Abstract
We report the first synthesis of the C-terminally spermine-conjugated stapled peptide-based inhibitors of the p53-Mdm2 interaction. Subsequent biological, biophysical and cellular uptake assays with the spermine-conjugated stapled peptides revealed that spermine conjugation minimally affects biological activity while significantly increases peptide helicity and cellular uptake without apparent cytotoxicity.
Keywords: spermine, peptides, helical structure, protein-protein interaction, cellular uptake
Peptide helices are found frequently at the interfaces of protein-protein interactions that mediate all facets of cellular processes including cell cycle control and apoptosis.1 As a result, there has been a growing interest recently aimed at translating these helical peptides into biological therapies targeting the intracellular protein-protein interactions.2 Since short peptides exhibit poor pharmaceutical properties, i.e., poor cell permeability and low metabolic stability, numerous strategies have been developed to increase their proteolytic stability as well as their cellular uptake. Among them, selective cross-linking of peptide side-chains (also referred to as peptide ‘stapling’) via selective chemical reactions, e.g., disulfide bond formation,3 olefin cross metathesis,4 lactam formation,5 and 1,3-dipolar cycloaddition reactions,6 has emerged to be one of the most promising approaches.7
Recently, we reported the discovery of a distance-matching cross-linker, 6,6′-bisbromomethyl-[3,3′]bipyridine (Bpy), that reacts with a pair of cysteines located at i,i+7 positions of a short helical peptide, resulting in a significant enhancement in cell permeability.8 While it is known that attachment of the positively charged groups to peptides generally leads to enhanced cellular uptake, most studies in the literature have focused on either direct fusion of polyarginine-like sequences9 such as TAT sequence or substitution of arginines at strategically selected non-continuous sites on the peptides.10 Herein, we report the first synthesis of polyamine-conjugated, stapled peptides and the characterization of the effect of polyamine conjugation on peptide bioactivity, helicity, and cell permeability.
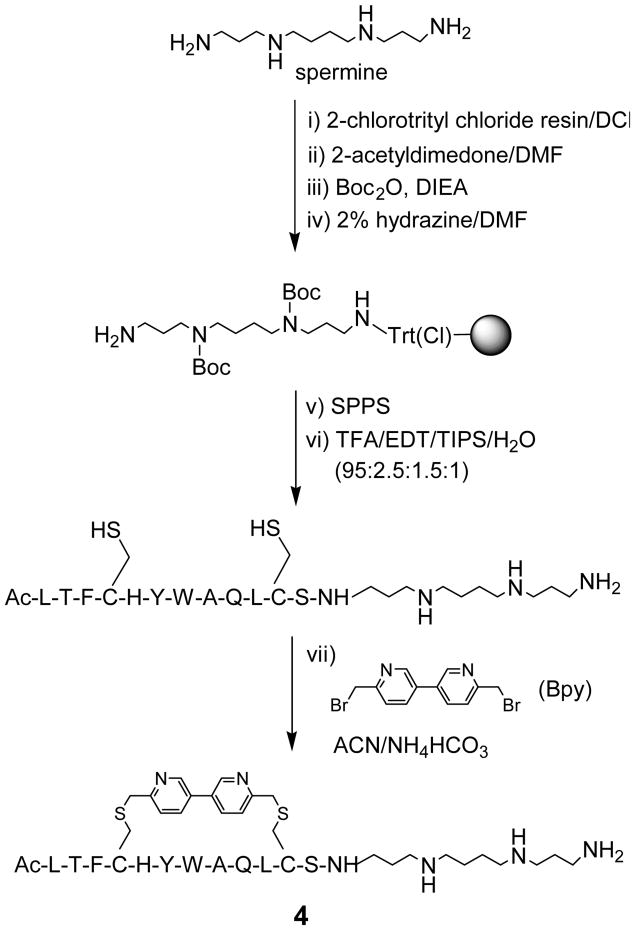
Polyamines such as spermine are positively charged at the physiological pH, and have been used through conjugation as an intracellular delivery aid for the lipophilic small-molecule drugs11 as well as the negatively charged siRNA.12 However, the effect of polyamine conjugation to synthetic peptides on cellular transport of peptides has not been reported.13 According to helical dipole model,14 placement of the positive-charged capping groups at the carboxyl termini of short helical peptides leads to increased helicity due to the favourable charge-dipole interactions.15 To probe whether this model is operative in the case of short stapled peptides, we conjugated spermine at the C-terminus of a dicysteine-containing peptide inhibitor of p53–Mdm2 interaction (sequence = LTFCHYWAQLCS), which was used previously as a model in our peptide stapling studies.8 Scheme 1 shows a representative synthesis of the spermine-conjugated, stapled peptide 4 in seven steps based on a solid phase procedure. Briefly, spermine was first immobilized onto solid support by treating 2-chlorotrityl chloride resin with excess amount of spermine in dichloromethane for 30 min. After extensive washing, selective protection of the secondary amines with Boc was achieved by first masking the terminal primary amine with 2-acetyldimedone16 to form the N-[1,(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl (Dde) derivative followed by treatment with di-tert-butyl dicarbonate. The Dde-protecting group was selectively removed by treating the resin with 2% hydrazine in DMF for 15 min. With the spermine-functionalized resin in hand, standard Fmoc-based solid phase peptide synthesis was performed and the linear cysteine-containing peptide was released from the resin after treatment with a TFA-containing cleavage cocktail. After HPLC purification, the fully deprotected linear peptide was incubated with 1.5 equiv of Bpy in a mixed acetonitrile/30 mM NH4HCO3 solution at pH 8.5. HPLC analysis of the crude product indicated that the stapling reaction of the spermine-conjugated peptide proceeded with greater than 95% conversion after 1.5 hours (Figure S1). Using this route, we also prepared spermine-conjugated, D,L-dicysteine-stapled peptide 8 because the D,L-dicysteine-stapled peptides have shown greater inhibitory activity towards Mdm2 in our previous work.8
Scheme 1.
Representative synthesis of a spermine-conjugated stapled peptide 4 on solid support.
To gauge how spermine conjugation affects stapled peptide's biological activity, we determined the inhibitory activities of the spermine-conjugated cross-linked peptides with ELISA and compared them to other stapled peptides reported previously8 (Figure S2 and Table 1). Interestingly, whereas substitution of two residues at solvent-exposed side of the helix with arginines (His5→Arg and Gln9→Arg) led to addition of +2 charge accompanied by substantial drop (6~20-fold) in inhibitory activity8 (compare 3 to 2; 7 to 6), spermine conjugation resulted in addition of +3 charge and significantly smaller reduction in inhibitory activity (compare 4 to 2; 8 to 6). Therefore, spermine conjugation appears to provide a less disruptive route to the addition of net positive charge.
Table 1. Sequence and Activity of Peptide-Based Inhibitorsa.
| peptide | sequenceb | charge | IC50, nM |
|---|---|---|---|
| 1 | LTFCHYWAQLCS | 0 | 57 ± 5 |
| 2 | LTFC′HYWAQLC′Sc | 0 | 35 ± 5 |
| 3 | LTFC′RYWARLC′S | +2 | 237 ± 16 |
| 4 | LTFC′HYWAQLC′S-Spd | +3 | 46 ± 6 |
| 5 | LTFcHYWAQLCSe | 0 | 13 ± 1 |
| 6 | LTFc′HYWAQLC′Sf | 0 | 5 ± 1 |
| 7 | LTFc′ RYWARLC′S | +2 | 102 ± 8 |
| 8 | LTFc′HYWAQLC′S-Sp | +3 | 18 ± 2 |
ELISA assays were repeated three times to obtain average IC50 values and standard deviations.
For non-spermine containing peptides, the N-termini were acetylated and the C-termini were amidated. For spermine-conjugated peptides, the N-termini were acetylated.
C′ denotes Bpy-linked cysteine.
Sp denotes spermine.
c denotes D-cysteine.
c′ denotes Bpy-linked D-cysteine.
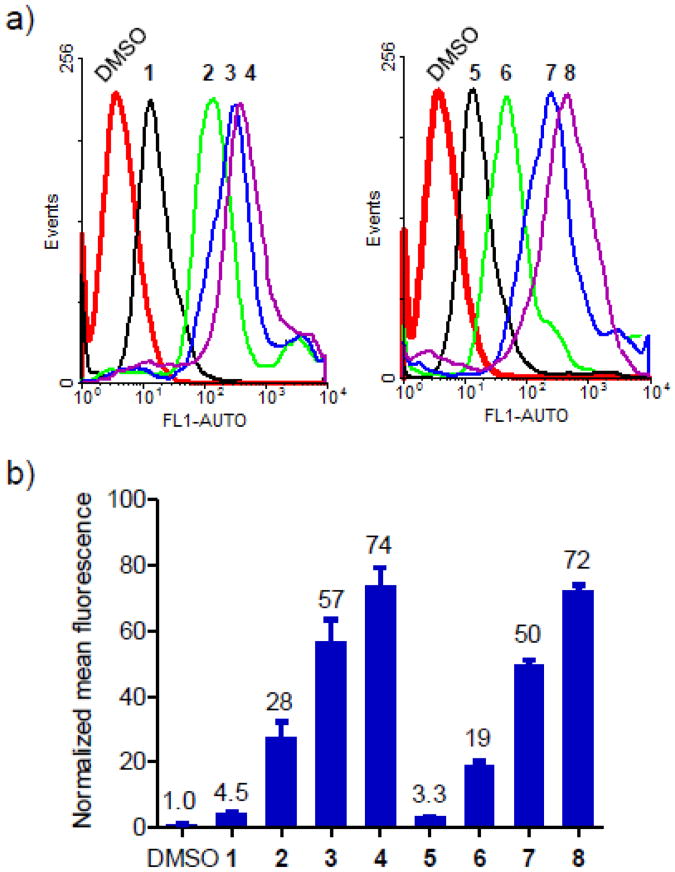
To examine how spermine conjugation affects cellular uptake, we synthesized the N-terminal fluorescein-labeled, spermine conjugated, stapled peptides 4 and 8 (Fluo-4 and -8, Table S1) and compared their uptake into HeLa cells as measured by fluorescence activated cell sorting (FACS) to the previously reported fluorescein-labeled stapled peptides8 (Fluo-1–3 and Fluo-5–7). As shown in Figure 1, peptide stapling significantly enhanced the cellular uptake (compare Fluo-2–4 to -1; Fluo-6–8 to -5). Among the stapled peptides, peptides carrying the positive charges (Fluo-3, -4, -7, and -8) showed significantly higher uptake than the charge-neutral peptides (Fluo-2 and -6); the spermine-conjugated stapled peptides Fluo-4 and -8 exhibited the highest uptake, yielding 16- and 22-fold increase, respectively, over their charge-neutral linear counterparts (compare Fluo-4 to -1; Fluo-8 to -5), indicating that spermine conjugation acts in concert with peptide stapling to further enhance the cellular uptake. The higher uptake observed for the spermine conjugated, stapled peptides Fluo-4 and -8 relative to the arginine-substituted, stapled peptides Fluo-3 and -7 could also be attributed to the localized positive charge at the C-termini of Fluo-4 and -8 versus the dispersed positive charge in Fluo-3 and -7.
Figure 1.
Flow cytometry analysis of HeLa cells after treatment with 10 μM fluorescein-conjugated peptides, Fluo-1–8: (a) Representative histograms; (b) Bar graph showing the normalized mean fluorescence. For simplicity, Fluo- prefixes were omitted in the stapled peptide denotations.
To probe structural changes induced by spermine conjugation, we determined the percent helicity of all stapled peptides with far-UV CD measurement (Figure 2). While cysteine cross-linking invariably led to modest increases in percent helicity (compare 2–4 to 1; 6–8 to 5), the spermine-conjugated stapled peptides showed additional 50% increases in percent helicity than the arginine-substituted stapled peptides (compare 4 to 3; 8 to 7). This enhancement in helicity is consistent with the helical dipole model15 in which the C-terminally conjugated, positive-charged spermine stabilizes helical dipole by forming additional hydrogen bonds with C-terminal unpaired carbonyl groups. A correlation between percent helicity and cell permeability appears to exist as peptides with higher percent helicity invariably exhibited higher cellular uptake.
Figure 2.
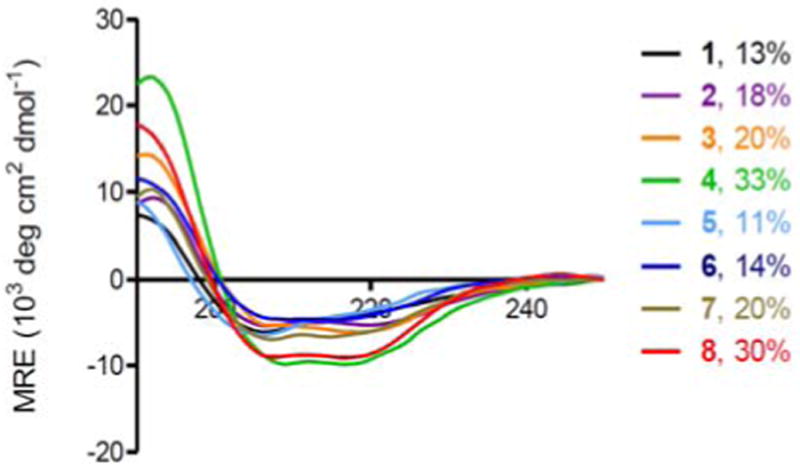
CD spectra of the linear and Bpy-stapled peptides with or without spermine conjugation and their calculated percent helicity values: The peptides were dissolved in 20% ACN/H2O for a final concentration of 50 μM. The percent helicity was calculated based on the [θ]222 value.
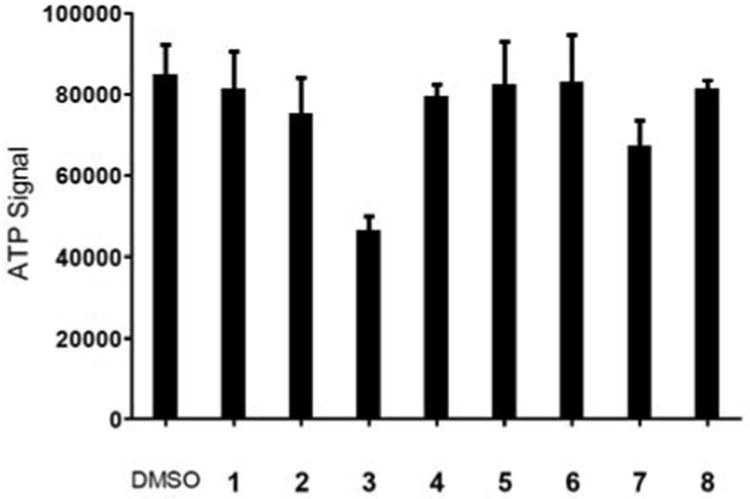
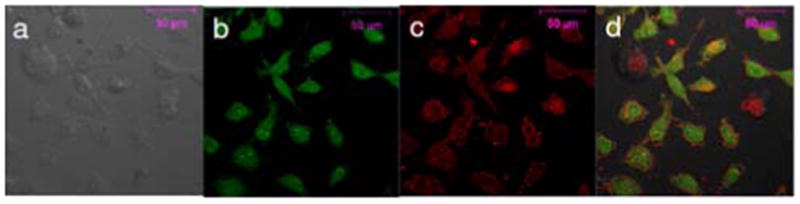
To corroborate the FACS results on cellular uptake, a confocal fluorescent microscopy study was performed. We found that while the positive-charged stapled peptides showed stronger intracellular fluorescence than the charge-neutral ones, Fluo-3 and -7 treated cells produced significant amount of cellular debris which was absent in Fluo-4 and -8 treated cells (Figure S3). This difference in apparent cytotoxicity was verified by ATP assay. Cells treated with the spermine-conjugated stapled peptides (4 and 8) were found to be completely viable whereas cells treated with the arginine-substituted peptides 3 and 7 showed 50% and 30% decrease in viability, respectively (Figure 3). The cytotoxicity of 3 and 7 could be attributed to the non-specific cell membrane insertion activity of amphipathic arginine-containing peptide helices with one side of the helix to be hydrophobic and the opposite side to be cationic.17 In contrast, the devoid of cytotoxicity for the spermine-conjugated stapled peptides could be attributed to their unique mode of helix stabilization. The possible mechanism for uptake of the spermine-conjugated stapled peptides was then probed with a fluorescence-based subcellular localization study (Figure 4). Fluo-4 showed a more diffusive intracellular distribution, with some overlap with Alexa Fluor 586 labeled transferrin, a fluorescent marker for the recycling endosomes (panel d in Figure 4), suggesting a possible endosome-mediated transport process.
Figure 3.
Spermine conjugation increases cellular uptake of the stapled peptides without apparent cytotoxicity analyzed by ATP assay. The CellTiter-Glo reagent (Promega) was used to measure HeLa cell viability after treatment with 10 μM of the unstapled (1 and 5) or the stapled peptides (2-4 and 6-8).
Figure 4.
Confocal fluorescent microscopy of live HeLa cells after treatment with 10 μM of fluorescein-conjugated 4 for 2 hr followed by incubation with 10 μg/mL Alexa Fluor 586 labeled transferrin for 30 min: (a) DIC channel; (b) FITC channel; (c) Fluor 585 channel; (c) merged images of channels a-c.
In summary, we have demonstrated a small-molecule approach in rendering stapled peptides cationic through the C-terminal spermine conjugation. The attachment can be readily integrated into the solid phase synthesis protocol and compatible with the peptide stapling conditions. Compared to the arginine-substitution approach, spermine conjugation minimally affected biological activity of the stapled peptides while significantly increased peptide helicity. Together with peptide stapling, spermine conjugation acted in concert to further enhance the cellular uptake of the bioactive peptides without apparent cytotoxicity frequently associated with arginine substitution. Inasmuch as the cationic TAT peptide,[9a] spermine conjugation should provide a simple, small-molecule strategy for intracellular delivery of bioactive peptides and other biotherapeutics.
Supplementary Material
Supplemental figures, experimental procedures, and characterization of peptides 4 and 8.
Acknowledgments
We gratefully acknowledge the Pardee Foundation (to Q.L.) and the National Institutes of Health (CA 109636 and CA 118210 to J.C.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Meador WE, Means AR, Quiocho FA. Science. 1992;257:1251. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]; (b) Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]; (c) Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 2.(a) Yin H, Hamilton AD. Angew Chem Int Ed. 2005;44:4130. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]; (b) Verdine GL, Walensky LD. Clin Cancer Res. 2007;13:7264. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J Am Chem Soc. 1991;113:9391. [Google Scholar]
- 4.(a) Blackwell HE, Grubbs RH. Angew Chem Int Ed. 1998;37:3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (b) Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891. [Google Scholar]
- 5.(a) Bracken C, Gulyas J, Taylor JW, Baum J. J Am Chem Soc. 1994;116:6431. [Google Scholar]; (b) Phelan CJ, Skelton NJ, Braisted AC, McDowell RS. J Am Chem Soc. 1997;119:455. [Google Scholar]; (c) Harrison RS, Shepherd NE, Hoang HN, Ruiz-Gomez G, Hill TA, Driver RW, Desai VS, Young PR, Abbenante G, Fairlie DP. Proc Natl Acad Sci USA. 2010;107:11686. doi: 10.1073/pnas.1002498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Cantel S, Isaad ALC, Scrima M, Levy JJ, DiMarchi RD, Rovero P, Halperin JA, D'Ursi AM, Papini AM, Chorev M. J Org Chem. 2008;73:5663. doi: 10.1021/jo800142s. [DOI] [PubMed] [Google Scholar]; (b) Madden MM, Rivera Vera CI, Song W, Lin Q. Chem Commun. 2009:5588. doi: 10.1039/b912094g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Madden MM, Muppidi A, Li Z, Li X, Chen J, Lin Q. Bioorg Med Chem Lett. 2011;21:1472. doi: 10.1016/j.bmcl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, Wahl GM, Walensky LD. Cancer Cell. 2010;18:411. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muppidi A, Wang Z, Li X, Chen J, Lin Q. Chem Commun. 2011;47:9396. doi: 10.1039/c1cc13320a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Fonseca SB, Pereira MP, Kelley SO. Adv Drug Deliv Rev. 2009;61:953. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]; (b) Myrberg H, Zhang L, Mae M, Langel U. Bioconjug Chem. 2008;19:70. doi: 10.1021/bc0701139. [DOI] [PubMed] [Google Scholar]
- 10.a Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J Am Chem Soc. 2007;129:2456. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith BA, Daniels DS, Coplin AE, Jordan GE, McGregor LM, Schepartz A. J Am Chem Soc. 2008;130:2948. doi: 10.1021/ja800074v. [DOI] [PubMed] [Google Scholar]
- 11.(a) Delcros JG, Tomasi S, Carrington S, Martin B, Renault J, Blagbrough IS, Uriac P. J Med Chem. 2002;45:5098. doi: 10.1021/jm020843w. [DOI] [PubMed] [Google Scholar]; (b) Hahn F, Schmitz K, Balaban TS, Brase S, Schepers U. ChemMedChem. 2008;3:1185. doi: 10.1002/cmdc.200800013. [DOI] [PubMed] [Google Scholar]
- 12.Nothisen M, Kotera M, Voirin E, Remy JS, Behr JP. J Am Chem Soc. 2009;131:17730. doi: 10.1021/ja908017e. [DOI] [PubMed] [Google Scholar]
- 13.Endogenous polyamine-peptide conjugates have been detected in human amniotic fluid, see: Seale TW, Chan WY, Shukla JB, Rennert OM. Clin Chim Acta. 1979;95:461. doi: 10.1016/0009-8981(79)90197-9.
- 14.(a) Shoemaker KR, Kim PS, York EJ, Stewart JM, Baldwin RL. Nature. 1987;326:563. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]; (b) Presta LG, Rose GD. Science. 1988;240:1632. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- 15.Forood B, Feliciano EJ, Nambiar KP. Proc Natl Acad Sci USA. 1993;90:838. doi: 10.1073/pnas.90.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash IA, Bycroft BW, Chan WC. Tetrahedron Lett. 1996;37:2625. [Google Scholar]
- 17.Scheller A, Oehlke J, Wiesner B, Dathe M, Krause E, Beyermann M, Melzig M, Bienert M. J Pept Sci. 1999;5:185. doi: 10.1002/(SICI)1099-1387(199904)5:4<185::AID-PSC184>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures, experimental procedures, and characterization of peptides 4 and 8.