Abstract
Objectives
The aims of this study were to determine the outcome and predictors of renal disease progression in Puerto Ricans with systemic lupus erythematosus (SLE) initially presenting mild renal involvement.
Methods
A retrospective cohort of 61 SLE patients (per American College of Rheumatology classification) with mild renal involvement was studied. Mild renal disease was defined as glomerular filtration rate (GFR) ≥ 90 ml/min in the presence of proteinuria (> 0.25g/day, but < 3.5 g/day), hematuria, and/or urinary cellular casts. Demographic parameters, clinical manifestations, serologic markers, comorbidities, pharmacologic treatments, disease activity and damage accrual were determined at onset of renal disease. Factors associated with renal disease progression were evaluated using recurrent event survival analysis.
Results
Of 61 patients, 55(90.2%) were women. The mean [standard deviation (SD)] age at renal onset was 29(11.2) and the mean (SD) follow-up period was 5.1(3.4) years. Thirty-eight patients had a decline in GFR: Thirty-two had a mild decline (GFR = 60–89 ml/min), five developed moderate to severe renal insufficiency (GFR = 15–59 ml/min), and one evolved to end-stage renal disease (GFR< 15 ml/min). In the Cox model, low C4 levels and proteinuria > 0.5g/day were associated with an earlier decline in GFR.
Conclusions
The majority of SLE Puerto Rican patients initially presenting with mild renal involvement had a decrease in GFR after an average of five years of kidney disease, although most had a mild dysfunction. Low C4 levels and proteinuria were predictors of an earlier decline in GFR. The awareness of these factors may contribute to early identification of individuals at risk of renal deterioration.
Key indexing terms: systemic lupus erythematosus, lupus nephritis, proteinuria, hypocomplementemia, Puerto Ricans, Hispanics
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown etiology that affects a wide variety of tissues and organs [1]. Renal disease occurs in approximately half of adult lupus patients significantly contributing to the morbidity and mortality related to the disease [2–5]. Previous studies have shown that prognosis of lupus nephritis (LN) depends on several factors including sociodemographic, clinical, serological and genetic features [6–8]. In addition, renal markers such as low creatinine clearance, proteinuria, and nephrotic syndrome are associated with poor prognosis among LN patients [2,8].
The Third National Health and Nutrition Examination Survey (NHANES III) reported the increasing prevalence of complications of chronic kidney disease (CKD) at lower levels of glomerular filtration rate (GFR) [9]. In addition, the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommended that treatment of comorbid conditions and adequate early intervention are essential to slow progression of kidney disease and should begin during early stages of CKD (GFR stages I and II) [9]. The burden of illness associated with early stages of CKD has not been systematically studied [10–11]. Thus, it is important to closely evaluate patients presenting normal GFR but with clinical signs of kidney disease because they are at increased risk for adverse outcomes of kidney disease [12].
Previous studies in lupus patients have not examined the outcome of different stages of renal involvement at LN presentation. Particularly, no data are available for patients that have a relatively mild onset of renal disease. Thus, the present study aimed to evaluate the outcome and predictors of renal disease progression in Puerto Rican SLE patients initially presenting mild clinical renal involvement.
MATERIALS AND METHODS
Patients Population
A retrospective cohort study of 61 Puerto Rican patients with mild LN followed at the University of Puerto Rico Medical Sciences Campus, San Juan, Puerto Rico from 1990 to 2005 was performed. All patients fulfilled at least 4 of the 11 American College of Rheumatology (ACR) criteria for SLE [13]. Mild renal disease was defined as a GFR ≥ 90 ml/min at renal onset, and the presence of one or more of the following criteria: (a) persistent proteinuria > 0.25g/day but < 3.5 g/day, or ≥2+ dipstick, (b) hematuria > 5 RBC/hpf attributed to SLE in two or more occasions, and (c) urinary cellular casts. The study was approved by the Institutional Review Board of the University of Puerto Rico, Medical Sciences Campus.
The total number of patients with LN during the study period consisted of 122 patients; 47 patients were not eligible because at renal presentation GFR was under the normal cutoff value (Stages II–V) (n=43) or proteinuria was ≥ 3.5g/day (n=4). Fourteen patients were excluded because serum creatinine levels were not available at onset of renal disease. The latter represented prevalent cases of LN at initial evaluation at our institution in which complete medical records of previous evaluations were not available. Therefore, the study group consisted of 61 eligible patients.
Patients of this cohort had routine visits at three months intervals. Additional visits were scheduled as needed per disease activity or complications. At each routine visit a structured questionnaire was completed to gather information about demographic parameters, health-related behaviors, clinical manifestations, laboratory tests [complete blood cell count, comprehensive metabolic panel, urine analysis, erythrocyte sedimentation rate (ESR), and lipid panel], pharmacologic treatment, disease activity and disease damage. For all patients, a lupus autoantibody panel was performed at the time of SLE diagnosis and afterwards as needed.
Variables
The outcome variable of interest was renal disease progression defined as a decline in renal function below the normal cutoff value of GFR (<90 ml/min), as described by the National Kidney Foundation, K/DOQI clinical practice guidelines [9]. GFR was estimated using the Cockcroft-Gault formula [14]. Given the fact that mild changes in renal function might not be of significant interest due to bias of the GFR estimation formula at higher levels; for the purpose of our study we considered as having renal disease progression only patients presenting at least three consecutive visits with GFR estimations under the normal GFR cutoff value, suggesting a mild but persistent renal function deterioration.
Study variables included factors from the demographic, clinical, serologic and pharmacologic domains at renal onset. The following demographic parameters were described: age at SLE diagnosis (per ACR criteria), age at renal onset, and gender. In addition, disease duration from SLE diagnosis to renal disease onset was calculated. The clinical domain included the assessment of SLE manifestations (per ACR criteria), serologic features, renal biopsy findings (per World Health Organization Classification System) [15], selected comorbid conditions (diabetes mellitus, arterial hypertension, and hyperlipidemia), disease activity and damage accrual. Disease activity was determined with the Systemic Lupus Activity Measure-Revised (SLAM-R) [16]. Disease damage was assessed using the Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI) [17]. Serologic markers included anti-nuclear (ANA), anti-double stranded DNA (dsDNA) antibodies, and serum complements (C3 and C4). The exposure to corticosteroids, hydroxychloroquine (HCQ), cyclophosphamide and azathioprine were also examined. In addition, the use of anti-hypertensive treatment [angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB)] was evaluated.
Statistical analysis
The database construction and the statistical analysis were performed using the statistical software STATA version 11 (STATA Corp, College Station, TX, USA). Descriptive statistics were used to describe the study population. Numerical variables were evaluated using measures of central tendency and dispersion (mean, standard deviation, and median); categorical variables were described using frequencies and percentages. Comparison of the baseline clinical parameters between patients preserving normal renal function and those presenting renal function deterioration at the end of the study period was performed using Student’s t-test (or Mann-Whitney U test) for continuous variables, and by Pearson chi-square (or Fisher’s exact test) for categorical variables.
Survival analysis for recurrent events was employed to evaluate the association between the study covariates at time of mild renal onset and the risk of renal disease progression. Individuals were included in the study if mild renal disease was presented during the period from January 1990 to December 2005. The beginning of the follow-up period initiated in January 1990 and was extended until March 2007. Follow-up period began at mild renal disease onset and ended after three consecutive visits presenting a decline in renal function. Censoring of data was employed in the presence of one of the following conditions: (a) lost to follow-up (censored at time of last study visit), and (b) no development of the outcome of interest (censored at the end of study period). Survival curves of study covariates were estimated using the Kaplan-Meier method for recurrent events and evaluated by the log-rank test. Graphical representations of the survival curves were estimated for the occurrence of the third recurrent event during the follow up period using the marginal approach (time from study entry to development of the third event).
Taking into account the presence of multiple events in one subject; a fitted Cox model was constructed using the counting process approach to account for the presence of multiple failure-time of the same outcome. Robust estimation was used to adjust the variances of the estimated regression coefficients for the correlation of different observations on the same subject. To perform this analysis, we employed the STATA command stcox identifying as the cluster variable the id number of each subject. All covariates with a p-value < 0.05 in bivariate analysis were included in the final Cox model and an adjustment was done by follow-up period to account for the observed marginal tendency (p=0.06) of a larger follow-up in patients entering into the cohort during the early and mid 1990’s thus; having more follow up information might influence estimates of study outcome. The evaluation of the potential confounding effect of gender and age were not considered in the final model given that GFR adjustment (outcome variable) considers these variables in its definition, therefore; they could not be considered as independent predictors of outcome.
RESULTS
Characteristics of patients with mild lupus nephritis
Of the 61 patients included in the study, 55 (90.2%) were females and 6 (9.8%) were males. The mean [standard deviation (SD)] age at SLE diagnosis and renal onset were 26.8 (11.5) and 29.3 (12.1) years, respectively. The mean (SD) duration between SLE diagnosis and renal onset was 2.4 (5.4) years. The mean (SD) follow-up period was 5.1 (3.4) years. At the end of the study, 23 (37.7%) patients remained with a normal renal function (GFR ≥ 90 ml/min) and 38 (62.3%) had a decline in GFR: thirty-two had a mild decline (GFR = 60–89 ml/min), three had a moderate decrease (GFR = 59-30 ml/min), two progressed to severe renal insufficiency (GFR = 15–29 ml/min), and one developed end-stage renal disease. Twenty-five patients had a renal biopsy performed during the study period. Mesangial (28.0%) and membranous (32.0%) glomerulonephritis were the most frequently reported histopathological findings; whereas the diffuse glomerulonephritis and advanced sclerosing were reported in 4% and 8% of the patients, respectively (data not shown).
As described in the methods section, 14 patients were excluded from the analysis because serum creatinine levels were not available at the time of renal disease onset. These patients had proteinuria and/or hematuria attributed to lupus; however, concomitant creatinine levels were missing. Thus, we evaluated other surrogate markers of kidney disease at renal onset such as proteinuria, hematuria, cellular casts, and hypocomplementemia (either C3 or C4), among other serological markers, between the 14 excluded patients and the 61 study patients. We found no significant differences between these groups.
Given that our definition of mild renal disease was based on laboratory findings, we performed an analysis stratifying patients in 2 groups: LN defined by histological findings on renal biopsy (n=25), and LN defined by laboratory findings (n=36). Both groups were similar regarding to age, disease duration, hypertension, presence of anti-dsDNA antibodies, complements levels (C3 and C4), activity, and damage index, as well as in renal parameters such as proteinuria level, presence of cellular casts and hematuria as expected by the definition used to identify mild cases of LN. A tendency of higher unadjusted creatinine levels was observed in patients with biopsy proven LN. However, only one patient in this group had a serum creatinine of 1.3 mg/dL at baseline and after adjustment for age and sex the GFR estimation was normal (data not shown).
Table 1 shows the baseline demographic parameters, clinical manifestations, serologic markers, pharmacologic treatments, disease activity and damage accrual of study participants by renal function group at study end. Patients with proteinuria (≥ 0.5 g/day) and low C4 levels at baseline were more likely to have a decline of GFR (p<0.05). No differences were found for demographic parameters, clinical manifestations or serologic abnormalities between renal function groups. In addition, no differences were noted for selected comorbidities, pharmacologic treatments and SLAM-R and SDI scores.
Table 1.
Baseline characteristics of mild lupus nephritis patients by normal renal function (GFR ≥ 90 ml/min) or renal function decline (GFR <89 ml/min) at the end of study period.
| Features | GFR ≥ 90 ml/min (n=23) | GFR < 90 ml/min (n=38) | p-value |
|---|---|---|---|
| Demographic characteristics | |||
| Gender, female (%) | 19 (82.6) | 36 (94.7) | 0.316 |
| Age at SLE diagnosis | |||
| mean years (SD) | 25.0 (10.1) | 27.8 (12.2) | 0.359 a |
| median | 21.0 | 26.0 | |
| Age at renal onset | |||
| mean years (SD) | 27.0 (10.2) | 30.6 (13.0) | 0.254 a |
| median | 23.0 | 28.0 | |
| Disease duration (from SLE diagnosis to renal onset) | |||
| mean years (SD) | 1.8 (3.7) | 2.7 (6.3) | 0.531 a |
| Follow-up period | |||
| mean years (SD) | 4.6 (2.4) | 6.1 (3.8) | 0.061 a |
| Clinical manifestations, n (%) | |||
| Malar rashc | 8 (36.4) | 11 (32.4) | 0.387 |
| Discoid lupusc | 1 (4.3) | 5 (13.2) | 0.136 |
| Photosensitivityc | 8 (36.4) | 13 (37.1) | 0.730 |
| Oral ulcersc | 5 (21.7) | 7 (18.4) | 0.895 |
| Arthritisc | 7 (30.4) | 10 (26.3) | 0.575 |
| Serositisc | 4 (17.4) | 7 (18.4) | 0.544 |
| Neurologic disorderc | 2 (8.7) | 3 (7.9) | 0.505 |
| Anemia, any etiology | 17 (73.9) | 23 (60.5) | 0.657 |
| Leukopeniac | 9 (39.1) | 11 (28.9) | 0.792 |
| Lymphopeniac | 5 (21.7) | 11 (28.9) | 0.234 |
| Thrombocytopeniac | 5 (21.7) | 6 (15.8) | 0.951 |
| Renal parameters, n (%) | |||
| Proteinuria > 0.5 g/dayc | 4 (17.4) | 10 (26.3) | 0.048 b |
| Hematuria | 16 (69.6) | 19 (50.0) | 0.117 |
| Cellular castsc | 0 (0) | 5 (13.2) | 0.161 |
| Immunological profile, n (%) | |||
| Anti-nuclear antibodies | 21 (91.3) | 33 (86.8) | 0.797 |
| Anti-dsDNA antibodies | 17 (73.9) | 29 (76.3) | 0.931 |
| Low C3 levels | 11 (47.8) | 18 (52.9) | 0.674 |
| Low C4 levels | 10 (43.5) | 24 (68.6) | 0.009 b |
| Comorbid conditions, n (%) | |||
| Arterial hypertension | 12 (52.2) | 16 (42.1) | 0.345 |
| Diabetes mellitus | 2 (8.7) | 1 (2.6) | 0.320 |
| Dyslipidemia | 5 (21.7) | 12 (31.6) | 0.597 |
| Treatment, n (%) | |||
| Corticosteroids (prednisone or equivalent) | 0.218 | ||
| Low-dose (≤ 10mg) | 3 (13.0) | 13 (34.2) | |
| Mid-dose (>10mg, < 60mg) | 14 (60.9) | 17 (44.7) | |
| High-dose (≥60mg) | 3 (13.0) | 4 (10.5) | |
| Hydroxychloroquine | 11 (47.8) | 16 (42.1) | 0.494 |
| Cyclophosphamide | 2 (8.7) | 4 (10.5) | 0.371 |
| Azathioprine | 6 (26.1) | 6 (15.8) | 0.447 |
| ACE inhibitors | 7 (30.4) | 8 (21.1) | 0.585 |
| ARB | 1 (4.3) | 1 (2.6) | 0.322 |
| SLAM-R, mean score (SD) | 9.7 (5.3) | 8.2 (3.8) | 0.225 |
| SDI, mean score | 0.2 (0.5) | 0.2 (0.5) | 0.834 |
Mann-Whitney U Test
Significant (p<0.05)
As defined by the American College of Rheumatology (ACR) classification criteria for SLE
ACE: Angiotensin-converting enzyme; ARB: Angiotensin II receptor blockers; SLAM-R: Systemic Lupus Activity Measure-Revised; SDI: Systemic Lupus International Collaborating Clinics/ACR Damage Index
Survival analysis
Twenty-three patients of the 61 patients were censored during the study period. Of them, 14 (23.0%) did not developed renal deterioration at the end of the follow-up period. The remaining 9 (14.8%) patients were lost to follow-up and thus were censored at the time of the last visit reported in the medical record. However, 5 of the 9 patients lost to follow-up had at least one visit during the last year of follow-up (from March 2006 to March 2007).
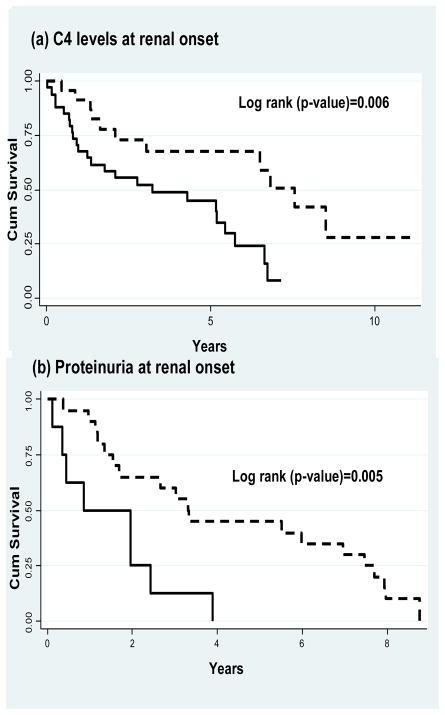
Survival curves were estimated for the occurrence of the third consecutive time that a decline in GFR was observed for those patients experiencing a mild renal deterioration. Figure 1 shows the Kaplan Meier (KM) survival curves for (a) C4 levels, and (b) proteinuria level. Patients with low C4 levels at baseline had a median survival time for renal function deterioration of 3.22 years compared to a median survival time of 7.56 years among individuals with normal C4 levels at renal onset (log-rank test=0.006). In addition, patients with proteinuria >0. 5 g/day had a median survival time for renal function deterioration of 0.84 years compared to a median survival time of 3.30 years among patients with proteinuria ≤ 0. 5 g/day (log-rank test=0.005).
Figure 1.
Kaplan-Meier (KM) plots for C4 levels and proteinuria. (a) KM survival curves showing cumulative survival of patients with normal C4 levels (dotted line) or low C4 (continuous line) at renal onset (log rank=0.006). (b) KM survival curves showing cumulative survival of patients with proteinuria ≤ 0.5 g/day (dotted line) or proteinuria > 0.5 g/day (continuous line) at renal onset (log rank=0.005).
Cox Proportional Hazards Fitted Model
Bivariate and multivariate analyses of factors associated with renal disease progression are presented in table 2. In the bivariate analysis, low C4 levels [HR 2.40, 95% CI 1.24–4.62], and proteinuria > 0.5 g/day [HR 2.65, 95% CI 1.39–5.03] at mild renal onset, were associated with an increased risk of renal deterioration. Other variables such as the presence of cellular casts [HR 1.18, 95%CI 0.39–3.60], SLAM score > 8 [HR 1.55, 95%CI 0.81–2.98], and dyslipidemia [HR 2.12, 95%CI 0.87–5.17], showed a tendency to be related with renal deterioration (data not shown). In addition, the use of SLE pharmacotherapy and anti-hypertensive drugs (ACE inhibitors) did not contribute to the potential risk or protective effect of renal deterioration: high-dose corticosteroids [HR 1.07, 95%CI 0.66–1.78], hydroxychloroquine [HR 1.30, 95%CI 0.67–2.54], cyclophosphamide [HR 1.66, 95%CI 0.57–4.81], azathioprine [HR 0.73, 95%CI 0.30–1.75], and ACE inhibitors [HR 0.84, 95%CI 0.38–1.85].
Table 2.
Univariate and multivariate analysis of factors associated with mild renaldisease progression (GFR <90 ml/min)
| Variable | Univariate analysis | Multivariate analysis† | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| C4 (Low levels) | 2.40 (1.24–4.62) | 0.009 | 1.95 (1.03–3.70) | 0.039 |
| Proteinuria (> 0.5 g/day) | 2.65 (1.39–5.03) | 0.003 | 2.32 (1.20–4.47) | 0.012 |
Mutivariate fitted Cox model was adjusted for follow-up period
Given the possibility that low C4 levels could be associated with other SLE manifestations, we compared patients with low C4 levels and those with normal C4 levels at renal onset. No significant differences were observed between these groups regarding SLE manifestations and other serologic markers except for a significant association with low C3 levels (data not shown). However, the association with low C3 was expected because the two covariates are highly correlated.
A significant association between C4 levels, and proteinuria with the development of renal disease progression was also observed in the multivariate fitted Cox analysis. After adjustment for follow-up period, patients with low C4 complement levels at mild renal involvement had 1.95 increased risk of renal function deterioration compared to individuals with normal C4 levels (95% CI 1.03–3.70). In addition, patients with proteinuria > 0.5 g/day had two times the risk of renal disease progression compared to patients with levels of proteinuria ≤ 0.5 g/day at mild renal onset (95% CI 1.20–4.47).
DISCUSSION
Renal disease is a known risk factor for poor prognosis in patients with SLE due to the risk of evolution into ESRD and its association with cardiovascular disease [18]. Even though survival of SLE patients with renal involvement has improved due to advances in disease knowledge and treatments during the past four decades, the morbidity and mortality related to chronic renal disease remain significant among SLE patients [19–20]. Renal manifestations in lupus patients are highly heterogeneous ranging from asymptomatic proteinuria to chronic renal failure [2,7]. However, disease progression in patients presenting with clinical signs of renal disease but with normal renal function at onset is not well known. The present study was performed among incident cases of LN Puerto Rican patients with mild clinical kidney disease at renal onset. The results showed that most patients presenting with mild clinical renal disease, remained with mild renal involvement during the first five years from renal onset. Of the 61 patients initially presenting with normal renal function (GFR ≥ 90 ml/min), a decline in renal function (GFR <90 ml/min) was observed in 62.3%. However; most (84%) of them had a mild decrease in GFR (GFR 60–89 ml/min) and only one patient progressed to ESRD. Multivariate analysis showed that low C4 levels and proteinuria > 0.5 g/day at LN onset were predictors of an early decline in GFR.
Hypocomplementemia was found to be a predictor of renal disease progression among this lupus cohort. Low C4 levels, but not low C3 levels, are linked to more aggressive forms of lupus nephritis (21). Furthermore, C4 activation is associated with the pre-flare period of renal disease in lupus patients [22]. C4 deficiency in SLE may be the result of an inherited deficiency and/or complement consumption due to active disease. Inheritance of one or more null alleles for C4 predisposes to the development of SLE [23]. Total deficiency of C4 is rare but is strongly related with SLE and renal disease [24]. C4 may impair the effective clearance of immune complexes and apoptotic cells leading aberrant tolerance induction and immune dysregulation [25]. The latter mechanism together with the deposition of complement in affected tissues and their participation in the inflammatory reaction of the disease may result in tissue and organ damage [25].
The association of proteinuria with a subsequent decline in GFR was expected for several reasons. Proteinuria appears to be both a marker of glomerular dysfunction and a direct mediator of renal disease progression. Protenuria may lead to hyperplasia of proximal tubular epithelium resulting in contraction of proximal tubular volume and impairment of glomerular filtration [26]. In addition, albuminuria seems to activate tubular cells to express the macrophage-directed chemokine monocyte chemoattractant protein-1, resulting in macrophage infiltration and subsequent renal damage [27]. Further damage of tubular cells may be induced by the heavy load of fatty acids that are bound to albumin [28]. In agreement with the results presented here, previous studies have shown that proteinuria is a prognostic factor of renal disease progression in SLE patients [29–30]. Thus, lupus patients with mild renal involvement should be routinely monitored with urine protein quantification and must be closely evaluated and treated for factors that are associated with worsening of proteinuria and its related decline in renal function such as arterial hypertension and dyslipidemia.
Our study has some limitations. The definition of mild renal disease was based on laboratory parameters of renal disease and not on kidney biopsy findings. However, we performed an analysis to evaluate if patients with biopsy proven LN differed from those with LN based on abnormal laboratory parameters and found that both groups were similar with regards to clinical signs of renal disease and other parameters related to disease activity. A study performed with a larger sample size did not find significant differences in disease duration, anti-dsDNA antibodies and albumin between patients with biopsy proven LN and those with clinical signs of LN; but found statistical differences in age and damage activity index [31]. In our study these differences were not observed making the groups more homogeneous.
We also recognize that any formula using serum creatinine concentrations to calculate GFR has limitations. These might not be suitable for estimation of GFR in patients with significant variations in dietary intake or muscle mass (e. g., malnutrition, muscle wasting), as these affect serum creatinine. Furthermore, variations in the results may occur if the measurement of creatinine is not accurately calibrated and standardized among different laboratories. Another limitation is that our study was based on medical record examination and given its retrospective nature, the evaluation of other factors and lifestyle behaviors was not possible. In addition, given to the limited study sample the power of certain covariates (such as high blood pressure and dyslipidemia) that have been linked with renal outcomes in lupus patients was limited. We were also unable to find differences by immunosupressive agents as well as a possible protective effect of hydroxychloroquine and ACE inhibitors due to the low number of patients being treated with these medications. Therefore, the role of these factors must be considered with a more suitable sample in future studies. Finally, the study included only Hispanics from Puerto Rico; thus, results are not intended to be generalized to other ethnic populations.
Finally, we recognize that the evaluation of adjusted mean serum C4 and urine protein levels as numeric variables is a better method to evaluate outcome. However, given the retrospective nature of the study and the data collection method used to perform the analysis (medical chart review), it was not possible to evaluate them as continuous variables. This limitation was due to the fact that tests were performed in different laboratories; thus, normal ranges varied according to the methodology and equipment used affecting the homogeneity of the tests results among subjects. Even though it would be more informative to report the results as continuous variables and to evaluate tests sensitivity and predictive values, the present study was not aimed to evaluate the predicting capacity of these tests as diagnostic tools for renal disease progression. However, future prospective studies should consider a receiving operating curves (ROC) analysis to precisely estimate absolute values of these parameters and their ability to predict outcomes. Unfortunately this evaluation was out of the scope for our study.
Despite these limitations, the present study has important clinical implications for lupus patients with mild renal involvement, especially since some studies suggest that the prevalence of LN is probably higher among SLE patients due to the fact that some individuals have renal disease without any clinical manifestations (silent lupus nephritis) [32–33]. To our knowledge, this is the first study evaluating renal outcome in a well-defined group of patients with mild renal disease. Our study showed that the majority of SLE Puerto Ricans patients initially presenting with mild renal involvement, had a decrease of GFR after an average of five years of renal disease; however, most of them presented only a mild decline. Proteinuria and low C4 complement were predictors of an early decrease in renal function in this group of Hispanic lupus patients. The awareness of these factors and more timely and effective interventions may help to prevent a decline in renal function.
Acknowledgments
Support: Supported by the National Center for Research Resources (NCRR/NIH) Grant 1U54RR026139-01A1, NIH-NCRR RCMI Grant G12RR03051, and by unrestricted educational grants from Bristol-Myers Squibb, Inc. and Abbott Laboratories, Inc.
References
- 1.Ramsey-Goldman R, Manzi S. Systemic Lupus Erythematosus. In: Goldman MB, Hatch M, editors. Women and Health. Academic Press; 2002. pp. 704–723. [Google Scholar]
- 2.Contreras G, Pardo V, Cely C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus. 2005;14:890–895. doi: 10.1191/0961203305lu2238oa. [DOI] [PubMed] [Google Scholar]
- 3.Mak A, Mok CC, Chu WP, et al. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus. 2007;16:28–34. doi: 10.1177/0961203306074469. [DOI] [PubMed] [Google Scholar]
- 4.Balow JE. Clinical presentation and monitoring of lupus nephritis. Lupus. 2005;14:25–30. doi: 10.1191/0961203305lu2055oa. [DOI] [PubMed] [Google Scholar]
- 5.Sidiropoulus PI, Kritikos HD, Boumpas DT. Lupus nephritis flares. Lupus. 2005;14:49–52. doi: 10.1191/0961203305lu2059oa. [DOI] [PubMed] [Google Scholar]
- 6.Alarcón GS, McGwin G, Petri M, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PloS Medicine. 2006;3:e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastian HM, Roseman JM, McGwin G, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–160. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 8.Mok CC, Mak A, Chu WP, et al. Long-term survival of southern Chinese patients with systemic lupus erythematosus: a prospective study of all age-groups. Medicine (Baltimore) 2005;84:218–224. doi: 10.1097/01.md.0000170022.44998.d1. [DOI] [PubMed] [Google Scholar]
- 9.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–S246. [PubMed] [Google Scholar]
- 10.Hsu CY, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1567–1576. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 11.Coladonato J, Klassen P, Owen WF., Jr Perception versus reality of the burden of chronic kidney disease in the United States [Editorial] J Am Soc Nephrol. 2002;13:1686–1688. doi: 10.1097/01.asn.0000019646.05890.ea. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 15.Churg J, Sobin LH. Lupus Nephritis: renal disease classification and atlas of glomerular disease. Igaku-Shoin. 1982:127–149. [Google Scholar]
- 16.Liang MH, Socher SA, Larson MG, et al. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107– 1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 17.Stoll T, Stucki G, Malik J, et al. Association of the Systemic Lupus Erythematosus International Collaborating Clinics/American College of Rheumatology Damage Index with systemic lupus erythematosus. J Rheumatol. 1997;24:309– 313. [PubMed] [Google Scholar]
- 18.Rahman A, Isenberg DA. Systemic Lupus Erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 19.Mok CC, Lau CS, Chan TM, et al. Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus. 1999;8:188–196. doi: 10.1191/096120399678847605. [DOI] [PubMed] [Google Scholar]
- 20.Bono L, Cameron JS, Hicks JA. The very long-term prognosis and complications of lupus nephritis and its treatment. QJM. 1999;92:211–218. doi: 10.1093/qjmed/92.4.211. [DOI] [PubMed] [Google Scholar]
- 21.Franco C, Yoo W, Franco D, Xu Z. Predictors of end stage renal disease in African Americans with lupus nephritis. Bull NYU Hosp Jt Dis. 2010;68(4):251–6. [PubMed] [Google Scholar]
- 22.Birmingham D, Irshaid F, Nagaraja HN, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus. 2010;19(11):1272–80. doi: 10.1177/0961203310371154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Chung EK, Zhou B, et al. The intricate role of complement component C4 in human systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:98–132. doi: 10.1159/000075689. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Lhotta K, Chung EK, et al. Complete complement components C4A and C4B deficiencies in human kidney diseases and systemic lupus erythematosus. J Immunol. 2004;173:2803–14. doi: 10.4049/jimmunol.173.4.2803. [DOI] [PubMed] [Google Scholar]
- 25.Gullstrand B, Mårtensson U, Sturfelt G, et al. Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells. Clin Exp Immunol. 2009;156:303–11. doi: 10.1111/j.1365-2249.2009.03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebert LA, Agarwal G, Sedmak DD, et al. Proximal tubular epithelial hyperplasia in patients with chronic glomerular proteinuria. Kidney Int. 2000;57:1962–7. doi: 10.1046/j.1523-1755.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- 27.Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–97. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 28.Arici M, Chana R, Lewington A, et al. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J Am Soc Nephrol. 2003;14:17–27. doi: 10.1097/01.asn.0000042167.66685.ea. [DOI] [PubMed] [Google Scholar]
- 29.Manger K, Manger B, Repp R, et al. Definition of risk factors for death, end-stage renal disease, and thromboembolic events in a monocentric cohort of 338 patients with systemic lupus erythematosus. Ann Rheum Dis. 2002;61:1065–1070. doi: 10.1136/ard.61.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christopher-Stine L, Siedner M, Lin J, et al. Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol. 2007;34:332–335. [PubMed] [Google Scholar]
- 31.Yip J, Aghdassi E, Su J, et al. Serum albumin as a marker for disease activity in patients with systemic lupus erythematosus. J Rheumatol. 2010 Jun 1; doi: 10.3899/jrheum.091028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Zabaleta-Lanz M, Vargas-Arenas RE, Tápanes F, Bianco NE, et al. Silent lupus nephritis in systemic lupus erythematosus. Lupus. 2003;12:26–30. doi: 10.1191/0961203303lu259oa. [DOI] [PubMed] [Google Scholar]
- 33.Zabaleta ME, Muñoz LE, Tapanes FJ, et al. Further description of early clinically silent lupus nephritis. Lupus. 2006;15:845–851. doi: 10.1177/0961203306070002. [DOI] [PubMed] [Google Scholar]