Abstract
Research into the neural basis of recognition memory has traditionally focused on the remembrance of visual stimuli. The present study examined the neural basis of object recognition memory in the dark, with a view to determining the extent to which it shares common pathways with visual-based object recognition. Experiment 1 assessed the expression of the immediate-early gene c-fos in rats that discriminated novel from familiar objects in the dark (Group Novel). Comparisons made with a control group that explored only familiar objects (Group Familiar) showed that Group Novel had higher c-fos activity in the rostral perirhinal cortex and the lateral entorhinal cortex. Outside the temporal region, Group Novel showed relatively increased c-fos activity in the anterior medial thalamic nucleus and the anterior cingulate cortex. Both the hippocampal CA fields and the granular retrosplenial cortex showed borderline increases in c-fos activity with object novelty. The hippocampal findings prompted Experiment 2. Here, rats with hippocampal lesions were tested in the dark for object recognition memory at different retention delays. Across two replications, no evidence was found that hippocampal lesions impair nonvisual object recognition. The results indicate that in the dark, as in the light, interrelated parahippocampal sites are activated when rats explore novel stimuli. These findings reveal a network of linked c-fos activations that share superficial features with those associated with visual recognition but differ in the fine details; for example, in the locus of the perirhinal cortex activation. While there may also be a relative increase in c-fos activation in the extended-hippocampal system to object recognition in the dark, there was no evidence that this recognition memory problem required an intact hippocampus.
Keywords: immediate-early genes, hippocampus, perirhinal cortex, recognition memory, retrosplenial cortex
Our understanding of the neural basis of recognition memory (detecting the novelty or familiarity of an event) has been considerably advanced by studies of spontaneous object recognition by rodents. In the most popular version of the task, a rodent first explores a sample object and then, after a delay, is exposed to that same object (now familiar) and a novel alternative (Ennaceur & Delacour, 1988). Normal rats prefer to explore the novel object, and so display their recognition memory. The overwhelming majority of spontaneous object experiments have tested rodents in the light, yet their natural ecology indicates that rats should be proficient at object recognition in the dark.
The very few studies of object recognition in the dark have, indeed, found that rats can readily distinguish novel from familiar objects, with performance levels comparable to those in the light (Albasser et al., 2011; Winters & Reid, 2010). Lesion studies have then shown that rats require parietal cortex, but not perirhinal cortex, to use tactile cues effectively for object recognition, but that the perirhinal cortex is needed when switching from sampling in the dark to recognition testing of the same object in the light or vice versa (Albasser et al., 2011; Winters & Reid, 2010). The effects of lesions in other areas, including the hippocampus, do not appear to have been tested in the rat.
One goal was to compare the neural activity associated with object recognition in the dark with that in the light. As this goal involved multiple sites and required high anatomical resolution, Experiment 1 mapped the expression of the immediate-early gene (IEG) c-fos following exposure to novel stimuli, treating it as an indirect marker for processes related to recognition memory (Aggleton, Brown, & Albasser, 2012). This rationale stems from the repeated finding that c-fos activity increases when rats are shown novel objects or novel visual images (Albasser, Poirier, & Aggleton, 2010; Wan, Aggleton, & Brown, 1999; Wan et al., 2004; Warburton et al., 2003, 2005; Zhu, Brown, McCabe, & Aggleton, 1995). Evidence of a direct link between c-fos expression and visual object recognition is shown by the finding that blocking Fos production in the perirhinal cortex disrupts the long term maintenance of object recognition information (Seoane, Tinsley, & Brown, 2012).
The present study used the “bow-tie maze” to examine object recognition in the dark. Testing with this apparatus is highly suitable for studies in the dark (Albasser et al., 2011) and also permits direct comparisons with studies of c-fos activity related to object recognition in the light (Albasser, Poirier et al., 2010). On the critical final session, one group of rats (Group Novel) was given pairs of objects to discriminate, one novel the other familiar. The control group (Group Familiar) was given the same pairs of objects, but they were all highly familiar, having been exposed to the rats on every previous test session. Attention focused not only on the perirhinal and parietal cortices, but also on prefrontal and hippocampal sites, as these additional regions have variously been implicated in forms of recognition memory (Barker, Bird, Alexander, & Warburton, 2007; Barker & Warburton, 2011a, 2011b; Clark, Zola, & Squire, 2000; Clark, West Zola, & Squire, 2001). Evidence of possible changes in c-fos activity in the hippocampus and related structures led to a second experiment.
Experiment 2 examined the impact of bilateral hippocampal lesions on object recognition in the dark using behavioral protocols very similar to those in Experiment 1. The rationale for this second experiment arose from the long-standing debate over whether the rat hippocampus is necessary for recognition memory (Brown & Aggleton, 2001; Mumby, 2001; Winters, Saksida, & Bussey, 2008). While many studies of object recognition in the light have found no apparent effects of hippocampal lesions (e.g., Aggleton, Hunt, & Rawlins, 1988; Albasser, Lin, Iordanova, Amin, & Aggleton, 2012; Forwood, Winters, & Bussey, 2005; Winters et al., 2008), other studies have reported recognition deficits (e.g., Broadbent, Squire, & Clark, 2004; Clark et al., 2000, 2001). A number of reviews have considered these apparently conflicting results (Brown, Warburton, & Aggleton, 2010; Mumby, 2001; Squire, Wixted, & Clark, 2007; Wixted & Squire, 2011), without reaching a consensus explanation. One potential explanation that has not been explored relates to the extent that nonvisual information is used to guide object recognition. If hippocampal lesions disrupt object recognition memory in the dark, this factor might help explain the variation across studies.
Experiment 1. Expression of c-fos Associated With Object Recognition Memory in the Dark
Materials and Methods
Subjects
Subjects were 20 naïve, male rats (Lister Hooded strain, Harlan, Bicester, U.K.). The rats were 12–14 weeks old at the beginning of the experiment. Rats were food-deprived up to 85% of their free-feeding body weight and were maintained at this level throughout the experiment. Water was available ad libitum. Rats were housed in pairs under diurnal conditions (14:10-h light−dark cycle), and testing occurred at a regular time during the light period. Rats were thoroughly habituated to handling before the study began. All experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act (1986) and associated guidelines.
Apparatus
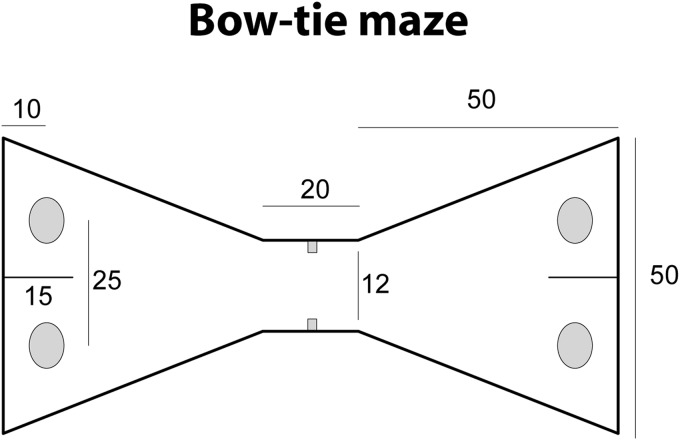
All training and testing was in a bow-tie shaped maze (see Figure 1) made with steel walls and a wooden floor (see Albasser, Chapman et al., 2010). The maze was 120 cm long, 50 cm wide, and 50 cm high. Each end of the apparatus was triangular, the apices of which were joined by a narrow corridor (12 cm wide). An opaque sliding door set in the middle of the corridor could be raised by the experimenter. The far wall of each triangle contained two recessed food wells, 3.5 cm in diameter and 2 cm deep. The food wells were separated by a short, opaque dividing wall that protruded 15 cm from the middle of the end wall. This wall ensured that the rats could not explore both objects at the same time, for example, with their vibrissae. Even so, all rats could readily step around the wall to reach the object on the other side. All food wells were covered by objects in the experiment proper.
Figure 1. Schematic drawing showing a plan, with dimensions, of the bow-tie maze. From “Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-fos imaging” by M. Albasser, G. L. Poirier, and J. P. Aggleton, 2010European Journal of Neuroscience, 31, 134-147. Copyright [2009] by John Wiley and Sons. Adapted with permission.
Objects
A total of 147 different pairs of junk objects were used in the study. The objects in each pair were identical, but across the pairs each object was unique with its own shape, texture, size and color. All objects were large enough to cover a circular food well (3.5 cm diameter) but light enough to be displaced by a rat. Any object with an obvious scent was excluded. All objects were cleaned with alcohol wipes after each session.
Behavioral testing
Rats were arbitrarily divided into two groups: Novel (n = 10) and Familiar (n = 10). Pairs of rats (one from Group Novel and one from Group Familiar) were housed together. The rats in these pairs were trained behaviorally, one immediately after the other. Likewise, the rats in a given pair were processed concurrently for immunohistochemistry. The critical difference between Group Novel and Group Familiar was whether they received novel objects every session (Group Novel) or whether they received the same set of objects, session after session, so that their test objects became increasingly familiar (Group Familiar).
Pretraining
Over seven daily sessions, rats were first habituated to eat in the bow-tie maze, and then trained; i) to run back and forth between the two ends of the maze, and ii) to displace an object covering a food well in order to reach a food reward (for fuller description see Albasser, Poirier et al., 2010). Four pairs of objects were used during pretraining, but these objects were not used in the experiment proper. For pretraining only, the rats were trained in the light. Illumination was provided by ceiling lights giving a mean light intensity of 581.0 lx in the center of the maze.
Dark condition
The design of the experiment was identical to that used to examine c-fos activation associated with object recognition in the light (Albasser, Poirier et al., 2010), except that the experiment proper (after pretraining) was run in complete darkness. All sources of light were switched off or blocked, resulting in a fully darkened room (light intensity of 0.11 lx in the center of the maze). The darkness was such that the experimenters could not see their hands in front of their eyes; consequently, they wore night vision goggles (Productive Firm Dipol Ltd, Belarus) and the session was recorded with two infrared cameras (Maplin Electronics, U.K.) fixed directly above the maze. Rats are unable to see in the infrared spectrum (Burn, 2008).
General training protocol
In order to provide informative comparisons between Groups Novel and Familiar it was necessary to match all training elements between the two groups with the exception of object novelty. All rats received 13 sessions of training over 7 consecutive days. The testing procedure for Session 1 was identical for both groups, and so this session is described below.
Session 1 contained 20 trials, and during each trial the animal could freely explore two objects, one novel the other familiar (Table 1 upper). At the start of each session (Trial 0), a rat was placed on one side of the maze, where a single object (object A1) covered a food well that contained a single sucrose pellet (45 mg; Noyes Purified Rodent Diet, Lancaster, NH). The rat was rewarded for pushing the object and was allowed to explore it freely during a period of 1 minute. For Trial 1, the central sliding door was then raised, and the rat ran to the opposite side of the maze. There, the rat had the free choice between object A2 (a duplicate of A1), which was now familiar, and novel object B1 (Trial 1; see Table 1 upper). Both objects A and B covered baited food wells that were concurrently available to the rat. As there were duplicates of each object, the rat could not mark an object for the next trial.
Table 1. Upper—Experiment 1. Schematic Table Showing the Testing Protocols for Group Novel and Group Familiar. Lower —Experiment 2. Testing Protocol for Object Recognition in the Dark.
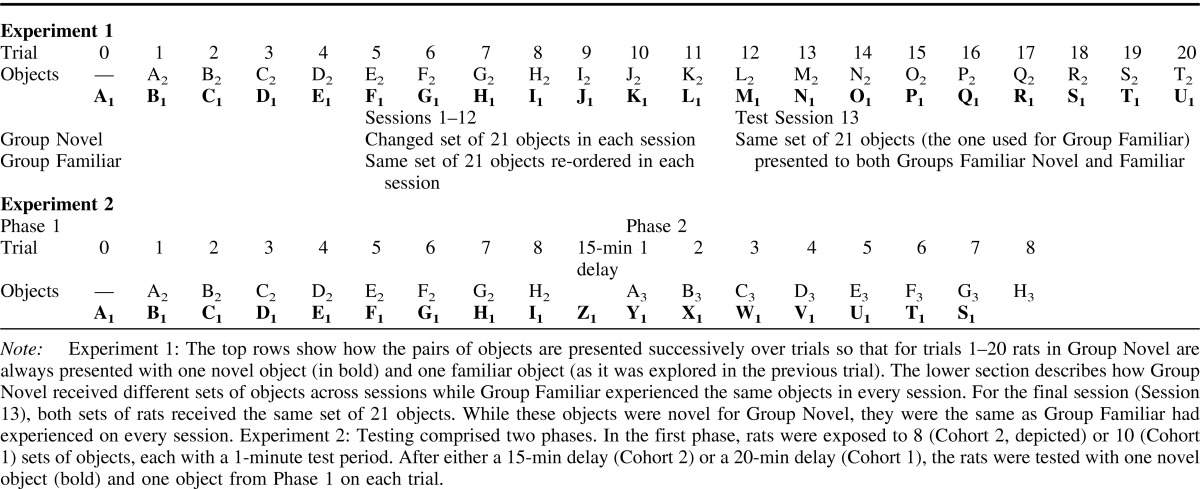
After 1 minute, the guillotine door was raised and Trial 2 began. The rat passed under the door (which was then closed) to explore object B2 (now familiar) and novel object C1 (Table 1 upper). After another minute the guillotine door was raised again (Trial 3), and the rats were exposed to object C2 (now familiar) and novel object D1 (Table 1 upper). Each session used 21 sets of objects (20 plus the object for Trial 0). Throughout training all objects, both novel and familiar, covered a single reward. This feature ensured the rats' continued approach to the objects, but did not affect the validity of the behavioral test of recognition as this relied on differential object exploration. Rats were video-recorded throughout the 13 sessions.
Specific behavioral protocol for Group Novel
Group Novel rats received 12 training sessions over 6 days (two sessions per day: morning and afternoon). Each trial consisted of presentations of two objects, one novel the other familiar. The familiar object was in fact the “novel” object from the previous trial; that is, it had just been explored and so was now familiar (see Table 1 upper). The placement of the novel object varied from left to right according to a pseudorandom schedule. The pool of 126 different pairs of objects was exhausted after six sessions (21 pairs per session), and so the complete pool was reused over the next six sessions (Sessions 7–12), but the order and pairings of the individual objects changed from that used in Sessions 1–6.
Specific behavioral protocol for Group Familiar
Group Familiar rats received 12 training sessions over 6 days (two sessions per day: morning and afternoon). This training was identical to that given to Group Novel, with one critical difference: the same set of 21 objects was used for all 13 sessions, from the first to the final session (see Table 1). This manipulation was designed to ensure that the rats were highly familiarized with every individual object. The order of the objects changed from session to session. The decision to give all rats 12 training sessions was principally to ensure that Group Familiar rats were fully familiar with every object in the set.
Final behavioral test session prior to c-fos immunohistochemistry (Session 13)
On the final session (Session 13), training for Group Novel was identical to that described above. Consequently, Group Novel received a completely new set of 21 pairs of objects; that is, a set that the rats had never experienced. The training procedure for Group Familiar was identical to that for Group Novel as it used the very same set of 21 pairs of objects. Critically, these particular objects were also the same objects used throughout all of the previous 12 sessions for Group Familiar, and so should be highly familiar for just this group (see Table 1). Object order was identical for the two groups.
Analysis of behavior
Rats were video-recorded throughout training, and exploration data were analyzed for Session 1 and Session 13. Object exploration was defined as directing the nose at a distance <1 cm from the object, with the vibrissae moving, and/or touching it with the nose or the paws. Object exploration was not scored when animals sat on the object, when rats used the object to rear upward with the nose of the rat facing the ceiling, or when chewing the object. The duration of exploration was determined by holding down a key pad on a computer during the bursts of exploration recorded on video.
For tests of object recognition, two performance indices were calculated, D1 and D2 (Ennaceur & Delacour, 1988). The D1 index is the duration of exploration time devoted to the novel object minus the exploration time devoted to the familiar object. The “cumulative D1” is the sum of the D1 scores across each trial in a session (or phase within a session). The D2 index also uses the difference in exploration times between the novel object and the familiar object (i.e., D1), but then divides D1 by the total duration of exploration given to both the novel and familiar objects. The resulting D2 ratio can vary between + 1 and −1, with a positive ratio showing a preference for novel objects and a ratio of 0 corresponding to no preference; that is, chance. The D2 index should better compensate for individual changes in amounts of exploration, and so provides the principal index. The D1 index was also calculated as D2 can give anomalous results when exploration levels are very low, and so providing both indices helps to cross-validate any results. The “updated D2” was the D2 ratio recalculated after each trial, combining the raw data from all previous trials. Throughout the experiment the behavioral scoring was blind; that is, the experimenter did not know the group allocation of the individual rats.
Fos immunohistochemistry
On the final test day, animals were processed using previously described immunohistochemical methods for Fos protein (Albasser, Poirier et al., 2010). Ninety minutes after completing the test session, rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, Euthatal, Rhone Merieux, Harlow, U.K.) and transcardially perfused with 0.1 m phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 m PBS (PFA). This interval (90 min) was selected as it is within the typical period of peak production (between 90 and 120 min) for Fos protein after a specific, initiating event (Zangenehpour & Chaudhuri, 2002). The brains were removed and postfixed in PFA for 4 h and then transferred to 25% sucrose overnight at room temperature with continuous rotation. Sections were cut at 40 μm on a freezing microtome in the coronal plane. One series (one-in-three sections) was collected in PBS. Sections were transferred to 10 mm citrate buffer (pH = 6) dissolved in deionized H2O and incubated in a water bath at 70 °C for 30 min. Endogenous peroxidase was blocked by incubating the sections in 0.3% hydrogen peroxide in PBST for 10 min, before rinsing several times with PBST. Sections were next incubated in PBST containing c-fos rabbit polyclonal antibody (1:5000; Ab-5, Oncogene Science, Cambridge, U.K.), for 48 h at 4 °C with periodic rotation. Sections were then washed with PBST and incubated for c-Fos in biotinylated goat antirabbit secondary antibody (diluted 1:200 in PBST; Vectastain, Vector Laboratories, Burlingame, CA) and 1.5% normal goat serum. Sections were then washed and processed with avidinbiotinylated horseradish peroxidase complex in PBST (Elite Kit, Vector Laboratories) for 1 h at room temperature, again with constant rotation. Sections were washed again in PBST and then in 0.05 m Tris buffer. The reaction was then visualized using diaminobenzidine (DAB Substrate Kit, Vector Laboratories) and finally stopped by washing in cold PBS. Sections were mounted on gelatin-coated slides, dehydrated through a graded series of alcohols and coverslipped.
Fos-positive cell counts
Estimates of c-fos activated cells were made using a semiautomated cell counting procedure. Images were viewed on a Leica DMRB microscope, photographed using an Olympus DP70 camera, and stored digitally. Stained nuclei were counted using the program AnalysisD̂ (Soft-Imaging Systems; Olympus, Southend, U.K.). This program makes it possible to select and count cells automatically without experimenter bias. In addition, counts were made without knowledge of group assignments; that is, blind.
In order to derive accurate, absolute cell counts it is necessary to use stereological methods (Coggeshall & Lekan, 1996), but the goal of the present study was to compare relative numbers of activated cells across two conditions. For this purpose, automated cell counting is appropriate when certain conditions are met. Key conditions are that there are no systematic changes in the volume or packing of the neurons in the two groups (Coggeshall & Lekan, 1996), as well as random tissue sampling (Mura, Murphy, Feldon, & Jongen-Relo, 2004). There is no a priori reason why both conditions should not be met in the present study.
Numbers of labeled cells in each region of interest were determined by counting cells (mean feret, a measure of particle size, of 4–20 μm) stained above an automatically determined threshold of greyscale intensity that was above background levels (software setting for histogram intensity phases = 3). The cortical counts were made in a frame area of 0.84 × 0.63 mm that enabled all laminae to be included in one image. For the hippocampus, image montages of the dentate gyrus, CA3, and CA1 fields were created from coronal sections at the septal, intermediate and temporal levels of the hippocampus (see Figure 2). For all brain areas analyzed, counts were taken from four consecutive immunoreacted sections from each hemisphere. As a consequence, each section was separated by120 μm in the anterior-posterior (AP) plane as the tissue came from a one-in-three (40 μm) series.
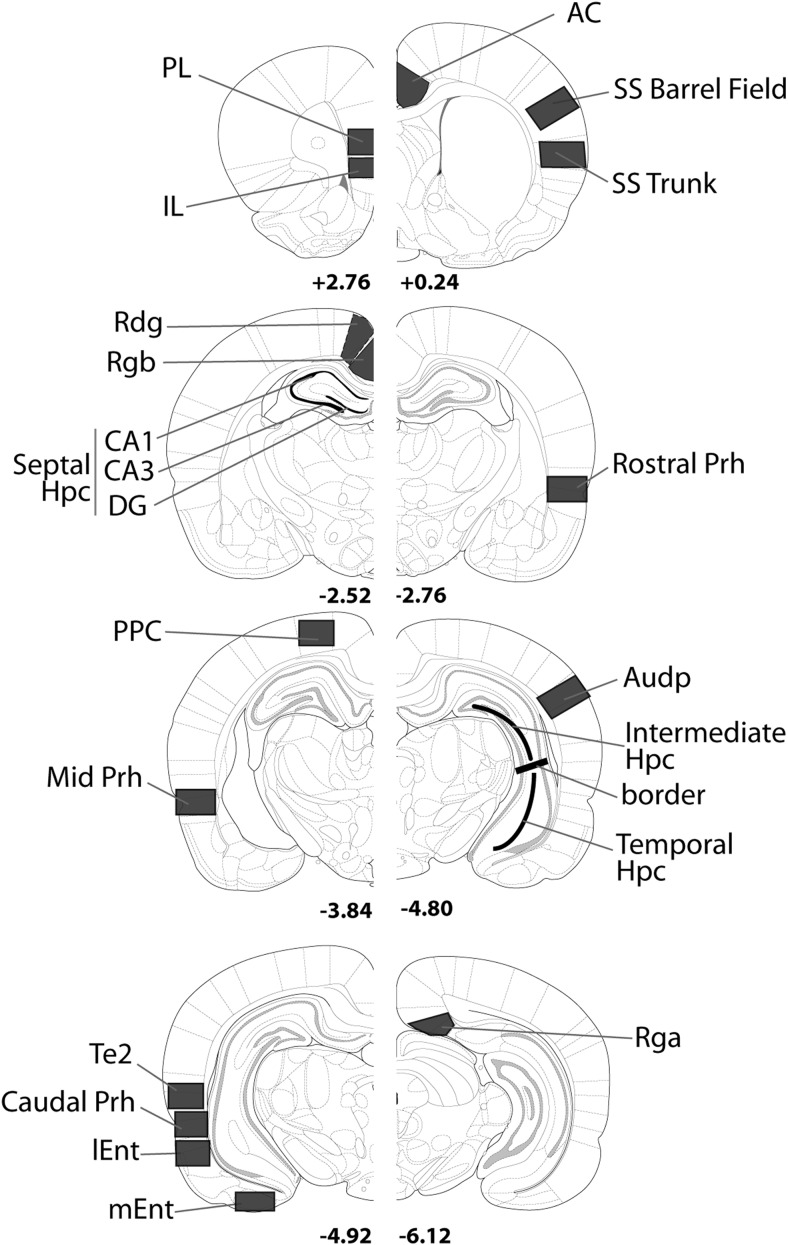
Figure 2. Coronal sections indicating the regions of interest. Abbreviations: AC = anterior cingulate cortex; Audp = primary auditory cortex; DG = dentate gyrus; dSub = dorsal subiculum; Hpc = hippocampus; IL = infralimbic cortex; lEnt = lateral entorhinal cortex; mEnt = medial entorhinal cortex; PL = prelimbic cortex; Prh = perirhinal cortex; Rdg = retrosplenial dysgranular cortex; Rga = retrosplenial granular; Rgb = retrosplenial granular; Te2 = cortical area Te2; Visp = primary visual cortex; vSub = ventral subiculum. The numbers refer to the distance (mm) from bregma. From The Rat Brain in Stereotaxic Coordinates (figures 12, 31, 54, 56, 65, 73, 74 and 84) by G. Paxinos and C. Watson, 2005.
Regions of interest (Figure 2)
Sites were primarily selected because of their previous implication in recognition memory processes, in either the light or the dark. Additional structures—for example, the various anterior thalamic nuclei and the retrosplenial cortex—were included because of their close involvement in memory processes dependent on hippocampal function. The AP and height positions of the various borders relative to bregma (see Figure 2) correspond to coordinates from the atlas by Paxinos and Watson (2005). A further series of cortical counts were taken from primary sensory areas in order to assess the effectiveness of the control condition. The rationale was that the sensory cues experienced by Groups Novel and Familiar should be closely matched, and so primary sensory areas might be expected to show little or no Fos difference across the two groups. The regions sampled comprise seven main groupings.
1) Perirhinal cortex and area Te2
The nomenclature and borders were taken from Burwell (2001). The perirhinal cortex was subdivided into three subregions: rostral from AP −2.76 to −3.84 relative to bregma), mid (AP −3.84 to −4.80) and caudal (from AP −4.80 to −6.30). These same three subregions were then further divided into areas 35 and 36 (Burwell, 2001), which make up the perirhinal cortex. It should be noted that the most rostral perirhinal region would include part of the caudal insular cortex as described by Shi & Cassell (1999), who proposed a much more restricted perirhinal region than Burwell (2001). Counts were also taken from the adjacent visual association cortex area Te2, which has been implicated in visual novelty detection (Ho et al., 2011; Wan et al., 1999; Zhu, McCabe, Aggleton, & Brown, 1996).
2) Hippocampus
Cytoarchitectonic subfields (dentate gyrus, CA1, CA3) within the hippocampus were subdivided into their septal (dorsal), intermediate, and temporal (ventral) components (Bast, 2007; Bast, Wilson, Witter, & Morris, 2009). The septal hippocampus counts (dentate gyrus, CA3 and CA1) were obtained from sections near AP level −2.52 from bregma. Counts for the intermediate part (dentate gyrus, CA1, CA3) and the temporal pole of the hippocampus (CA1, CA3) were obtained from sections near AP level −4.80 from bregma. The division between the intermediate and temporal hippocampus corresponded to −5.0 dorsoventrally from bregma (Paxinos & Watson, 2005).
3) Entorhinal cortex
Separate cell counts were taken from the lateral and medial entorhinal cortices as described by Swanson (1992).
4) Frontal cortex
Three distinct regions were examined (Swanson, 1992); the prelimbic (PL), infralimbic (IL), and anterior cingulate (AC) cortices (see Figure 2).
5) Retrosplenial cortex
The retrosplenial cortex (areas 29, 30) can be subdivided into granular b (Rgb), granular a (Rga), and dysgranular cortex (Rdg) (see van Groen & Wyss, 1990). Separate counts were made in all three subregions. Furthermore, separate counts were made for the superficial (layer II and upper III) and deep (lower layer III to VI) layers of Rdg, Rgb and Rga, but these data are not presented as they did not reveal any differential laminar effects.
6) Thalamic nuclei
Fos-positive cells were counted in the three anterior thalamic nuclei: the anterodorsal (AD), anteroventral (AV), and anteromedial (AM) nucleus. In addition, counts were made across the extent of the medial dorsal thalamic nucleus (MD).
7) Cortical sensory areas
Counts were taken from three cortical areas described by Swanson (1992); the posterior parietal cortex (PPC), the primary auditory area (Audp), and the primary somatosensory cortex (SS). Separate counts were made in the barrel field and the trunk areas of the somatosensory cortex.
Statistical analyses
The behavioral findings from Sessions 1 and Sessions 13 were subjected to a mixed ANOVA, with the between factor (group) and the within factor (session). When there was an interaction, simple effects were examined. One-sample Student t tests (one-tailed) determined if discrimination performance was above chance (a score of zero).
For statistical analyses of regional c-fos activity, cell counts were first normalized according to the matched pairs of animals (one Novel, one Familiar). This normalization procedure reduces the impact of any staining variability from batch to batch of animals and, hence, should reduce variance. In addition, it counters the fact that there are very different baseline levels of c-fos activity in different brain structures that would lead to scaling errors when considering any interactions. Normalization involved dividing the mean number of activated neurons in a given animal for a given site by the combined mean of the two animals in each matched pair, and expressing this result as a percentage. The Fos-positive cell counts were then analyzed in seven separate regional groupings to reduce Type I errors: (1) perirhinal cortex and area Te2, (2) hippocampal subfields, (3) entorhinal cortices, (4) frontal cortex, (5) retrosplenial cortex, (6) anterior thalamic nuclei and (7) cortical sensory areas. The normalized cell counts were analyzed using a one-between (groups) by one-within (subregions) design. When there was a significant group × region interaction, simple effects were examined. Failure to meet these conditions did not, however, preclude all further analyses (see Howell, 1995, p. 355). For those multiple comparisons at the regional level, the significance level was further adjusted using the modified Bonferonni test (Keppel, 1991). As a consequence, the probability level of ≤0.05 was taken as being statistically significant only when the number of sites (simple effects) to be compared was four or less. For the region including the perirhinal cortex and area Te2 (seven comparisons) and for the hippocampal subfields (eight comparisons) the significance level was adjusted to ≤0.029 and ≤0.025, respectively (Keppel, 1991). Those sites identified by these analyses as being altered by the behavioral procedure were then subject to an additional statistical comparison as the normalization procedure constrains the scores so that each pair sum to 100. Now, the raw (absolute) cell counts were compared in matched (within-subject) t tests (one-tailed). A significance level of p < .05 determined those sites with changes in Fos levels associated with novel object exploration in the dark.
Results
Behavioral measures of object recognition in the dark (Sessions 1 and 13)
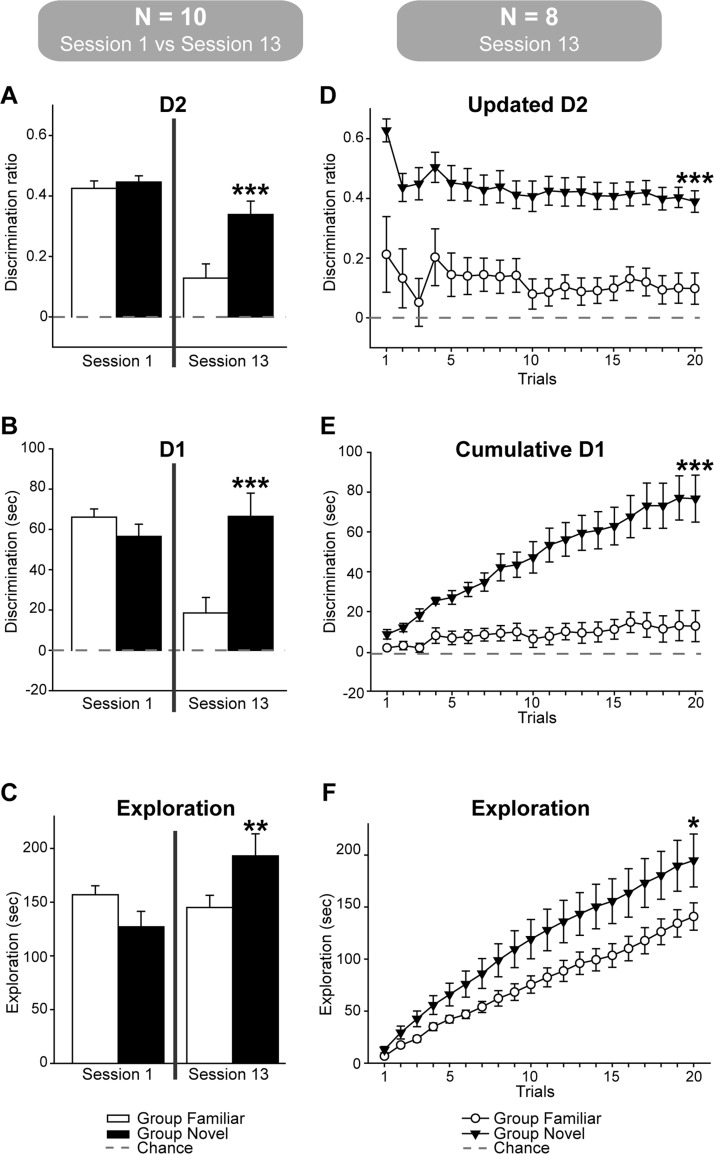
While the initial (Session 1) object recognition scores (D1, D2) of the two groups appeared closely matched, they looked very different by Session 13 (Figure 3 left). This pattern was expected as in Session 13 the Group Novel rats explored one novel and one familiar object per trial. In contrast, Group Familiar rats were given two familiar objects per trial (as they had been explored on all preceding sessions), resulting in much lower D1 and D2 scores. This pattern was confirmed by more formal analyses.
Figure 3. Object recognition performance of Group Novel and Group Familiar on Session 1 and Session 13. The recognition performance (mean +/− one standard error) of the 10 rats from each group is shown on the left, where the discrimination ratios (D2, A.) and the exploration differences (D1, B.) are shown. The bottom left graph (C.) shows the total amounts of object exploration (irrespective of whether to novel or familiar objects). The graphs on the right (n = 8) show the corresponding trial by trial data (D1, D.; D2, E.; total exploration, F.) for the revised groups that used only those Group Novel rats (n = 8) showing clear object recognition in the final session. For all graphs on the right side of the figure the data are cumulative, i.e., they are not independent across trials. Those conditions leading to a significant group difference by the end of 20 trials are indicated: * p < 0.05, ** p < 0.01, *** p < 0.001.
Starting with the D2 recognition index (Figure 3 upper left), the data from Sessions 1 and 13 revealed a group-by-session interaction F(1, 18) = 6.39, p = .021, reflecting the lower performance on Session 13 by Group Familiar. This pattern is supported by the simple effects as there was no group difference on Session 1, F<1, but a very clear difference by Session 13, F(1, 36) = 17.2, p < .001. Comparable analysis using the D1 recognition index (Figure 3 mid-left) produced exactly the same pattern of results [group by session interaction F(1, 18) = 17.8, p < .001; simple effects Session 1 (F<1), Session 2 F(1, 36) = 18.6, p < .001].
The next comparison concerned the total amounts of object exploration (Figure 3, lower left), irrespective of whether the object was novel or familiar. While there was no overall group difference, F<1, there was a group by session interaction, F(1, 18) = 6.74, p = .018, reflecting the higher amounts of exploration in the final session by Group Novel, F(1, 36) = 5.60, p = .024.
A final set of analyses determined whether the discrimination indices of the two groups were above chance. For Session 1, both groups strongly preferred the novel objects as measured by either the updated D2 score, Group Novel: t(9) = 21.7, p < .001; Group Familiar: t(9) = 17.4, p < .001, or the cumulative D1 score, Group Novel: t(9) = 9.26, p < .001; Group Familiar: t(9) = 16.4, p < .001. For Session 13, the updated D2 scores and the cumulative D1 scores remained above chance for Group Novel, t(9) = 7.68, p < .001; t(9) = 5.75, p < .001, respectively. On Session 13, Group Familiar also preferred the less familiar objects, updated D2: t(9) = 2.75, p < .05, cumulative D1: t(9) = 2.43, p < .05.
Session 13 performance and c-fos analysis
A principal goal was to examine IEG expression associated with novel object recognition in the dark compared with IEG expression after a matched experience with familiar objects in the dark. Inspection of the data showed that for two pairs of rats, the animal from Group Familiar had higher D1 and D2 scores than its matched partner in Group Novel. The decision was, therefore, taken to remove these two pairs from all subsequent immediate-early gene analyses as the Group Novel rats had barely discriminated the novel objects in this final session (with D2 scores of 0.128 and 0.146). (The corresponding scores for the Group Familiar rats were 0.308 and 0.194, respectively.)
The behavioral findings for the remaining eight pairs of rats on Session 13 (Figure 3, right) were, therefore, reanalyzed to confirm whether Group Novel still recognized the novel objects and whether Group Familiar treated some objects as less familiar. Unsurprisingly, Group Novel outperformed Group Familiar, updated D2: t(14) = 4.58, p < .001; cumulative D1: t(14) = 4.53, p < .001, while the difference in overall exploration times for the two groups was at borderline significance, t(14) = 1.88, p = .041 (one-tailed). Subsequent analyses showed that Group Novel discriminated above chance, updated D2: t(7) = 10.77, p < .001; cumulative D1: t(7) = 6.51, p < .001, while Group Familiar were at borderline levels above chance; updated D2: t(7) = 1.88, p = .05; cumulative D1: t(7) = 1.63, p = .075, all one-tailed.
Counts of Fos-positive cells (Session 13)
All c-fos data are based on the eight pairs of rats where the Group Novel rat outperformed the Group Familiar rat.
Perirhinal cortex and area Te2
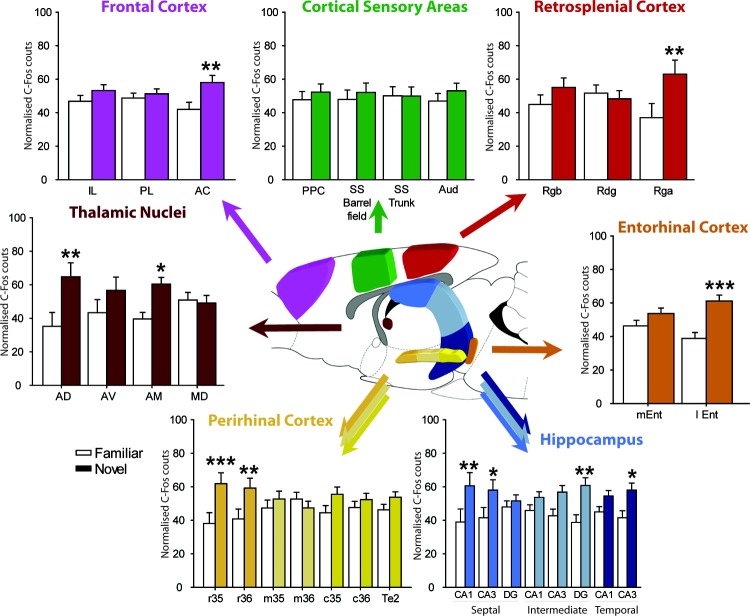
While exposure to novel objects (Group Novel vs. Group Familiar) did not produce a significant overall increase in Fos-positive cells across these areas, F(1, 14) = 3.24, p = .094 (Figure 4, lower left), there were significantly different patterns of c-fos activity within the perirhinal cortex (group by subregion interaction: F(6, 84) = 4.37, p < .001) reflecting selective responses to novel stimuli. Simple effects found a significant increase of Fos-positive cells in rostral perirhinal areas 35 and 36, rostral area 35, F(1, 98) = 12.6, p < .001; rostral area 36, F(1, 98) = 12.6, p < .001). In both sites these changes were supported by the results of a matched t test based on the raw scores, rostral area 35 t(7) = 2.24, p = .031; rostral area 36 t(7) = 1.93, p = .048, one-tailed. In none of the other sites did the simple effects indicate a significant change, mid area 35, F < 1, mid area 36, F < 1; caudal area 35, F(1, 98) = 2.70, p = .10; caudal area 36, F < 1; area Te2, F(1, 98) = 1.25, p = .27.
Figure 4. Histograms showing the normalized Fos counts for Group Novel (dark) and Group Familiar (light) for the seven grouped regions of interest. Because the data are normalized they sum to 100. Abbreviations: AC = anterior cingulate cortex; AD = anterior dorsal thalamic nucleus; AM = anterior medial thalamic nucleus; Audp = primary auditory cortex; AV = anterior ventral thalamic nucleus; DG = dentate gyrus; dSub = dorsal subiculum; Hpc = hippocampus; I = infralimbic cortex; lEnt = lateral entorhinal cortex; MD = medial dorsal thalamic nucleus; mEnt = medial entorhinal cortex; PL = prelimbic cortex; Perirhinal cortex (r = rostral; m = mid; c = caudal, areas 35 and 36); PPC = posterior parietal cortex; Rdg = retrosplenial dysgranular cortex; Rga = retrosplenial granular a; Rgb = retrosplenial granular b; SS = somatosensory cortex; Te2 = cortical area Te2; Visp = primary visual cortex; vSub = ventral subiculum. * p < 0.05, ** p < 0.01, *** p < 0.001 (simple effects).
Hippocampus
The hippocampus was divided in septal, intermediate, and temporal regions (Bast et al., 2009), and the different subfields (CA1, CA3 and DG) were counted separately within these regions (Figure 4, lower right). Group Novel had higher overall Fos counts in the hippocampus compared to Group Familiar, F(1, 14) = 7.24, p < .025. Although there was a borderline group by subregion interaction, F(7, 98) = 1.91, p = .077, this did not reach significance (note also the required Bonferroni correction). However, in light of Experiment 2, further analyses determined if any site showed changed Fos count levels as derived from the analysis of the raw counts, as well as the simple effects. Using these criteria, the intermediate dentate gyrus was the only site to show a significant Fos increase according to both matched t tests using raw cell counts, t(7) = 2.93, p = .011, and the simple effects analyses, F(1, 112) = 10.4, p < .01. Although other sites showed c-fos activity increases in Group Novel according to the simple effects; septal CA1, F(1, 112) = 10.05, p < .01); septal CA3, F(1, 112) = 5.82, p < .025; and temporal CA3, F(1, 112) = 5.79, p < .025, none of these were significant when the raw scores were considered (p > .1). Finally, the remaining subregions did not show significant group differences according to the simple effects from the normalized scores (p > .1).
Entorhinal cortex
Object novelty induced a relative increase of activity in the entorhinal cortices, F(1, 14) = 10.27, p < .006, (Figure 4, mid-left), but this change was not constant throughout the entorhinal cortex as revealed by the group by subregion interaction, F(1, 14) = 25.1, p < .001. A significant increase of c-fos activity in Group Novel was found for the lateral entorhinal but not the medial entorhinal cortex, simple effects; lEnt: F(1, 28) = 21.1, p < .001; mEnt: F(1, 28) = 2.26, p = .14, (Figure 4, mid right). This lateral entorhinal increase was supported by the matched t test comparison using the raw cell counts, t(7) = 3.41, p = .0055, one-tailed.
Frontal cortex
There was no overall group effect in the frontal cortices, F(1, 14) = 3.96, p = .067 (Figure 4, upper left), however, a group by subregion effect was found, F(2, 28) = 3.80, p < .05. Subsequent analyses revealed that this interaction reflected the significant increase of Fos-positive cells in Group Novel in the anterior cingulate cortex, simple effects; F(1, 42) = 9.90, p < .01, but not in the prelimbic cortex F(1, 42) = 1.58, p = .21 or infralimbic cortex, F < 1. This anterior cingulate increase was supported by the matched t test comparison using the raw cell counts, t(7) = 1.87, p = .052, one-tailed.
Retrosplenial cortex
There was no overall group difference across this cortical area, F(1, 14) = 1.36, p = .26; Figure 4 upper right. There was, however, a group by subregion interaction, F(4, 56) = 5.06, p < .001, reflecting the increase of c-fos activity for Group Novel in the retrosplenial granular a cortex, simple effect: F(1, 70) = 8.44, p < .01. This particular change was not, however, significant when the raw scores were considered, t(7) = 1.62, p = .075, one-tailed. Counts from the superficial lamina were also compared with those from the deep lamina, but are not presented as the pattern of results was the same for both superficial and deep layers.
Thalamic nuclei
A relative increase of Fos-positive cells was found in Group Novel across the four nuclei of the thalamus, F(1, 14) = 5.25, p < .05; Figure 4 mid-left. Likewise, a group-by-nucleus interaction was found, F(3, 42) = 3.55, p < .05, and further analyses showed that there was a significant increase of Fos-positive cells in the anterodorsal nucleus, simple effects; F(1, 56) = 10.54, p < .01, and the anteromedial nucleus F(1, 56) = 5.21, p < .05. There was no significant effect in the anteroventral nucleus, F(1, 56) = 2.16, p = .15, nor in the medial dorsal nucleus, F < 1. This anteromedial change was supported by the matched t test comparison using the raw cell counts, t(7) = 2.16, p = .034, but this change was not significant for the anterodorsal thalamic nucleus (p > .1).
Cortical sensory areas
Immediate-early gene activity across the four cortical regions failed to distinguish the Novel from the Familiar conditions, F < 1 (Figure 4, upper), and there was no evidence of a group-by-subregion interaction, F < 1.
Experiment 2: Spontaneous Object Recognition in the Dark: Effects of Hippocampal Lesions
Materials and Methods
Two cohorts of rats, both including rats with bilateral hippocampal lesions, were tested on object recognition in the dark. Testing involved multiple trials within a session, with two blocks of trials in two separate phases. Each trial in Phase 1 lasted for 1 minute, so that object recognition memory was tested after a retention delay of no more than 1 minute (the same as Group Novel, Experiment 1). The second phase made it possible to examine object recognition after longer retention intervals. The interval between Phase 1 and Phase 2 was set at 20 minutes for Cohort 1, and set at 15 minutes for Cohort 2. The replication tested the reliability of the results from Cohort 1, as the D2 results for the 1 min retention condition hinted at a possible lesion effect. The longer retention delay was reduced for Cohort 2 from 20 min to 15 min, and the number of trials slightly reduced, to counter any potential floor effects.
Subjects
Cohort 1 and Cohort 2 comprised, respectively, 44, and 25 male, Lister Hooded rats. All rats were housed in pairs under diurnal conditions (12-hr light–dark cycle), and water was provided ad libitum throughout the study. Animals were food-deprived up to 85% of their free-feeding body weight and maintained above this level during behavioral testing. Rats were 11–12 months (Cohort 1), and 7 months (Cohort 2) old at the start of the study. All rats in Cohort 1 had previously been trained on place discriminations in a curtained water tank that involved the rats distinguishing plain walls of differing lengths or walls with different patterns. The rats in Cohort 2 had previously been trained on digging tasks to examine spatial discriminations and configural learning (Albasser et al., 2012). It is assumed that these previous experiences did not interfere with the present task as the apparatus, stimuli, and task requirements were markedly different from those in the recognition task. All experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act (1986) and associated guidelines.
Surgery
Rats in each cohort received bilateral hippocampal lesions (HpcC1 = 23 and HpcC2 = 13) made by injecting ibotenic acid. Rats were first anesthetized using an isoflurane-oxygen mix. The rat was then placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), with the incisor bar set at −3.3 mm, and the rat administered with 0.1 mg/kg of the analgesic Metacam subcutaneously. A sagittal incision was made in the scalp, and the skin retracted to expose the skull. A dorsal craniotomy was made directly above the target region and the dura cut to expose the cortex. The rats with hippocampal lesions received injections of ibotenic acid (Biosearch Technologies, San Rafael, CA) dissolved in phosphate-buffered saline (pH 7.4) to provide a solution with a concentration of 63 mM. The injections were made through a 2 μl Hamilton syringe held with a microinjector (Kopf Instruments, Model 5000). Fourteen infusions per hemisphere were made at an infusion rate of 0.10 μl/min and a diffusion time of 2 min. The injection coordinates and volumes have been published (Iordanova, Burnett, Good, Aggleton, & Honey, 2009). The control groups (ShamC1 = 21 and ShamC2 = 12) received identical treatments except that the dura was repeatedly perforated with a 25-gauge Microlance3 needle (Becton Dickinson, Drogheda, Ireland) and no solution was infused into the brain. All rats were 3–4 months old at the time of surgery.
Histological procedure
On completion of behavioral testing, all rats received a lethal overdose of sodium pentobarbital (60 mg/kg, Euthatal, Rhone Merieux). The rats from Cohort 1 were transcardially perfused, first with 0.9% saline and then with 10.0% formal-saline. Their brains were extracted, postfixed for 24 h, and then transferred to 25% distilled water sucrose solution in which they remained for a further 24 h. The rats from Cohort 2 were transcardially perfused with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde in 0.1M PBS (PFA), so that their tissue could be used for immunohistochemistry. All brain sections were cut at 40 μm on a freezing microtome in the coronal plane. The sections were collected on gelatin-coated slides, left to dry in room temperature over 24 h, and then stained with cresyl violet, a Nissl stain.
The amount of damage in the hippocampus (dentate gyrus and CA fields but not including the subiculum) was measured separately with the program AnalysisD̂ (Soft-Imaging Systems, Olympus). First, the total area of the region of interest was measured from 10 coronal sections corresponding to −2.12, −2.80, −3.30, −3.80, −4.30, −4.80, −5.30, −5.80, −6.30, −6.80 relative to bregma (Paxinos & Watson, 2005) in a surgical control. Then, using the same protocol, the extent of hippocampal damage and cortical damage was quantified for each animal that received a hippocampal lesion.
Apparatus
The apparatus (bow-tie maze, Figure 1) and lighting conditions were identical to those used in Experiment 1.
Objects
A total of 21 different pairs of junk objects were used to test Cohort 1, while 17 different pairs of junk objects were used for Cohort 2. For those objects used in Phase 1, triplicate copies were required. As before, any object with an obvious scent was excluded and all objects were cleaned with alcohol wipes after each session.
Behavioral testing: Pretraining
Over 7 days, animals were habituated to eat in the bow-tie maze, and then trained; i) to run back and forth between the two ends of the maze, and ii) to displace an object covering a food well in order to reach a food reward, as described for Experiment 1. Four pairs of objects were used during pretraining, but these objects were not used in the experiment proper. For pretraining only, the animals were tested in the light.
Behavioral testing: Object recognition in the dark
All rats were first transported from the holding room to the darkened test room in individual opaque containers made of aluminum. An individual rat was then placed in the bow-tie maze in the dark. The testing procedure was modeled on that used for Group Novel in Experiment 1. The principal difference was that the rats received one test session, divided into two phases (Table 1 lower).
Cohort 1: The first phase of the session comprised 10 trials, along with an initial trial (Trial 0) to familiarize the rats with the first object (object A1). Each trial lasted one minute and as in Experiment 1, each object was set above a food well that contained one sucrose pellet. On each of the 10 trials in Phase 1 the rat was allowed to explore freely between two objects, one familiar from the previous trial, the other novel; for example, A2 versus B1, Trial 1; B2 versus C1, Trial 2; C2 versus D1, Trial 3; and so forth (see Table 1 lower). Between trials the rat shuttled back and forth across the maze.
At the completion of Phase 1 the rat was removed from the bow-tie maze and transported to an adjacent room that was also in the dark. This room contained individual cages similar to the home cages, also filled with sawdust. Rats remained in their cages for 20 minutes and were then transported back in the carry box to the testing room for Phase 2 (Table 1 lower). In Phase 2 the rats again received 10 trials, each lasting 1 minute. On each trial the rat was presented with two objects. One object was novel the other was familiar as it was a copy of an object used in Phase 1 (e.g., familiar object A3 vs. novel object Z; familiar object B3 vs. novel object Y; see Table 1 lower). In this way, Phase 1 examined object recognition memory with a retention period of less than 1 min, while Phase 2 examined object recognition memory with a retention period of 31 minutes for each familiar object (20 mins plus the 11 mins taken to complete Phase 1). Scoring of the exploration behavior matched that used for Experiment 1.
Cohort 2: The testing procedure was essentially identical to that used for Cohort 1. The only differences were that the first and second phases of the test session each comprised eight trials (rather than 10 trials), and the interval between the two phases was reduced to 15 minutes (from 20 minutes). Consequently, Phase 1 examined object recognition memory in the dark with a retention period of less than 1 min, while Phase 2 examined object recognition memory in the dark with a retention period of 23 minutes for each familiar object (15 mins plus the 8 mins taken to complete Phase 1).
Statistical analyses
As with Experiment 1, all behavioral scoring was conducted by an observer who was unaware of the group identity of each rat; that is, scored blind. For both Cohort 1 and Cohort 2, the behavioral findings from the two different delay conditions were subject to a mixed ANOVA (one between factor [group] and one within factor [session]). When there was an interaction, simple effects were examined. One-sample Student t tests (one-tailed) determined if discrimination performance was above chance (a score of zero).
Results
Histology
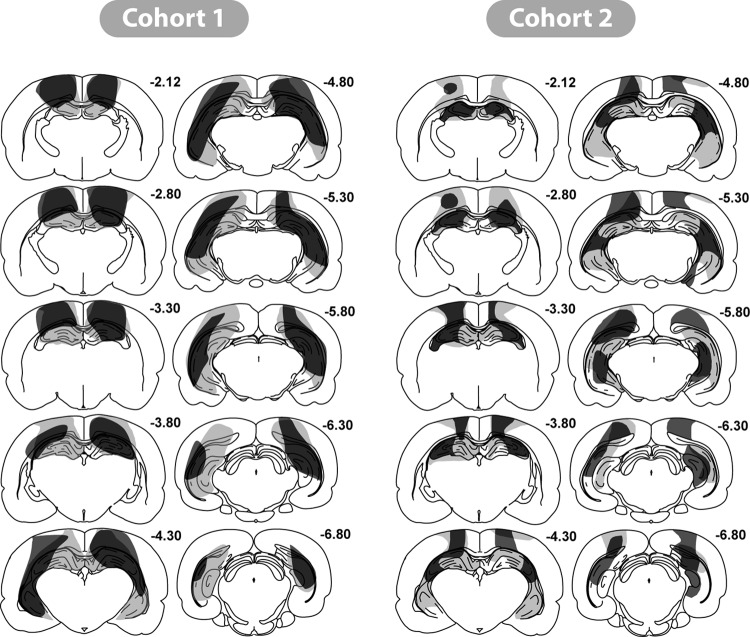
Cohort 1: Figure 5 depicts a series of coronal sections (based on Paxinos & Watson, 2005) showing the maximum and minimum extent of hippocampal damage. Histological analyses revealed that four rats had appreciable sparing of the hippocampus (less than 50% volume loss), and all four rats were excluded from the behavioral analyses. The remaining 18 HpcC1 rats had extensive damage to the hippocampus that was typically more complete in the dorsal rather than the ventral hippocampus. Three rats had over 85% volume loss of the dorsal hippocampus, with eight more rats having more than 70% damage to the dorsal hippocampus. In these rats the only consistent sparing was restricted to the most medial part of the dorsal hippocampus, in particular the most medial part of the blade of the dentate gyrus. The remaining seven rats had from 50% to 70% cell loss in the dorsal hippocampus, with most sparing of the medial parts of the dorsal hippocampus.
Figure 5. Coronal sections illustrating the hippocampal lesions in those cases in Cohort 1 and 2 with the smallest (dark gray) and largest (light gray) region of cell loss. The numbers refer to the approximate distance of each section in mm caudal to bregma. From The Rat Brain in Stereotaxic Coordinates (figures 26, 29, 31, 33, 35, 37, 39, 41, 43, 45) by G. Paxinos and C. Watson, 1997.
Of the 18 rats, six had over 70% volume loss in the ventral hippocampus, five rats had between 55% and 70% damage, and seven rats had between 30% and 55% damage. In the more ventral parts of the hippocampus, all rats showed some partial sparing of the cell layers of the lateral blade of the dentate gyrus, as well as the most ventral part of CA1 and CA3.
The hippocampal lesions were confined such that no damage was visible to more ventral structures, leaving the thalamus intact. All rats did, however, sustain bilateral, cortical damage dorsal to the hippocampus that invaded parts of the primary somatosensory area and adjacent cortex within the parietal area (see Figure 5). At posterior levels, parts of the primary and rostrolateral visual areas were sometimes damaged. Very limited damage to the dysgranular retrosplenial cortex was seen in all rats. The cohort also included 21 Sham lesioned rats.
Cohort 2: Of the 14 rats with hippocampal lesions, four were excluded from further analysis. In two of these cases there was excessive hippocampal sparing (less than 40% damage). A further animal was excluded because of widespread cortical damage, while the lesion in the fourth case was largely unilateral. In the remaining nine HpC2 cases, the volume loss for the entire hippocampus was between 42%−79% (see Figure 5). As before, the cell loss was greater in the dorsal hippocampus where six cases had more than 70% damage. In the remaining three cases, the dorsal hippocampus sparing (range: 48%−53%) extended into lateral CA3, and sometimes into the medial portion of CA1. The only subfield to show any consistent partial sparing was the dentate gyrus, but here the subfield was always markedly diminished in volume despite spared granule cells. The dorsal subiculum was damaged in all cases, often being extensively damaged.
Tissue loss in the ventral hippocampus ranged from 27%−69%, with any sparing in the most ventral part of CA1 and CA3, as well as in the dentate gyrus. The ventral subiculum was typically spared. It should be added that in all cases the hippocampus was markedly shrunken in all three planes, and so it is likely that the coronal reconstructions underestimated the extent of tissue loss. In eight cases the lesions just encroached into the dorsal thalamus. Five of these cases had partial damage to the laterodorsal nucleus, which in one case included unilateral damage to the most dorsal part of the anterior ventral thalamus. Finally, all rats also had some bilateral cell loss in cortex dorsal to the hippocampus. As before, the damage involved parts of the primary and secondary motor areas, the primary somatosensory area, and the parietal region of the posterior association area. There was also some restricted damage in dysgranular retrosplenial cortex. The cohort also included 12 Sham lesioned rats.
Recognition memory in the dark
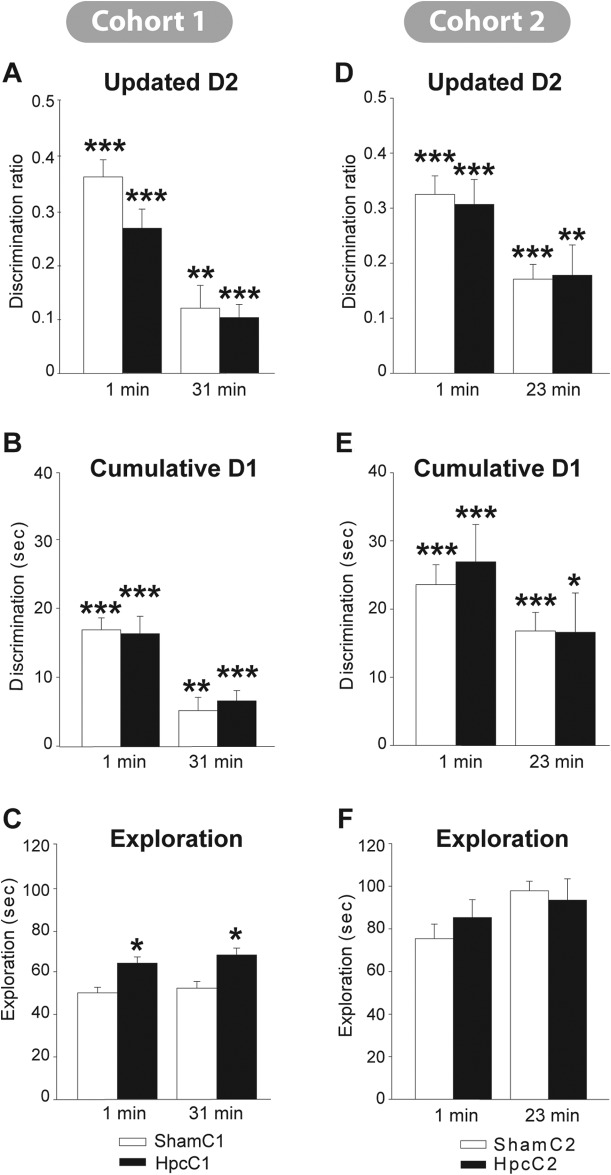
Cohort 1: The recognition scores of the two surgical groups were first compared using the “updated” D2 score across the 10 trials of Phase 1 (1 min retention) and the 10 trials of Phase 2 (31 min retention delay). A mixed Anova showed that performance declined between the 1 min and 31 min delay conditions, F(1, 37) = 56.8, p < .001 (see Figure 6), but that the scores of the HpcC1 and ShamC1 groups did not differ, F < 1, and there was no interaction between group and delay condition, F(1, 37) = 2.05, p = .16. One-sample t tests (one-tailed) confirmed that both groups performed above chance in both test phases. For the one minute condition all Group D1 and D2 scores were above chance, all p < .001. For the delay condition the scores were lower but still above chance, D2, ShamC1 p = .005, HpcC1 p < .001; D1, ShamC1 p = .008, HpcC1 p < .001. Comparisons based on the “cumulative” D1 scores (see Figure 6) again found no evidence of an effect of surgical group, F < 1, a very clear effect of retention delay, F(1, 37) = 38.4, p < .001, but no interaction with delay, F < 1. The final analyses concerned the total object exploration times for Phases 1 and 2 (see Figure 6). While these exploration times did not differ across the two Phases, F(1, 37) = 1.17, p = .29, the HpcC1 rats showed higher overall levels of object exploration, F(1, 37) = 21.4, p < .001. There was no interaction between test phase and surgical group, F < 1.
Figure 6. Object recognition performance in the dark after retention delays of 1 min, 23 mins (Cohort 2), or 31 mins (Cohort 1). The data from Cohort 1 are shown in the left half of the figure, with updated D2 (A.), cumulative D1 (B.), and total exploration times (C.). The corresponding data from Cohort 2 are shown in the right half of the figure (D.E.F.). The black bars show the mean scores (± 1 standard error) of the rats with hippocampal lesions, the white bars show the corresponding data for the sham controls. For D1 and D2 (A, B, D, E) the asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001) refer to whether performance is above chance. For total exploration (C, F), the asterisks signify the presence of a group difference (* p < 0.05).
Cohort 2: Initial group comparisons used the “updated” D2 score across the eight trials of Phase 1 (1 min retention) and the eight trials of Phase 2 (23 min retention delay). While performance again declined between the 1 min and 23 min delay conditions, F(1, 16) = 18.2, p = .002 (see Figure 6), the scores of the HpcC2 and ShamC2 groups did not differ, F < 1. Once again, one-sample t tests (one-tailed) confirmed that both groups performed above chance in both test phases. For the one minute condition all Group D1 and D2 scores were above chance, p < .001. For the 23-min delay condition the scores were lower but remained above chance, D2, ShamC2 p < .001, HpcC2 p = .007; D1, ShamC2 p < .001, HpcC2 p = .012. There was no interaction between group and retention delay, F < 1. Group comparisons based on the “cumulative” D1 scores (see Figure 6) produced a similar set of results, as there was no effect of group, F < 1, nor any interaction with delay, F < 1, but with this measure the effect of delay was at borderline significance, F(1, 16) = 3.93, p = .065. The final analyses concerned the total object exploration times for Phases 1 and 2. While total exploration was slightly higher in Phase 2, F(1, 16) = 4.26, p = .056, there was no evidence of a lesion effect on this measure, F < 1.
Discussion
Much is known about the neural basis of visual recognition memory in animals (Brown & Aggleton, 2001; Brown et al., 2010; Dere, Huston, & De Souza Silva, 2007; Murray, 1996; Winters et al., 2008) but corresponding knowledge about recognition memory for other modalities remains sparse. When in the dark, the sensory information that rats could use to recognize the previous occurrence of an object presumably consists of tactile and olfactory information. Previous research has indicated that in monkeys the perirhinal cortex is involved in tactile object recognition (Goulet & Murray, 2001; Murray & Mishkin, 1984; see also Suzuki, Zola-Morgan, Squire, & Amaral, 1993). While the rat perirhinal cortex also appears to be involved in tactile recognition, it is more specifically implicated when information is switched from tactile to visual modes of performance or vice versa (Albasser et al., 2011; Winters & Reid, 2010). Instead, lesion studies with rats have highlighted the importance of the parietal cortex for tactile recognition (Winters & Reid, 2010). Meanwhile, the contribution of the hippocampus for tactile-based recognition remains largely unknown. There is, however, evidence that normal olfactory recognition memory may rely on the perirhinal plus entorhinal cortices (Otto & Eichenbaum, 1992), with the hippocampus only becoming critical for olfactory recency memory (Agster, Fortin, & Eichenbaum, 2002; Fortin, Agster, & Eichenbaum, 2002).
The goal of the present study was to enlarge our understanding of nonvisual recognition memory. The first experiment sought to determine whether similar combinations of brain structures show c-fos activation for object recognition in the dark as found for object recognition in the light (Aggleton & Brown, 2005; Aggleton et al., 2012). It was for this reason that the study was closely modeled on a previous study of c-fos expression where rats were given recognition memory problems in the light (Albasser, Poirier et al., 2010). Both studies used the bow-tie maze (Albasser, Chapman et al., 2010) as this apparatus is particularly suitable for recognition experiments that require multiple trials. Also, as the test objects cover food rewards that are in fixed locations, the objects are readily located in both the light and in the dark. The immediate-early gene c-fos was examined because this IEG is rapidly upregulated after rats experience novel visual stimuli in a reliable, site specific manner (Albasser, Poirier et al., 2010; Wan et al., 1999, 2004; Warburton et al., 2003, 2005; Zhu et al., 1995). Furthermore, evidence of a direct functional association between c-fos and recognition memory comes from temporarily blocking the expression of this IEG in the perirhinal cortex, a manipulation that disrupts long term recognition memory (Seoane et al., 2012). This result is supported by the repeated finding that manipulations which disrupt object recognition also block Fos increases in the perirhinal cortex (Wan et al., 2004; Warburton et al., 2003, 2005). It is this pattern of evidence that underpins the rationale for focusing on this particular immediate-early gene (Aggleton et al., 2012), while remembering that it is not a direct marker of neuronal activity (Herdegen, 1996; Kovács, 2008).
On the very first training session, Group Familiar showed levels of object recognition comparable to those of Group Novel, as expected. After more sessions, the groups diverged as the rats tested with the same set of objects on every session (Group Familiar) displayed appreciably less discrimination between the test objects, despite maintaining a slight preference to avoid the object from the previous trial of that session. Group Novel, by contrast, maintained much higher levels of object discrimination, as shown by their performance on Session 13. Even so, the sensory experiences of both groups on this final session should have been very similar as identical objects were used in the same order for both groups. Aside from their respective recognition index scores (D1 and D2), the only demonstrable difference was that the Group Novel rats spent somewhat more time overall exploring objects. This time difference is presumably a consequence of repeating for Group Familiar the same objects across every previous session (see Table 1). Indeed, this decrease in spontaneous exploration helps to confirm that the rats in this group correctly perceived the repeated objects as familiar.
The decision to remove two pairs of rats, where the Group Novel D2 scores in the final session were appreciably less than their counterparts in Group Familiar, had three benefits for the c-fos analyses. The first was that the rationale arose from evidence of a functional link between c-fos activation and recognition memory (Seoane e tal., 2012), leading to the likelihood that these two pairs of rats would have had atypical relative patterns of Fos expression in key sites. The second was that the total object exploration times of the two groups in the final session became more comparable, with only a borderline difference when using a one-tailed test. Consistent with this result was the lack of a Group Fos difference in the three cortical sensory areas (two somatosensory, one auditory), which also suggests that these two subgroups were closely matched. The third benefit was that the eight Group Familiar rats only performed at borderline levels above chance on this same final session, that is, these rats largely failed to discriminate the objects on the basis of recency. This final issue is relevant as the goal was to have a control group that matched Group Novel in all respects but did not discriminate novel from familiar objects (or discriminate recency differences, as this would suggest other memory demands).
The principal finding in Experiment 1 is that there are general similarities between recognition memory in the light and in the dark as mapped by c-fos expression, but there are also potentially important differences of detail. In the light and in the dark, novelty is strongly associated with increased c-fos expression. One difference, however, was that in the dark the largest perirhinal change was in rostral areas 35 and 36, while with visual recognition memory it is the caudal parts of areas 35 and 36 that most reliably show a Fos increase (Albasser, Poirier et al., 2010; see also Albasser, Davies, Futter, & Aggleton, 2009; Wan et al., 1999, 2004; Warburton et al., 2003, 2005; Zhu et al., 1995). The significance of this rostral perirhinal location in rodents can be linked to its cortical inputs, as within the perirhinal cortex it is rostral areas 35 and 36 that receive the highest proportion of inputs from both parietal and pyriform cortices (Furtak, Wei, Agster, & Burwell, 2007), regions likely to provide somatosensory and olfactory inputs, respectively. Visual inputs are more focused on caudal areas 35 and 36 (Furtak et al., 2007). The Fos increase in the lateral entorhinal cortex is also notable given the close anatomical links between this area and the perirhinal cortex (Aggleton, 2012; Naber, Witter, & Da Silva, 1999), although a lateral entorhinal increase has not been reported in previous studies of c-fos expression and visual recognition memory (Wan et al., 1999, 2004; Warburton et al., 2003, 2005; Zhu et al., 1995). One possibility is, therefore, that this entorhinal change partially reflects the contribution of this area to olfactory memory (Otto & Eichenbaum, 1992; Wirth, Ferry, Di Scalla, 1998; Ramus, & Eichenbaum, 2000).
Perirhinal lesions in rats can spare object recognition in the dark as shown by two studies (Albasser et al., 2011; Winters & Reid, 2010). Furthermore, in both studies the perirhinal lesions included the rostral part of perirhinal cortex that showed the clearest c-fos changes in the present study. These findings are not, however, in conflict as the same lesion studies revealed the importance of the perirhinal cortex when there is a switch from sampling stimuli in the dark and recognizing them in the light, and vice versa (Albasser et al., 2011; Winters & Reid, 2010). One interpretation is that the perirhinal cortex helps to create multimodal representations of objects, which are required if recognition remains reliant on visual cues at either sampling or test (Albasser et al., 2011). An unresolved issue is why the posterior parietal cortex did not show significant Fos increases with novel objects in the dark given its involvement in tactile object recognition (Winters & Reid, 2010). Possible explanations include the need for additional subdivisions within posterior parietal cortex prior to Fos counting, along with the potential importance of connected adjacent sites, including the retrosplenial cortex. It is also possible that the posterior parietal cortex is an automatic processor of somatosensory information, leaving it difficult to detect any additional role in novelty detection using the current methods given the close sensory match across Groups Novel and Familiar.
Evidence of increased Fos counts associated with novel object recognition was found across a number of hippocampal subfields (dentate gyrus, CA3, and CA1) but these changes often failed to reach significance when the raw counts were analyzed, aside from the dentate gyrus in the intermediate hippocampus. Object recognition in the light in the bow-tie maze seems more reliably associated with hippocampal changes, with Fos increases found in CA3 and CA1, but Fos decreases in dentate gyrus (Albasser, Poirier et al., 2010). It is, however, important to note that these hippocampal Fos changes are selectively associated with those studies that have involved active exploration of novel objects (in the bow-tie maze) as they are not found when rats are passively shown novel visual stimuli (Zhu et al., 1995, 1996; Wan et al., 1999; Warburton et al., 2003, 2005; Wan et al., 2004). The implication is that object exploration promotes hippocampal c-fos activity but this hippocampal activity is not required for effective recognition memory.
It was precisely this prediction that was tested in Experiment 2, where two studies examined the effects of extensive bilateral hippocampal lesions on object recognition with delays of 1, 23, and 31 minutes. There was very little evidence that hippocampal lesions impair object recognition under these conditions, and no group differences emerged. There was, however, evidence from Cohort 1 that hippocampal damage can increase overall levels of object exploration, a result that may reflect the hyperactivity sometimes associated with hippocampal damage (Davidson & Jarrard, 2004). This increased exploration was not, however, found in Cohort 2, where again there was no suggestion of a lesion-induced recognition memory deficit. These null results match the repeated outcome of object recognition tests in the bow-tie maze in the light where again, hippocampal lesions spare object recognition (Albasser, Chapman et al., 2010; Albasser et al., 2012). As noted in the Introduction, there are conflicting results concerning whether hippocampal lesions in rats impair or spare test of object recognition memory (e.g., Aggleton et al., 1986; Albasser et al., 2012; Broadbent et al., 2004; Clark et al., 2000, 2001; Forwood et al., 2005; Winters et al., 2008). One potential explanation might be that the hippocampus is important for nonvisual object recognition, and that these experiments vary in the demands they make upon this aspect of recognition memory. The present results show, however, that this is most unlikely to be the case. At the same time, deficits after hippocampal lesions have, however, often been found for object recency (Albasser et al., 2012; see also Agster et al., 2002; Barker & Warburton, 2011a; Charles, Gaffan, & Buckley, 2004), pointing to a potential role in linking specific objects to their associated attributes (Aggleton et al., 2012; Gilbert & Kesner, 2003; Warburton & Brown, 2010).
Other areas to show evidence of increased c-fos expression with novel objects in the dark included three sites all located within interlinked regions, the granular a retrosplenial cortex, the anterior medial thalamic nucleus, and the anterior cingulate cortex. Aside from one previous study that noted increased Fos levels in the anterior cingulate cortex with object novelty (Zhu et al., 1995), the other sites have not so far been associated with novelty detection in the light. Given the strong anatomical interactions that the retrosplenial cortex and anterior thalamic nuclei have with the hippocampus, it is tempting to speculate that these regions assist in the acquisition of associated information about objects rather than support the detection of novelty. One example would be learning object location, which rats acquire spontaneously (Dix & Aggleton, 1999; Save, Poucet, Foreman, & Buhbot, 1992). Indeed, lesion studies indicate that the integrity of the retrosplenial cortex, anterior thalamic nuclei, and hippocampus are all required for spontaneous object-location learning (Barker & Warburton, 2011b; Save et al., 1992; Vann & Aggleton, 2002; Wilton, Baird, Muir, Honey, & Aggleton, 2001). Likewise, hippocampal c-fos changes are consistently seen in those tests of “associative recognition” where a rat recognizes the novel repositioning of a familiar object within a familiar space (Aggleton et al., 2012; Amin, Pearce, Brown, & Aggleton, 2006; Jenkins, Amin, Pearce, Brown, & Aggleton, 2004; Wan et al.., 1999; see also Vann, Brown, & Aggleton, 2000; Vann, Brown, Erichsen, & Aggleton, 2000). Increased IEG activation in the anterior thalamic nuclei and retrosplenial cortex is also seen in some studies of spatial reorganization (Vann, Brown, & Aggleton., 2000; Vann, Brown, Erichsen et al., 2000, but see Jenkins et al., 2004). The implication is that object novelty can raise Fos levels in this extended-hippocampal system, and that this activation may reflect the role of this IEG in stabilizing experience-induced plastic changes (Guzowski et al., 2005). A consequence would be the improved long-term retention of information linked to specific objects, such as their spatial and temporal properties.
Acknowledgments
This work was supported by the Wellcome Trust [WT087855].
References
- Aggleton J. P. (2012). Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neuroscience and Biobehavioral Reviews, 36, 1579–1596. doi:10.1016/j.neubiorev.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., & Brown M. W. (2005). Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology, 58, 218–233. doi:10.1080/02724990444000131 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Brown M. W., & Albasser M. M. (2012). Contrasting brain activity patterns for item recognition memory and associative recognition memory: Insights from immediate-early gene imaging. Neuropsychologia, 50, 3141–3155. doi:10.1016/j.neuropsychologia.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Hunt P. R., & Rawlins J. N. P. (1986). The effects of hippocampal lesions upon spatial and nonspatial tests of working memory. Behavioural Brain Research, 19, 133–146. doi:10.1016/0166-4328(86)90011-2 [DOI] [PubMed] [Google Scholar]
- Agster K. L., Fortin N. J., & Eichenbaum H. (2002). The hippocampus and disambiguation of overlapping sequences. The Journal of Neuroscience, 22, 5760–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Amin E., Iordanova M. D., Brown M. W., Pearce J. M., & Aggleton J. P. (2011). Separate but interacting recognition memory systems for different senses: The role of the rat perirhinal cortex. Learning & Memory, 18, 435–443. doi:10.1101/lm.2132911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Chapman R. J., Amin E., Iordanova M. D., Vann S. D., & Aggleton J. P. (2010). New behavioral protocols used to extend our knowledge of rodent object recognition memory. Learning & Memory, 17, 407–419. doi:10.1101/lm.1879610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M. M., Davies M., Futter J. E., & Aggleton J. P. (2009). Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behavioral Neuroscience, 123, 115–124. doi:10.1037/a0013829 [DOI] [PubMed] [Google Scholar]
- Albasser M. M., Lin T-C. E., Iordanova M. D., Amin E., & Aggleton J. P. (2012). Evidence that the rat hippocampus has contrasting roles in object recognition memory and object recency memory. Behavioral Neuroscience, 126, 659–669. doi:10.1037/a0029754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser M., Poirier G. L., & Aggleton J. P. (2010). Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-fos imaging. European Journal of Neuroscience, 31, 134–147. doi:10.1111/j.1460-9568.2009.07042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin E., Pearce J. M., Brown M. W., & Aggleton J. P. (2006). Novel temporal configurations of stimuli produce discrete changes in immediate early gene expression in the rat hippocampus. European Journal of Neuroscience, 24, 2611–2621. doi:10.1111/j.1460-9568.2006.05131.x [DOI] [PubMed] [Google Scholar]
- Barker G. R. I., Bird F., Alexander V., & Warburton E. C. (2007). Recognition memory for objects, places, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience, 27, 2948–2957. doi:10.1523/JNEUROSCI.5289-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G. R. I., & Warburton E. C. (2011a). Evaluating the neural basis of temporal order memory for visual stimuli. European Journal of Neuroscience, 33, 705–716. doi:10.1111/j.1460-9568.2010.07555.x [DOI] [PubMed] [Google Scholar]
- Barker G. R. I., & Warburton E. C. (2011b). When is the hippocampus involved in recognition memory? The Journal of Neuroscience, 31, 10721–10731. doi:10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T. (2007). Toward an integrated perspective on hippocampal function: From a rapid encoding of experience to adaptive behaviour. Revues in Neuroscience, 18, 253–281. doi:10.1515/REVNEURO.2007.18.3-4.253 [DOI] [PubMed] [Google Scholar]
- Bast T., Wilson I. A., Witter M. P., & Morris R. G. M. (2009). (2009). From rapid place learning to behavioural performance: A key role for the intermediate hippocampus. PLoS Biology, 7, e1000089. doi:10.1371/journal.pbio.1000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent N. J., Squire L. R., & Clark R. E. (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences, 101, 14515–14520. doi:10.1073/pnas.0406344101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. W., & Aggleton J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. doi:10.1038/35049064 [DOI] [PubMed] [Google Scholar]
- Brown M. W., Warburton E. C., & Aggleton J. P. (2010). Recognition memory: Material, processes, and substrates. Hippocampus, 20, 1228–1244. doi:10.1002/hipo.20858 [DOI] [PubMed] [Google Scholar]
- Burn C. C. (2008). What is it like to be a rat? Rat sensory perception and its implications for experimental design and rat welfare. Applied Animal Behaviour Science, 112, 1–32. doi:10.1016/j.applanim.2008.02.007 [Google Scholar]
- Burwell R. D. (2001). Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. Journal of Comparative Neurology, 437, 17–41. doi:10.1002/cne.1267 [DOI] [PubMed] [Google Scholar]
- Charles D. P., Gaffan D., & Buckley M. J. (2004). Impaired recency judgements and intact novelty judgments after fornix transection in monkeys. The Journal of Neuroscience, 24, 2037–2044. doi:10.1523/JNEUROSCI.3796-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. E., West A. N., Zola S. M., & Squire L. R. (2001). Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus, 11, 176–186. doi:10.1002/hipo.1035 [DOI] [PubMed] [Google Scholar]
- Clark R. E., Zola S. M., & Squire L. R. (2000). Impaired recognition memory in rats after damage to the hippocampus. The Journal of Neuroscience, 20, 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E., & Lekan H. A. (1996). Methods for determining numbers of cells and synapses: A case for more uniform standards of review. Journal of Comparative Neurology, 364, 6–15. doi:10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- Davidson T. L., & Jarrard L. E. (2004). The hippocampus and inhibitory learning: A ‘Gray' area? Neuroscience and Biobehavioral Reviews, 28, 261–271. doi:10.1016/j.neubiorev.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Dere E., Huston J. P., & De Souza Silva M. A. (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and Biobehavioral Reviews, 31, 673–704. doi:10.1016/j.neubiorev.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Dix S. L., & Aggleton J. P. (1999). Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behavioural Brain Research, 99, 191–200. doi:10.1016/S0166-4328(98)00079-5 [DOI] [PubMed] [Google Scholar]
- Ennaceur A., & Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. doi:10.1016/0166-4328(88)90157-X [DOI] [PubMed] [Google Scholar]
- Fortin N. J., Agster K. L., & Eichenbaum H. B. (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood S. E., Winters B. D., & Bussey T. J. (2005). Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus, 15, 347–355. doi:10.1002/hipo.20059 [DOI] [PubMed] [Google Scholar]
- Furtak S. C., Wei S-M., Agster K. l., & Burwell R. D. (2007). Functional neuroanatomy of the parahippocampal region in the rat: The perirhinal and postrhinal cortices. Hippocampus, 17, 709–722. doi:10.1002/hipo.20314 [DOI] [PubMed] [Google Scholar]
- Gilbert P. E., & Kesner R. P. (2003). Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behavioral Neuroscience, 117, 1385–1394. doi:10.1037/0735-7044.117.6.1385 [DOI] [PubMed] [Google Scholar]
- Goulet S., & Murray E. A. (2001). Neural substrates of crossmodal association memory in monkeys: The amygdala versus the anterior rhinal cortex. Behavioral Neuroscience, 115, 271–284. doi:10.1037/0735-7044.115.2.271 [PubMed] [Google Scholar]
- Guzowski J. F., Timlin J. A., Roysam B., McNaughton B. L., Worley P. F., & Barnes C. A. (2005). Mapping behaviourally relevant neural circuits with immediate-early gene expression. Current Opinion in Neurobiology, 15, 599–606. doi:10.1016/j.conb.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Herdegen T. (1996). Jun, Fos, and CREB/ATF transcription factors in the brain: Control of gene expression under normal and pathophysiological conditions. The Neuroscientist, 2, 153–161. doi:10.1177/107385849600200310 [Google Scholar]
- Ho J. W-T., Narduzzo K. E., Outram A., Tinsley C. J., Henley J. M., Warburton E. C., & Brown M. W. (2011). Contributions of area Te2 to rat recognition memory. Learning & Memory, 18, 493–501. doi:10.1101/lm.2167511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. C. (1995). Fundamental statistics for the behavioral sciences (3rd ed.). Belmont, CA: Duxbury Press. [Google Scholar]
- Iordanova M. D., Burnett D., Good M., Aggleton J. P., & Honey R. C. (2009). The role of the hippocampus in mnemonic integration and retrieval: Complementary evidence from lesion and inactivation studies. European Journal of Neuroscience, 30, 2177–2189. doi:10.1111/j.1460-9568.2009.07010.x [DOI] [PubMed] [Google Scholar]
- Jenkins T. A., Amin E., Pearce J. M., Brown M. W., & Aggleton J. P. (2004). Novel spatial arrangements of familiar stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices; a c-fos expression study. Neuroscience, 124, 43–52. doi:10.1016/j.neuroscience.2003.11.024 [DOI] [PubMed] [Google Scholar]
- Keppel G. (1991). Statistics design and analysis: A researcher's handbook (3rd ed.). Englewood Cliffs, NJ: Prentice-Hall, Inc. [Google Scholar]
- Kovács K. J. (2008). Measurement of immediate-early gene activation- c-fos and beyond. Journal of Neuroendocrinology, 20, 665–672. doi:10.1111/j.1365-2826.2008.01734.x [DOI] [PubMed] [Google Scholar]
- Mumby D. G. (2001). Perspective on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research, 127, 159–181. doi:10.1016/S0166-4328(01)00367-9 [DOI] [PubMed] [Google Scholar]
- Mura A., Murphy C. A., Feldon J., & Jongen-Relo A. L. (2004). The use of stereological counting methods to assess immediate early gene immunoreactivity. Brain Research, 1009, 120–128. doi:10.1016/j.brainres.2004.02.054 [DOI] [PubMed] [Google Scholar]
- Murray E. A. (1996). What have ablation studies told us about the neural substrates of stimulus memory? Seminars in the Neurosciences, 8, 13–22. doi:10.1006/smns.1996.0003 [Google Scholar]
- Murray E. A., & Mishkin M. (1984). Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. Journal of Neuroscience, 4, 2565–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber P. A., Witter M. P., & da Silva F. H. L. (1999). Perirhinal cortex input to the hippocampus in the rat: Evidence for parallel pathways, both direct and indirect. A combined physiological and anatomical study. European Journal of Neuroscience, 11, 4119–4133. doi:10.1046/j.1460-9568.1999.00835.x [DOI] [PubMed] [Google Scholar]
- Otto T., & Eichenbaum H. (1992). Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behavioral Neuroscience, 106, 762–775. doi:10.1037/0735-7044.106.5.762 [DOI] [PubMed] [Google Scholar]
- Paxinos G., & Watson C. (1997). The rat brain in stereotaxic coordinates (3rd ed.). San Diego: Academic Press. [Google Scholar]
- Paxinos G., & Watson C. (2005). The rat brain in stereotaxic coordinates (5th ed.). Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Ramus S. J., & Eichenbaum H. (2000). Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. The Journal of Neuroscience, 20, 8199–8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E., Poucet B., Foreman N., & Buhot N. (1992). Object exploration and reactions to spatial and non-spatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behavioral Neuroscience, 106, 447–456. doi:10.1037/0735-7044.106.3.447 [PubMed] [Google Scholar]
- Seoane A., Tinsley C. J., &. Brown M. W. (2012). Interfering with Fos expression in rat perirhinal cortex impairs recognition memory. Hippocampus, 22, 2101–2113. doi:10.1002/hipo.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C. J., & Cassell M. D. (1999). Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. Journal of Comparative Neurology, 406, 299–328. doi:10.1002/(SICI)1096-9861(19990412)406:3<299::AID-CNE2>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- Squire L. R., Wixted J. T., & Clark R. E. (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience, 8, 872–883. doi:10.1038/nrn2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki W. A., Zola-Morgan S., Squire L. R., & Amaral D. G. (1993). Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactile modalities. The Journal of Neuroscience, 13, 2430–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. (1992). Brain maps: Structure of the rat brain. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- U.K. Animals (Scientific Procedures) Act. (1986). The Stationery office, Norwich, U.K. [Google Scholar]
- van Groen T., & Wyss J. M. (1990). Connections of the retrosplenial granular a cortex in the rat. Journal of Comparative Neurology, 300, 593–606. doi:10.1002/cne.903000412 [DOI] [PubMed] [Google Scholar]
- Vann S. D., & Aggleton J. P. (2002). Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behavioral Neuroscience, 116, 85–94. doi:10.1037/0735-7044.116.1.85 [PubMed] [Google Scholar]
- Vann S. D., Brown M. W., & Aggleton J. P. (2000). Fos expression in the rostral thalamic nuclei and associated cortical regions in response to different spatial memory tasks. Neuroscience, 101, 983–991. doi:10.1016/S0306-4522(00)00288-8 [DOI] [PubMed] [Google Scholar]
- Vann S. D., Brown M. W., Erichsen J. T., & Aggleton J. P. (2000). Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activity in response to different spatial memory tasks. The Journal of Neuroscience, 20, 2711–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Aggleton J. P., & Brown M. W. (1999). Different contributions of the hippocampus and perirhinal cortex to recognition memory. The Journal of Neuroscience, 19, 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Warburton E. C., Zhu X. O., Koder T. J., Park Y., Aggleton J. P., Cho K., Bashir Z. I., Brown M. W. (2004). Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. European Journal of Neuroscience, 20, 2214–2224. doi:10.1111/j.1460-9568.2004.03688.x [DOI] [PubMed] [Google Scholar]
- Warburton E. C., & Brown M. W. (2010). Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia, 48, 2262–2272. doi:10.1016/j.neuropsychologia.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Warburton E. C., Glover C. P. J., Massey P. V., Wan H., Johnson B., Bienemann A., et al. Brown M. W. (2005). CREB phosphorylation is necessary for perirhinal LTP and recognition memory. The Journal of Neuroscience, 25, 6296–6303. doi:10.1523/JNEUROSCI.0506-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E. C., Koder T., Cho K., Massey P. V., Duguid G., Barker G. R. I., et al. Brown M. W. (2003). Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron, 38, 987–996. doi:10.1016/S0896-6273(03)00358-1 [DOI] [PubMed] [Google Scholar]
- Wilton L. A. K., Baird A. L., Muir J. L., Honey R. C., & Aggleton J. P. (2001). Loss of the thalamic nuclei for ‘head direction' produces impaired spatial memory in rats. Behavioral Neuroscience, 115, 861–869. doi:10.1037/0735-7044.115.4.861 [PubMed] [Google Scholar]
- Winters B. D., & Reid J. M. (2010). A distributed cortical representation underlies crossmodal object recognition in rats. The Journal of Neuroscience, 30, 6253–6261. doi:10.1523/JNEUROSCI.6073-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B. D., Saksida L. M., & Bussey T. J. (2008). Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and Biobehavioral Reviews, 32, 1055–1070. doi:10.1016/j.neubiorev.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Wirth S., Ferry B., & Di Scala G. (1998). Facilitation of olfactory recognition by lateral entorhinal cortex lesion in rats. Behavioural Brain Research, 91, 49–59. doi:10.1016/S0166-4328(97)00102-2 [DOI] [PubMed] [Google Scholar]
- Wixted J. T., & Squire L. R. (2011). The medial temporal lobe and the attributes of memory. Trends in Cognitive Sciences, 15, 210–217. doi:10.1016/j.tics.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangenehpour S., & Chowdhari A. (2002). Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Molecular Brain Research, 109, 221–225. doi:10.1016/S0169-328X(02)00556-9 [DOI] [PubMed] [Google Scholar]
- Zhu X. O., Brown M. W., McCabe B. J., & Aggleton J. P. (1995). Effects of the novelty or familiarity of visual stimuli on the expression of the intermediate early gene c-fos in the rat brain. Neuroscience, 69, 821–829. doi:10.1016/0306-4522(95)00320-I [DOI] [PubMed] [Google Scholar]
- Zhu X. O., McCabe B. J., Aggleton J. P., & Brown M. W. (1996). Mapping recognition memory through the differential expression of the immediate early gene c-fos induced by novel or familiar visual stimulation. NeuroReport, 7, 1871–1875. doi:10.1097/00001756-199607290-00037 [DOI] [PubMed] [Google Scholar]