupont et al. published results of their prospective evaluation of IgG, IgM, and IgA blots for routine use for diagnosis of congenital toxoplasmosis (26). These investigators used a modification of the method described by Remington et al. (3, 19) with sera from 126 infants born to mothers who had seroconverted during gestation. Twenty-three infants were proven on follow-up for 1 year to have congenital toxoplasmosis, and for 103 infants infection was ruled out by disappearance of IgG antibodies on follow-up during the first year of life. Standard serologic tests were used during the first year of life, and Western blots were performed at day 0 and/or day 5 and at 1 and 3 months of life. Western blots proved more sensitive than the IgM immunosorbent agglutination assay (ISAGA) (86.9 versus 69.6%); specificities were 96.1 and 92.2%, respectively. Sensitivity for early diagnosis of the congenital infection increased to 91.3% when both these tests were used in combination. Use of IgA Western blots was least sensitive; the combination of IgG and IgM Western blots was most sensitive. Results similar to those of Tissot-Dupont et al. were reported by Pinon et al. in a study involving 14 laboratories supported by the European Community Biomed 2 program (16).
When serum obtained in the first few days of life is used, a negative result on Western blots must be interpreted with caution since a positive result may not be demonstrable for some weeks after birth. Cases have been observed in which IgM antibody tests were negative but Western blots were positive in the first months of life. In addition, prenatal treatment during gestation as well as postnatal treatment of the baby may result in false-negative results. The observation of greater intensity of bands in an infant's blot compared to the identical bands in the blots of the mother's serum should be considered an equivocal result since this pattern has been observed in noninfected newborns.
In the United States, where systematic serologic screening of pregnant women is uncommonly performed, the Western blot method appears to be a welcome addition to the more commonly used tests for diagnosis of congenital toxoplasmosis in the early months of life. It is important to point out that the method should always be used in combination with other serologic tests such as the IgM and IgA ELISA or ISAGA. In addition, no single or combination of serologic tests or the Western blot method allows for diagnosis of the congenital infection in all cases. Thus, in the absence of such early diagnosis, it is critical that all infants at risk be carefully monitored by repeated testing. Of note is that in some cases, a 4- to 5-month delay in synthesis of IgG T. gondii antibodies may be the sole biologic marker of congenital infection (20). Use is being made in Europe of a Western blot kit for IgG and IgM toxoplasma antibodies produced by LDBIO Diagnostic in Lyon, France. Rilling et al. (21) in Germany reported their results with this LDBIO kit in a retrospective study of 175 infants and noted a sensitivity of 67% at birth and a specificity of 96%. When combined with other serologic methods, the sensitivity increased to 78% at birth and to 85% within the first 3 months of life. Overall, the combination of conventional serologic tests with Western blots detected 94% of congenitally infected infants within the first 3 months of life. In another retrospective study of 17 neonates, Robert-Gangneux et al. (22), using their own laboratory method for IgG and IgM blots, reported a sensitivity of 88.2% within the first 2 months of life and a specificity of 100%. Similarly, using the LDBIO IgG and IgM Western blot kit at birth, Franck et al. reported a positive predictive value (PPV) of 100% and a negative predictive value (NPV) of 89.3%; for the combined results of the IgM and IgA ISAGAs, the PPV and NPV were 95.2 and 88.2%, respectively. At 90 days of life PPV and NPV for the IgG and IgM blots were 100 and 98.7%, respectively; for the combined results of the IgM and IgA ISAGAs, the PPV and NPV were 100 and 89.3% (M. Paul, Abstr. VIII Eur. Multicolloq. Parasitol., p. 6, 2000). They stated that false-negative results at birth were primarily observed in newborns treated with pyrimethamine-sulfonamides in utero. The importance of follow-up in newborns at risk is highlighted by an infant observed by V. Medroni and her colleagues (personal communication to J. S. Remington [2003]). In their series of 20 infected neonates they observed 1 in whom conventional serologies for IgM and IgA antibodies were negative at birth. The results of the blots using the LDBIO kit in the baby further demonstrate the value of this method. At birth, the IgG blot revealed only a few pale bands, none of which differed from those in the IgG blot of the mother. Whereas there were no bands in the IgM blot of the baby at birth, they appeared at 15 days of age, at which time the serologic tests remained negative. Bands in the infant's IgG blot first appeared at 30 days, and at that time conventional serologies also became positive. The mother had acquired her infection very late in gestation and was not treated.
Of note is that observations by a number of our colleagues in reference laboratories in France have not found blots of mother-baby serum pairs performed using Western blotting more helpful than conventional serologic methods which include testing for IgG, IgM, and IgA antibodies. Western blotting of mother-baby serum pairs is likely most useful in newborns in whom IgA and/or IgM toxoplasma antibodies are not demonstrable by conventional methods but whose mothers had definite or highly suspect acute acquired infection during gestation.
PCR
The PCR has been successfully used to diagnose congenital and ocular toxoplasmosis and toxoplasmosis in immunocompromised patients. For this purpose PCR with amniotic fluid, placental and brain tissues, whole blood, cerebrospinal fluid, urine, vitreous fluid, aqueous humor, bronchoalveolar lavage fluid, and pleural and peritoneal fluids has proved of value. The most common use of PCR is for prenatal diagnosis of the congenital infection using amniotic fluid.
Most laboratories use the 35-fold-repeated B1 gene (8). Some laboratories in Europe are switching to the AF146527 sequence, a DNA fragment that is repeated 200- to 300-fold in the T. gondii genome (11, 18). An evaluation of three targets (18S ribosomal DNA, B1, and AF146527) in parallel has recently been performed by the toxoplasmosis group in Strasbourg, France (6). The performance of the three PCR targets and mouse inoculation were compared against each other in 44 newborns (83 samples) born to mothers who were infected during gestation. The differences in the sensitivity and specificity of these three PCR techniques were not found to be statistically significant (6). Further studies are needed to assess whether the AF146527-based PCR assay has an advantage over the most commonly used B1 gene assays.
There is a wide variety of protocols used by different laboratories that employ the three-stage conventional PCR procedure (extraction, amplification, and detection) (2). Specific procedural steps for which significant divergence among different groups of investigators has been reported include those used for DNA extraction, selection of primers, use of the enzyme uracyl-DNA-glycosylase to prevent carryover contamination, use of hot start, the size of the PCR product, and use of internal control or nested PCR (2).
Prenatal diagnosis of congenital toxoplasmosis is primarily based on ultrasonography and PCR with amniotic fluid. PCR with amniotic fluid for the detection of T. gondii-specific DNA performed from 18 weeks of gestation is more sensitive, more rapid, and safer than conventional diagnostic procedures involving fetal-blood sampling (10). Amniotic fluid testing by PCR is indicated in all pregnant women with serologic test results diagnostic or highly suggestive of acute infection acquired during gestation and also if there is evidence of fetal damage by ultrasound examination (e.g., hydrocephalus and/or calcifications). Hohlfeld et al. reported the performance of a competitive PCR test performed on amniotic fluid from 339 consecutive women from Paris, France, who acquired acute T. gondii infection during pregnancy (10). In this study amniocentesis was performed between 18 and 38 weeks. PCR with amniotic fluid had a PPV of 100% and an NPV of 99.7% (10). In a subsequent and more recent study, Romand et al. reported the performance of the same PCR method used by Hohlfeld et al. for 271 pregnant women from Paris, Lyon, and Marseille, France, who were diagnosed with acute T. gondii infection during gestation (24). In this study, amniocentesis was performed in most patients at least 4 weeks after the estimated date of infection (systematic serologic screening at monthly intervals is performed in all pregnant women in France) but not prior to 18 weeks of gestation. PCR with amniotic fluid had a PPV of 100%, a specificity of 100%, an NPV of 88%, and a sensitivity of 64% (24). It is important to emphasize that the PCR method was the same in both studies, but the evaluation of sensitivity and NPV differed. In the study by Romand et al., sensitivity and NPV were evaluated in comparison with the definitive diagnosis in the infants obtained by serologic follow-up during the first year of life. As expected it revealed that all cases of congenitally transmitted toxoplasmosis cannot be detected prenatally. In the study by Hohlfeld et al. such follow-up was not performed. The primary aim of their study was to demonstrate that PCR with amniotic fluid was safer, more rapid, and more sensitive than the conventional approaches to diagnosis. Sensitivity and NPV were estimated in comparison with prenatal results of conventional methods and ultrasound but not with whether the infant definitely was infected.
The reliability of the PCR test when performed before 18 weeks of gestational age is unknown (10, 24). The specificity and PPV of amniotic fluid PCR for prenatal diagnosis of congenital toxoplasmosis have been reported to be close to 100% (10, 24). In contrast, the sensitivity and NPV vary significantly according to gestational age at which maternal infection was acquired (24). Sensitivity of prenatal diagnosis with PCR is significantly higher when maternal infection occurs between 17 and 21 weeks compared with infection occurring before 17 or after 21 weeks of gestation (P < 0.02) (24).
Children born to mothers who acquired the primary infection during gestation should be evaluated at birth for the possibility of congenital toxoplasmosis. Offspring of mothers chronically infected with the parasite but who are immunologically compromised (e.g., those with human immunodeficiency virus infection or those receiving high-dose immunosuppressive drugs) should also undergo a thorough diagnostic workup to rule out the possibility of congenital toxoplasmosis. PCR with cerebrospinal fluid, whole blood, and urine has been successfully used and could be included in the evaluation of these newborns (7, 20, 27).
Real-time PCR has recently been introduced for the diagnosis of toxoplasmosis (4, 5). It combines the steps of amplification and PCR product detection in a single phase, thereby shortening the turnaround time from 24 to 48 h to less than 4 h. Real-time PCR uses a fluorescence-labeled oligonucleotide probe, which eliminates the need for post-PCR processing. DNA extraction methods have also recently been automated. Using magnetic-bead technology, DNA can be purified from a variety of clinical samples, thereby eliminating the need for centrifugation, vacuum pumps, and other steps with high risk for contamination (13). It is likely that the use of real-time PCR and automated methods for DNA extraction will result in a decrease in the interlaboratory variability observed with the conventional three-stage PCR. In addition real-time PCR can be used to estimate the concentration of parasites in amniotic fluid, which may be helpful for physicians to assess neonatal outcome (i.e., maternal infections acquired before 20 weeks with a parasite of load of >100/ml of amniotic fluid were found by Romand et al. to have the highest risk of severe fetal outcome [23]).
The three methods discussed above have significantly contributed to our ability to diagnose toxoplasmosis. It should be understood, however, that the ultimate usefulness of these tests will depend on a number of factors, including the dependability of the method employed (e.g., quality control of commercial kits), the reliability of the laboratory performing the test, and the accuracy of the interpretation of results according to the specific clinical situation under consideration.
Acknowledgments
This work was supported by U.S. Public Health Service grant AI04717 from the National Institutes of Health.
REFERENCES
- 1.Ashburn, D., A. W. Joss, T. H. Pennington, and D. O. Ho-Yen. 1998. Do IgA, IgE, and IgG avidity tests have any value in the diagnosis of toxoplasma infection in pregnancy? J. Clin. Pathol. 51:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretagne, S. 2003. Molecular diagnostics in clinical parasitology and mycology: limits of the current polymerase chain reaction (PCR) assays and interest of the real-time PCR assays. Clin. Microbiol. Infect. 9:505-511. [DOI] [PubMed] [Google Scholar]
- 3.Chumpitazi, B., A. Boussaid, H. Pelloux, C. Racinet, M. Bost, and A. Goullier-Fleuret. 1995. Diagnosis of congenital toxoplasmosis by immunoblotting and relationship with other methods. J. Clin. Microbiol. 33:1479-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa, J. M., P. Ernault, E. Gautier, and S. Bretagne. 2001. Prenatal diagnosis of congenital toxoplasmosis by duplex real-time PCR using fluorescence resonance energy transfer hybridization probes. Prenat. Diagn. 21:85-88. [DOI] [PubMed] [Google Scholar]
- 5.Costa, J.-M., C. Pautas, P. Ernault, F. Foulet, C. Cordonnier, and S. Bretagne. 2000. Real-time PCR for diagnosis and follow-up of toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J. Clin. Microbiol. 38:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filisetti, D., M. Gorcii, E. Pernot-Marino, O. Villard, and E. Candolfi. 2003. Diagnosis of congenital toxoplasmosis: comparison of targets for detection of Toxoplasma gondii by PCR. J. Clin. Microbiol. 41:4826-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuentes, I., M. Rodriguez, C. J. Domingo, F. Del Castillo, T. Juncosa, and J. Alvar. 1996. Urine sample used for congenital toxoplasmosis diagnosis by PCR. J. Clin. Microbiol. 34:2368-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover, C. M., P. Thulliez, J. S. Remington, and J. D. Boothroyd. 1990. Rapid prenatal diagnosis of congenital Toxoplasma infection by using polymerase chain reaction and amniotic fluid. J. Clin. Microbiol. 28:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedman, K., M. Lappalainen, I. Seppala, and O. Makela. 1989. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-739. [DOI] [PubMed] [Google Scholar]
- 10.Hohlfeld, P., F. Daffos, J.-M. Costa, P. Thuylliez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 11.Homan, W. L., M. Vercammen, J. De Braekeleer, and H. Verschueren. 2000. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 30:69-75. [DOI] [PubMed] [Google Scholar]
- 12.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 13.Loeffler, J., K. Schmidt, H. Hebart, U. Schumacher, and H. Einsele. 2002. Automated extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA Pure LC system. J. Clin. Microbiol. 40:2240-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya, J. G. 2002. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185:S73-82. [DOI] [PubMed] [Google Scholar]
- 15.Montoya, J. G., and J. S. Remington. 1995. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin. Infect. Dis. 20:781-790. [DOI] [PubMed] [Google Scholar]
- 16.Pinon, J. M., H. Dumon, C. Chemla, J. Franck, E. Petersen, M. Lebech, J. Zufferey, M. H. Bessieres, P. Marty, R. Holliman, J. Johnson, V. Luyasu, B. Lecolier, E. Guy, D. H. Joynson, A. Decoster, G. Enders, H. Pelloux, and E. Candolfi. 2001. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J. Clin. Microbiol. 39:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinon, J. M., and N. Gruson. 1982. Interest of ELISA specific and compared immunological profiles in the early diagnosis of congenital toxoplasmosis. Lyon Med. 248:27-30. [Google Scholar]
- 18.Reischl, U., S. Bretagne, D. Kruger, P. Ernault, and J. M. Costa. 2003. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect. Dis. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remington, J. S., F. G. Araujo, and G. Desmonts. 1985. Recognition of different Toxoplasma antigens by IgM and IgG antibodies in mothers and their congenitally infected newborns. J. Infect. Dis. 152:1020-1024. [DOI] [PubMed] [Google Scholar]
- 20.Remington, J. S., R. McLeod, P. Thulliez, and G. Desmonts. 2001. Toxoplasmosis, p. 205-346. In J. S. Remington and J. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 21.Rilling, V., K. Dietz, D. Krczal, F. Knotek, and G. Enders. 2003. Evaluation of a commercial IgG/IgM Western blot assay for early postnatal diagnosis of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:174-180. [DOI] [PubMed] [Google Scholar]
- 22.Robert-Gangneux, F., M. F. Gavinet, T. Ancelle, J. Raymond, C. Tourte-Schaefer, and J. Dupouy-Camet. 1999. Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J. Clin. Microbiol. 37:2893-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romand, S., M. Chosson, J. Franck, M. Wallon, F. Kieffer, K. Kaiser, H. Dumon, F. Peyron, P. Thulliez, and S. Picot. Usefulness of quantitative PCR in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am. J. Obstet. Gynecol., in press. [DOI] [PubMed]
- 24.Romand, S., M. Wallon, J. Franck, P. Thulliez, F. Peyron, and H. Dumon. 2001. Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet. Gynecol. 97:296-300. [DOI] [PubMed] [Google Scholar]
- 25.Thulliez, P., F. Daffos, and F. Forestier. 1992. Diagnosis of Toxoplasma infection in the pregnant woman and the unborn child: current problems. Scand. J. Infect. Dis. Suppl. 84:18-22. [PubMed] [Google Scholar]
- 26.Tissot Dupont, D., H. Fricker-Hidalgo, M. P. Brenier-Pinchart, C. Bost-Bru, P. Ambroise-Thomas, and H. Pelloux. 2003. Usefulness of Western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:122-125. [DOI] [PubMed] [Google Scholar]
- 27.van de Ven, E., W. Melchers, J. Galama, W. Camps, and J. Meuwissen. 1991. Identification of Toxoplasma gondii infections by BI gene amplification. J. Clin. Microbiol. 19:2120-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]