Abstract
Background
Muscle-biased therapies (MBT) are commonly used to treat pain, yet several reviews suggest evidence for the clinical effectiveness of these therapies is lacking. Inadequate treatment parameters have been suggested to account for inconsistent effects across studies. Pain sensitivity may serve as an intermediate physiologic endpoint helping to establish optimal MBT treatment parameters. The purpose of this review was to summarize the current literature investigating the short-term effect of a single dose of MBT on pain sensitivity in both healthy and clinical populations, with particular attention to specific MBT parameters of intensity and duration.
Methods
A systematic search for articles meeting our prespecified criteria was conducted using Cumulative Index to Nursing and Allied Health Literature (CINAHL) and MEDLINE from the inception of each database until July 2012, in accordance with guidelines from the Preferred Reporting Items for Systematic reviews and Meta-Analysis. Relevant characteristics from studies included type, intensity, and duration of MBT and whether short-term changes in pain sensitivity and clinical pain were noted with MBT application. Study results were pooled using a random-effects model to estimate the overall effect size of a single dose of MBT on pain sensitivity as well as the effect of MBT, dependent on comparison group and population type.
Results
Reports from 24 randomized controlled trials (23 articles) were included, representing 36 MBT treatment arms and 29 comparative groups, where 10 groups received active agents, 11 received sham/inert treatments, and eight received no treatment. MBT demonstrated a favorable and consistent ability to modulate pain sensitivity. Short-term modulation of pain sensitivity was associated with short-term beneficial effects on clinical pain. Intensity of MBT, but not duration, was linked with change in pain sensitivity. A meta-analysis was conducted on 17 studies that assessed the effect of MBT on pressure pain thresholds. The results suggest that MBT had a favorable effect on pressure pain thresholds when compared with no-treatment and sham/inert groups, and effects comparable with those of other active treatments.
Conclusion
The evidence supports the use of pain sensitivity measures by future research to help elucidate optimal therapeutic parameters for MBT as an intermediate physiologic marker.
Keywords: muscle-biased therapy, pain sensitivity, pressure pain threshold
Introduction
Muscle-biased therapies belong to the National Center for Complementary and Alternative Medicine category of manipulative and body-based practices. Muscle-biased therapy (MBT) includes a variety of interventions that manipulate the soft tissues of the body, through manual (eg, hands-on) or instrument-assisted techniques, and by applying fixed or movable pressure to the skin, muscles, and connective tissues. A considerable amount of variation exists in the way MBT treatments are provided in clinical practice and across research studies. Treatment variation includes the manner in which soft tissues are manipulated, the pressure intensity perceived by the patient (eg, light, deep, painful yet tolerable), the duration of sustained pressure, the duration of a single session of MBT, and the dosage of MBT (number of sessions). Nonetheless, MBT interventions are currently used by a number of health care professionals and have a long history as a therapeutic modality.1
In the US, MBT is widely used to manage pain associated with a variety of muscular and nonmuscular pain conditions,2–6 and its popularity is growing. From 2002 to 2007, visits to health care professionals providing MBT increased, despite an overall decrease in the number of visits to complementary and alternative medicine practitioners during the same time period.2,5 In 2007, back pain was the most common problem for which adults sought MBT care, and roughly 60% of those adults experienced a “great deal” of benefit in reducing pain intensity following manipulative and body-based treatment, including MBT.2,4 However, a number of systematic reviews have reported similar themes of inconsistent, inconclusive, and insufficient evidence for the clinical effectiveness of MBT. These reviews focused on the effect of MBT on various pain-related conditions, including musculoskeletal pain,7,8 headache,9 neck pain,10 shoulder disorders,11 low back pain,12–14 cancer-related pain,15 and exercise-induced pain (ie, delayed-onset muscle soreness).16 A universal recommendation from those reviews for future MBT research has been to articulate the optimal therapeutic technique and dosing regimen.7–9,12–18 Currently, there are no clear definitions of what constitutes an adequate MBT treatment in terms of intensity or duration.9,17,18
We suggest that treatment parameters for MBT may be established through the use of intermediate physiologic endpoints. Studies that examine other modalities, such as transcutaneous electrical nerve stimulation, have addressed the lack of evidence for optimal intervention parameters through such means. For example, the optimal stimulation intensity for transcutaneous electrical nerve stimulation was identified using pain sensitivity as an intermediate physiologic outcome. The transcutaneous electrical nerve stimulation intensity of “strong but comfortable” was shown to produce the optimal analgesic effects compared with other intensities.19–22 We suggest using a similar approach to develop optimal parameters of MBT. Based on current models of the mechanisms underlying therapeutic benefit, intermediate biologic and psychologic endpoints might include measures of physical and mental relaxation, changes in pain sensitivity, changes in local blood perfusion, changes in local biochemical milieu, and shifts in the autonomic nervous system.12,13
Changes in pain sensitivity have been measured in several MBT randomized controlled trials and are recommended as objective outcomes.9,23,24 Further, a recent systematic review and meta-analysis of a joint-biased therapy found a small but favorable increase in pressure pain thresholds following a single session of spinal manipulation when compared with all other therapies combined.25 However, the authors did not examine whether the favorable changes in pain sensitivity were related to a clinical endpoint. Muscle-biased and joint-biased therapies, although distinguished by their targeted tissues, belong to the same National Center for Complementary and Alternative Medicine category of manipulative and body-based practices and are suggested to share similar neurophysiologic mechanisms underlying pain relief.26 However, there are no systematic reviews summarizing the effect of MBT on pain sensitivity and whether these effects are related to clinical pain or are influenced by treatment parameters.
Thus, the purpose of this review was to summarize and critically evaluate the current literature investigating the short-term effect of a single dose of MBT in randomized controlled trials on pain sensitivity in both pain-free and clinical pain populations. We aimed to address three primary questions within this review, ie, whether MBT affects pain sensitivity, whether MBT intensity and/or duration influences pain sensitivity, and whether there are short-term changes in pain sensitivity associated with changes in clinical pain over similar time points. To address the first question, we used meta-analysis to estimate the pooled effect of MBT across trials within a pain sensitivity measure. We planned two a priori subgroup analyses. The first subgroup analysis examined the effect of MBT on pain sensitivity compared with interventions stratified by type (ie, no intervention, sham intervention, or active intervention). The second subgroup analysis stratified responses following MBT by population (ie, clinical or healthy). The results of these analyses will establish whether MBT has an overall effect on pain sensitivity and whether these differ based on population or comparison group.
To address the second question, we categorized MBT interventions based on intensity (eg, whether pain was induced during an MBT session or not) and duration (eg, based on application time) and examined the associations between these two variables and pain sensitivity changes. These results will implicate the importance of these parameters on pain sensitivity. Finally, to address the third question, we explored the congruence between short-term changes in pain sensitivity and changes in clinical pain over similar time points within studies involving clinical samples. These results will provide an indirect indication of whether pain sensitivity changes are clinically meaningful. Our results will also potentially provide preliminary evidence to direct future research addressing the gap in our understanding of optimal duration and intensity of MBT.
Materials and methods
A similar methodologic approach was used as our previously published systematic review and meta-analysis, which was conducted in accordance with the guidelines from Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA).25,27
Eligibility criteria
Study eligibility was based on study design, population, MBT intervention, comparison group, and outcome. We included randomized controlled trials that investigated the effects of a single session of MBT on pain sensitivity. Randomized controlled trials could be either multigroup (between-subject) or single-group (within-subject) crossover trials. We excluded descriptive and nonexperimental observational studies such as case series.
The sample populations of interest were human adults (>18 years of age) with or without a clinical pain condition. We did not limit the sample to participants with a specific musculoskeletal pain condition, because MBT is applied to relieve pain in patients with a variety of clinical conditions.
We included studies that listed MBT as the primary intervention. We adopted the classification system of manipulative and body-based practices reported by Bialosky et al.26 The MBT interventions included in this review were those directed at the muscle and soft tissues of the body. Examples of MBT included Swedish, Thai, Shiatsu, and deep tissue massage (stroking and kneading of the skin and underlying soft tissue using various amount rhythmic of pressure), myofascial release (applying sustained pressure to elongate soft and connective tissue), trigger point therapy (deep pressure to areas of local tenderness), and cross-friction massage (deep pressure applied transversely to specific tissues).26 Excluded therapies included joint-biased (eg, joint mobilization and manipulation) and nerve-biased (eg, passive, combined movements of the spine and extremities, within their normal range of motion, in ways to elongate or tension specific nerves) techniques.26 The intervention may have been applied to a single region (ie, back or lower extremity) or to multiple regions (ie, full body). Studies with cointerventions were included if the cointerventions were part of the comparison treatment to allow for the difference in treatment effect to be attributed to the addition of MBT in the experimental group. Studies that included multiple sessions of MBT were included if the short-term changes in pain sensitivity could be discerned following the first treatment session.
The comparison group included any form of active or nonactive interventions or no treatment controls. Examples of active interventions were physical modalities and other forms of manual therapy. Nonactive interventions included inert or sham interventions. No treatment control groups included those receiving advice, home instructions, or quiet rest, without any other intervention.
The primary outcome of interest was a change in a pain sensitivity measure. Studies needed to employ a between-group or within-subject repeated-measures design with the measurements taken before and after the intervention. Specific characteristics of the pain sensitivity measure of interest included the experimental sensory modality used, the psychophysical response, and the location of the stimulus application. The experimental sensory modality could have included chemical, electrical, ischemic, mechanical, and thermal stimuli. The psychophysical response could have been either static (ie, threshold, tolerance, or suprathreshold rating) or dynamic (ie, change in perception to repeated stimuli) measures. The location of the experimental stimulus application was considered in relation to the region where MBT was applied. If the location was in the same anatomical region receiving the MBT, it was considered local. If the location was outside the anatomical region receiving the MBT, it was considered remote.
Data sources
Relevant articles were identified by performing a comprehensive search for studies in MEDLINE (PubMed) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) from the inception of each database until July 2012. Search terms included: musculoskeletal manipulations [MESH; major topic], “manual therapy”, “massage”, “alternative therapies”, “myofascial release”, “myofascial therapy”, “trigger point therapy”, pain [MESH; major topic], “pain measurement” [MESH; major topic], “quantitative sensory testing”, “thermal pain”, “electrical pain”, “pressure pain”, “mechanical pain”, “pain threshold”, “muscle pain”, “experimental pain”, and “trigger point”. The search strategy for both databases is listed in Table 1. MEDLINE limits were: “abstracts”, “humans”, “clinical trial”, “randomized controlled trial”, and “English language.” CINAHL limits were: “abstract available”, “English language”, “peer reviewed”, “research article”, “human”, and “all adult”. Database searches were conducted on August 1, 2012. The search strategy used for both databases is listed in Table 1. In addition, manual searches through reference lists of relevant articles and previously published systematic reviews were performed to identify additional articles.
Table 1.
Database search strategy performed August 1, 2012
| Number | Search terms | MEDLINE results | CINAHL results |
|---|---|---|---|
| Database search strategy | |||
| 1 | Musculoskeletal manipulations [MESH; major topic] | 7032 | 7 |
| 2 | “Manual therapy” | 1740 | 2586 |
| 3 | “Massage” | 10,457 | 7432 |
| 4 | “Alternative therapies” | 2833 | 18,775 |
| 5 | “Myofascial release” | 76 | 227 |
| 6 | “Myofascial therapy” | 20 | 24 |
| 7 | “Trigger point therapy” | 45 | 143 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 18,803 | 27,581 |
| 9 | “Pain” [MESH; major topic] | 174,141 | 52,073 |
| 10 | “Pain measurement” [MESH; major topic] | 7773 | 3444 |
| 11 | “Quantitative sensory testing” | 728 | 189 |
| 12 | “Thermal pain” | 596 | 91 |
| 13 | “Electrical pain” | 144 | 34 |
| 14 | “Pressure pain” | 1033 | 420 |
| 15 | “Mechanical pain” | 417 | 81 |
| 16 | “Pain threshold” | 10,014 | 1553 |
| 17 | “Muscle pain” | 2153 | 1293 |
| 18 | “Experimental pain” | 1059 | 203 |
| 19 | “Trigger point” | 546 | 766 |
| 20 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 | 183,382 | 53,510 |
| 15 | 8 AND 20 | 2087 | 2635 |
| Search limits | |||
| MEDLINE limits: “abstracts”, “humans”, “clinical trial”, “randomized controlled trial”, and “English” | 582 | ||
| CINAHL limits: “abstract available”, “English language”, “peer reviewed”, “research article”, “human”, and “all adult” after removing duplicates | 502 | ||
Abbreviation: CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Study search and selection
All articles were screened for eligibility into this review by the primary author (CWG). A manual search of reference lists was conducted by all members of the research team to identify additional studies. A systematic screening procedure was used to identify relevant articles. First, article titles were reviewed within each database search list. Second, abstracts were screened if the title did not yield adequate information for study inclusion. Based on title and abstract, articles were deemed as potentially relevant and further screened, while all other were excluded. Finally, two authors (MJA and CWG) independently reviewed the full texts of all potentially relevant articles for final inclusion in the review. Any disagreements regarding article inclusion were resolved by consensus. If a consensus could not be reached, a third author (MEH) was recruited to resolve disagreement.
Data extraction
Two authors (MJA and CWG) independently extracted data from each of the included articles using a standardized Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA) template. Results of each author’s extraction were compared to ensure accuracy of the data. Each article was reviewed for key information: type of randomized trial; participant characteristics, including total number of subjects, number of subjects within each group, gender, and clinical condition; type of intervention within groups, including cointerventions; duration of treatment; perceived intensity of treatment (eg, painful or nonpainful); pain sensitivity outcome and region in which stimulus was applied; and results of the study (pre-post mean values and standard deviation for each measure within each group). The primary author of the respective article was contacted if any of the above information was unobtainable. If a response was not provided within 14 days of being contacted, the information was not included in the review. Two authors were contacted for additional information28,29 and one provided a response.29
Methodologic quality assessment
Risk of potential bias was assessed by using the recommendations provided by the “Cochrane Handbook for Systematic Reviews of Interventions”.30 Each article was assessed for potential bias in five domains: selection bias, performance bias, attrition bias, reporting bias, and detection bias.30 A 12-item scale was used to assess the internal validity of each study. Articles were then individually categorized as: low risk of bias (plausible bias unlikely to alter the results seriously) if there was low risk of bias for all key domains; unclear risk of bias (plausible bias that raises some doubt about the results) if unclear risk of bias for one or more key domains; or high risk of bias (plausible bias that seriously weakens confidence in the results) if high risk of bias for one or more key domains.30 Prior to assessing the quality of the included studies, a practice trial of scoring was performed by two authors (MJA and CWG) on two articles (not included in this analysis) to ensure understanding of the quality criteria. The two authors independently rated the quality of each article. A second meeting was needed to clarify the grading of articles for four items within the risk of bias criteria (item 6, 7, 8, and 11). These items are not always fully addressed in the methods and results sections for immediate effects studies, because they are more relevant to longitudinal trials. After completion of independent grading, the authors met to finalize the scores for each article. Disagreements regarding article quality were resolved by consensus. If consensus could not be reached, a third author (MEH) was recruited to resolve discrepancy.
Data analysis
We used Microsoft Excel 2007 and PASW Statistics, version 20 (SPSS Inc, Chicago, IL) to examine and compile individual study descriptive statistics, congruence between changes in pain sensitivity and clinical pain, and reviewer agreement. For meta-analytical procedures, we used Comprehensive Meta-Analysis, Version 2 (Biostat Inc, Englewood, NJ). Alpha was set at the 0.05 level for statistical significance.
We estimated congruence between changes in pain sensitivity and clinical pain using Kappa agreement and 95% confidence interval (CI). We examined the agreement for article methodologic quality using intraclass correlation coefficient and 95% CI. We used guidelines for interpreting Kappa and intraclass correlation coefficient values proposed by Landis and Koch et al31 and Portney and Watkins et al,32 where values < 0 indicate no agreement and 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement.31,32
Conducting the meta-analysis was dependent on having an adequate number of trials (eg, more than two) that used similar study methodology. We based this decision primarily on the pain sensitivity measure where studies needed to use the same experimental sensory modality (ie, pressure, heat, electricity) and measure a similar psychophysical response (ie, threshold, tolerance). In some studies, multiple immediate measurements of the same pain sensitivity outcome were obtained from the same anatomical region (ie, low back or upper extremity). In these cases, we generated a single composite effect for that region using the methods for combining multiple outcomes described by Borenstein.33 We computed individual effect size estimates (Hedges g) for each group within each study using information provided in the articles.
We generated separate random-effects models for each pain sensitivity outcome. The primary comparison was a mean difference (eg, pre-to-post change) on the pain sensitivity measure between the group receiving MBT and the comparison group or groups. In cases where multiple treatment arms were included in a single study, we used the following approach. We collapsed comparison group data if groups were in the same category. We categorized comparison groups as active, sham, or control (no treatment) using the definitions in eligibility criteria. We examined dissimilar comparison groups separately. When multiple MBT treatment groups were included in a study, we examined these groups separately within the meta-analysis. We computed an overall pooled Hedges g effect size estimate, with 95% CI as the measure of effect for MBT. Effect size estimates were considered small (0.20), medium (0.50), or large (0.80).34 Last, we evaluated homogeneity of the estimated effects using a measure of inconsistency (I2), where large values of I2 suggest heterogeneity.
We performed two planned subgroup analyses. We planned a stratified analysis by population (healthy versus clinical) and comparison treatment (active versus sham versus control). We categorized population groups as “healthy” (asymptomatic participants) or “clinical” (symptomatic participants) based on the study description. Studies that induced delayed onset muscle soreness or included subjects based on the presence of trigger points were not considered strictly clinical or healthy, and thus were withheld based on the difficulty of placing them into a category. We also stratified our analysis by comparison group where the effects of MBT were examined separately for active, sham, and control group comparisons.
Results
Study selection
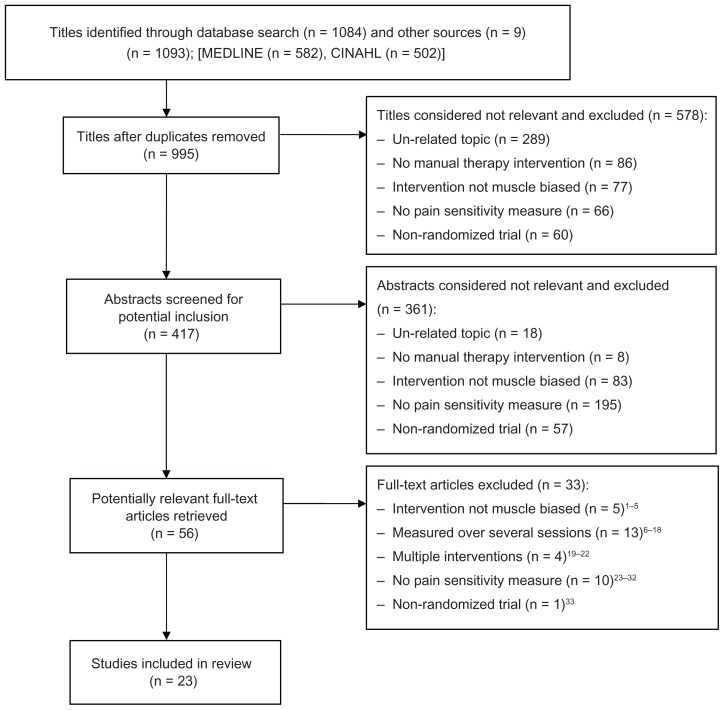
We identified a total of 1084 articles from the systematic search of MEDLINE (n = 582) and CINAHL (n = 502), and nine articles from a review of reference lists. After removing duplicates, we screened and assessed 995 titles. Of these, we excluded 939 articles after title or abstract screening. We selected the full texts of 39 articles to be screened by two independent reviewers. Twenty-three studies were excluded based on study design,35 inability to compare the effects of a single session of MBT,36–52 intervention was not considered MBT,53–57 or no pain sensitivity measure.58–67 As a result, 23 articles representing 24 studies were identified as meeting the criterion for inclusion in this review. Figure 1 depicts a flow diagram of the study selection process with reasons for exclusion at each stage. Hou et al reported on two separate study designs that included separate samples, and both studies were included. Thirteen of the studies compared two groups.68–80 Nine of the studies compared three groups.28,81–87 Two studies compared six groups.88
Figure 1.
Flow chart of study identification, selection, and inclusion.
Abbreviation: CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Characteristics of studies
Table 2 provides characteristics of each study, including the sample population, description of muscle-biased interventions and comparison groups, method of assessing pain sensitivity, and outcomes.
Table 2.
Characteristics of included studies
| Author (study design) | Population (n) | Induced pain | Duration (minutes) | Measure | Raw mean difference (SD) | Pain sensitivity | Clinical pain |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Study interventions | Sample size | ||||||
| Aguilera et al81 (RCT) | Healthy (n = 68) | ||||||
| MBT, ischemic compression | 24 | + | 1.5 | STR* | 8.23 (14.78) | + | NA |
| Com AT, ultrasound | 22 | 4 | 7.50 (7.86) | + | NA | ||
| Com IT, sham ultrasound | 22 | 5 | 1.50 (7.62) | − | NA | ||
| Aparicio et al (RCT)68 | Healthy (n = 70) | ||||||
| MBT, manual pressure | 36 | − | 2 | PPTa,b | 0.36 (1.43) | − | NA |
| Com AT, distraction of facial joints | 34 | 2 | 0.05 (1.00) | − | NA | ||
| Arroyo-Morales et al69 (RCT) | Healthy subjects/lower extremity DOMS (n = 62) | ||||||
| MBT, massage | 32 | − | 40 | PPTa,b | 0.35 (0.66) | + | NA |
| Com IT, sham ultrasound | 30 | 40 | 0.20 (0.67) | + | NA | ||
| Blikstad and Gemmell28 (RCT) | Neck pain with MTrPs (n = 45) | ||||||
| MBT, myofascial therapy | 15 | − | 2 | PPTb | UTC | UTC | UTC |
| MBT, activator (mechanical device) | 15 | − | 2 | UTC | UTC | UTC | |
| Com IT, sham ultrasound | 15 | 2 | UTC | UTC | UTC | ||
| Buttagat et al70 (RCT) | Scapulocostal syndrome (n = 20) | ||||||
| MBT, Thai massage | 10 | + | 30 | PPTb | 0.90 (0.85) | + | + |
| Com AT, hot pack and ultrasound | 10 | 30 | 0.10 (0.85) | − | − | ||
| Buttagat et al71 (RCT) | Low back pain with MTrPs (n = 36) | ||||||
| MBT, Thai massage | 18 | + | 30 | PPTb | 1.40 (1.03) | + | + |
| Com NT, rest | 18 | 30 | 0.00 (1.00) | − | − | ||
| Chatchawan et al72 (RCT) | Low back pain (n = 180) | ||||||
| MBT – Swedish massage | 90 | + | 30 | PPTb | 0.20 (1.10) | + | + |
| MBT – Thai massage | 90 | + | 30 | 0.30 (1.10)) | + | + | |
| Farasyn et al82 (RCT) | Low back pain (n = 60) | ||||||
| MBT, roptrotherapy (instrument) | 20 | + | 30 | PPTa,b | 1.03 (1.03) | + | + |
| Com IT, sham endermology | 20 | 30 | 0.00 (1.38) | − | − | ||
| Com NT, rest | 20 | 30 | −0.10 (0.49) | neg | − | ||
| Farasyn and Meeusen73 (RCT) | Low back pain (n = 58) | ||||||
| MBT, roptrotherapy (instrument) | 42 | + | 30 | PPTa,b | 1.03 (1.34) | + | + |
| Com IT, sham endermology | 20 | 30 | 0.00 (1.27) | − | NR | ||
| Fernández-de-las-Peñas et al74 (RCT) | Neck pain (n = 40) | ||||||
| MBT, ischemic compression | 20 | + | 1.5 | PPTb | 0.40 (0.55) | + | + |
| MBT, transverse friction massage | 20 | + | 3 | 0.35 (0.40) | + | + | |
| Fernandez-Lao et al75 (RCOT) | Breast cancer survivors (n = 20) | ||||||
| MBT, myofascial release | 20 | − | 40 | PPTa,b | 6.20 (60.86) | − | NA |
| Com NT, attention control | 20 | 40 | 3.30 (54.81) | − | NA | ||
| Frey Law et al29 (RCT) | Healthy subjects/upper extremity DOMS (n = 39) | ||||||
| MBT, deep touch treatment | 13 | − | 6 | PPTb | 13.30 (70.3) | + | NA |
| MBT, light touch treatment | 15 | − | 6 | 11.10 (61.35) | + | NA | |
| Com NT, rest | 11 | 6 | −7.3 (65.9) | − | NA | ||
| Fryer and Hodgson76 (RCT) | Healthy (n = 37) | ||||||
| MBT, myofascial release | 20 | + | 1 | PPTb | 2.05 (1.70) | + | NA |
| Com IT, touch control | 17 | 1 | 0.08 (1.70) | − | NA | ||
| Gemmell and Allen 2008a77 (RCT) | Neck pain with MTrPs (n = 52) | ||||||
| MBT, ischemic compression | 25 | + | 1 | PPTb | 1.1 (1.9) | + | + |
| MBT, activator (mechanical device) | 27 | − | 1 | 1.4 (1.2) | + | + | |
| Gemmell et al 2000b83 (RCT) | Subjects with MTrPs in trapezius mm (n = 45) | ||||||
| MBT, ischemic compression | 15 | + | 1 | PPTb | 1.06 (1.43) | + | NA |
| MBT, trigger point therapy | 15 | − | 1.5 | 0.97 (1.48) | + | NA | |
| Com IT, sham ultrasound | 15 | 2 | 0.77 (1.23) | + | NA | ||
| Hamilton et al84 (RCT) | Healthy (n = 90) | ||||||
| MBT, muscle energy technique | 30 | − | 1 min | PPTb | 42.04 (162.62) | − | NA |
| Com AT, joint-biased therapy | 35 | 1 min | 39.37 (132.82) | − | NA | ||
| Com IT, touch control | 25 | 1 min | 15.88 (181.96) | − | NA | ||
| Hou et al88 (RCT) | Neck pain (n = 48) | ||||||
| MBT, pain threshold pressure | 8 | + | 0.5 | PPTb; Ptolb | 0.00 (0.99); 0.06 (1.40) | −; − | + |
| MBT, pain threshold pressure | 8 | + | 1 | 0.07 (0.64); 0.07 (0.86) | −; − | + | |
| MBT, pain threshold pressure | 8 | + | 1.5 | 0.79 (1.02); 0.85 (1.18) | +; + | + | |
| MBT, pain tolerance pressure | 8 | + | 0.5 | 0.67 (0.87); 0.10 (0.98) | +; + | + | |
| MBT, pain tolerance pressure | 8 | + | 1 | 0.72 (0.76); 0.71 (0.99) | +; + | + | |
| MBT, pain tolerance pressure | 8 | + | 1.5 | 1.02 (0.71); 0.98 (1.33) | +; + | + | |
| Hou et al88 (RCT) | Neck pain (n = 71) | ||||||
| MBT, control + ischemic compression | 13 | + | 13.5 | PPTb; Ptolb | 0.42 (0.74) | +; + | + |
| Com AT, multiple modalitiesa | 58 | 25 | 0.63 (0.38) | +; + | + | ||
| Lewis et al85 (RCT) | Low back pain (n = 28) | ||||||
| MBT, strain-counterstrain | 28 | − | 1.5 | PPTa; EDTa; EPTa | 93.40 (268.89); 1.80 (28.35); 15.70 (198.43) | +; −; − | − |
| Com IT, sham strain-counterstrain | 28 | 1.5 | 48.30 (207.61); 1.30 (30.78); 6.90 (240.28) | +; −; − | − | ||
| Com NT, rest | 28 | 6 | 30.70 (219.35); 6.80 (24.43); 18.20 (188.58) | +; +; + | − | ||
| Mancinelli et al78 2006 (RCT) | General muscle soreness (n = 22) | ||||||
| MBT, massage | 11 | − | 17 | PPTa,b | 1.40 (6.05) | − | + |
| Com NT, rest | 11 | 17 | −0.15 (6.71) | − | − | ||
| Meseguer et al86 (RCT) | Neck pain (n = 54) | ||||||
| MBT, classic strain-counterstrain | 18 | + | 5 | STR** | 2.6 (1.4) | + | NR |
| MBT, modified strain-counterstrain | 18 | + | 5 | 2.6 (1.8) | + | NR | |
| Com NT, rest | 18 | 5 | 0.03 (0.3) | − | NR | ||
| Oliveira-Campelo et al87 (RCT) | Subjects with MTrP in masseter muscle (n = 122) | ||||||
| MBT, muscle inhibition technique | 41 | − | 2 | PPTb | 0.0 (0.70) | − | NR |
| Com AT, joint-biased therapy | 41 | 2 | 0.20 (0.70) | + | NR | ||
| Com NT, rest | 40 | 2 | −0.10 (0.70) | − | NR | ||
| Saiz-Llamosas et al79 (RCT) | Healthy (n = 35) | ||||||
| MBT, myofascial induction | 19 | − | 5 | PPTa,b | 5.95 (98.06) | − | NA |
| PPTa,c | −6.40 (285.59) | ||||||
| Com IT, touch control | 16 | 5 | PPTa,b | 15.80 (76.01) | − | NA | |
| PPTa,c | −15.85 (184.53) | ||||||
| Toro-Velasco et al80 (RCOT) | Chronic tension-type headache (n = 11) | ||||||
| MBT, massage | 11 | − | 40 min | PPTa,b | −8.7 (46.96) | − | + |
| Com IT, sham ultrasound | 11 | 40 min | 0.80 (56.60) | − | − | ||
Notes:
STR, suprathreshold rating to 2.5 kg pressure;
STR, suprathreshold rating to 4.5 kg pressure;
Composite measure;
local measure;
remote location;
both remote and local measurements; Induced pain = Did the intervention induce pain during the session? (+) = Yes; (−) = No; Duration, length of MBT session, reported in minutes; Measure, pain sensitivity measure; Diff in means (SE), estimated within-group difference in pre-intervention and post-intervention mean (standard error); Pain sensitivity = within group effect on pain sensitivity. (+) = favorable; (−) = no change; (neg) = unfavorable effect; Clinical pain = within group effect on clinical report of pain. (+) = favorable; (−) = no change.
Abbreviations: RCT, randomized controlled trial; RCOT, randomized crossover trial; MTrP, myofascial trigger point; PPT, pressure pain threshold; Ptol, pressure pain tolerance; EDT, electrical detection threshold; EPT, electrical pain threshold; NR, not reported; NA, not applicable; UTC, unable to compute; MBT, muscle-biased therapy; Com IT, comparative group inert treatment; Com NT, comparative group no treatment; Com ATT, comparative group active treatment; DOMs, delayed-onset muscle soreness.
Sample population
A total of 1318 participants (62% female) were enrolled in the included studies. Five studies (n = 300, 57% female) included healthy participants.68,76,79,81,84 Two studies (n = 106, 44% female) included healthy participants and then exposed them to eccentric exercise in a controlled laboratory setting to induce delayed onset muscle soreness.29,69 Sixteen studies (n = 912, 66% female) included a clinical population. The clinical conditions included low back pain (five studies),71–73,82,85 neck pain (five studies),28,74,77,86,88 tension-type headache (one study),80 individuals with myofascial trigger points (two studies),83,87 breast cancer survivors (one study),75 shoulder pain (one study),70 and preseason sport-related muscle soreness (one study).78
MBT characteristics
A variety of MBT was described across the 23 articles. A total of 36 groups (n = 823) received an MBT treatment. Sixteen studies included a single MBT group,68–71,73–76,78,79,81,82,84,85,87 seven studies included two MBT groups,28,29,72,74,77,83,86 and one study employed six MBT groups.88 One study (two of the 36 MBT groups) did not provide adequate descriptive statistics to discern group changes in pain sensitivity over time.28 Twenty-five (74%, n = 609) of the 34 MBT groups reported a favorable change, (ie, reduced pain sensitivity). No change in pain sensitivity was reported for nine of the 34 groups (26%, n = 184).
Intensity of MBT
Twenty-three of the 24 studies provided adequate information to discern whether the MBT induced pain or not. One study (two MBT groups, n = 30) did not.28 Twenty-one (62%, n = 491) of the 34 MBT treatments provoked pain during the treatment session. Nineteen (90%, n = 475) of the 21 pain-induced groups had a positive effect on pain sensitivity, and two (10%, n = 16) had no effect. Thirteen (38%, n = 302) of the 34 MBT treatments did not provoke pain during the treatment. Five of the 13 nonpain groups (38%, n = 134) had a positive effect on pain sensitivity and seven (54%, n = 168) had no effect. A significantly greater proportion of the MBT treatments that induced pain during the session had a positive effect on pain sensitivity than MBT treatments that did not induce pain during the session, (χ2 = 9.17, P < 0.01).
Duration of MBT
All of the studies provided adequate information to discern the approximate duration of the MBT session. Twenty-six of the MBT treatments (72%, n = 479) lasted less than 8 minutes (short duration). The duration of treatment in the remaining 10 groups (28%, n = 344) lasted 16 minutes or more (long duration). None of the included studies used an MBT technique lasting between 8 and 16 minutes (medium duration). One study, with two groups (n = 30) receiving short-duration interventions, did not report mean changes in pain sensitivity over time.28 Eighteen (75%, n = 307) of the 24 short-duration sessions had a positive effect on pain sensitivity, and six (25%, n = 142) had no effect. Seven (70%, n = 302) of the 10 long-duration sessions had a positive effect on pain sensitivity and three (30%, n = 48) had no effect. There was no difference in the proportion of studies reporting a positive effect on pain sensitivity based on session duration (ie, short versus long, χ2 = 0.091, P > 0.05).
Comparison interventions
A total of 29 (n = 575) comparison groups were described across the 23 articles. Eight of the groups (n = 166) were no treatment control groups. Seven of those rested for a comparable amount of time as the active MBT intervention, and one group received 40 minutes of back pain education, including home exercise instruction. Six (75%, n = 118) of eight groups showed no change (ie, no effect) in pain sensitivity. One group (12.5%, n = 20) showed a negative effect on pain sensitivity (ie, increase in pain sensitivity), and one group (12.5%, n = 28) showed a favorable effect on pain sensitivity (ie, decrease in pain sensitivity).
Eleven of the groups (n = 219) received an inert/sham intervention. The inert/sham interventions included inactive ultrasound (five groups), a touch control of similar duration as the active intervention (four groups) and inactive endermology (light skin suction, two groups). One study (n = 15) did not provide adequate descriptive statistics to discern group changes in pain sensitivity over time.28 Three (30%, n = 73) of ten groups showed a positive effect on pain sensitivity and seven (70%, n = 131) groups had no effect.
Ten of the groups (n = 200) received an active intervention. Active interventions included single or combinations of physical modalities (seven groups) and joint-biased interventions (three groups). Seven (70%, n = 121) of the ten groups showed a positive effect on pain sensitivity and three (30%, n = 79) groups had no effect.
Pain sensitivity measures
There were different characteristics of pain sensitivity outcomes reported across the studies. Only two types of sensory modalities were reported, ie, mechanical pressure (24 studies) and electricity (one study).85 The psychophysical responses were primarily static measures of pain processing, pain threshold (22 studies), pain tolerance (two studies),88 and a pain intensity rating of a fixed suprapain threshold stimulus (two studies).81,86 Eighteen studies measured changes in pain sensitivity within the same anatomical region where the intervention was performed. Three studies obtained measures in an anatomical region remote from the region receiving treatment.68,80,87 Two studies obtained measurements in both the region being treated and in a remote location.69,79 Eleven studies reported on both changes in clinical pain and pain sensitivity measures.70–74,77,78,80,82,85,88 In those studies, irrespective of random group assignment, there was a moderately strong congruence between changes in pain sensitivity and changes in clinical pain, kappa = 0.446 (P = 0.01), 95% CI (0.269–0.623).31
Methodologic quality
The assessment of potential risk of bias for the 23 articles is shown in Table 3. Quality score agreement between the two primary raters (MJA and CWG) was good with an intraclass correlation coefficient of 0.67 (95% CI 0.21–0.86). The involvement of a third rater (MEH) was needed to resolve disagreements over criteria item 2, concealment of treatment allocation, for three studies.28,77,81 Of the 23 studies included in the review, none were considered at low risk of bias (ie, high quality), 18 of the studies were considered at unclear risk of bias (plausible bias for one or more key domains), and five studies at high risk of potential bias (ie, low quality). Across studies, there was consistent plausible risk of performance and detection bias contributing to the overall quality being assessed as “unclear risk of bias”, meaning that most information was gathered from studies at low or unclear risk of bias. Insufficient blinding of patients and practitioners, and the inability to account for other potential threats to validity were the major methodologic limits throughout the 23 studies. The five studies rated as at high risk of bias additionally failed to blind the assessor and/or report on baseline characteristics of the groups being compared (increasing the potential detection and selection bias, respectively). In general, due to the nature of the studies (ie, immediate pre-post design) there was low risk of attrition bias. It is difficult to assess for reporting bias because the majority of studies used pain sensitivity tests as secondary outcome measures and not primary clinical measures.
Table 3.
Methodological quality assessment of included studies
| Article/criteria | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total | Domains | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||
| A | B | C | D | E | ||||||||||||||
| Aguilera et al81 | + | − | − | − | − | + | + | ? | + | + | + | + | 7 | U | U | U | L | ? |
| Aparicio et al68 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Arroyo-Morales et al69 | + | − | − | − | − | + | − | ? | + | + | + | + | 6 | L | U | U | L | ? |
| Blikstad and Gemmell28 | + | − | − | − | + | + | + | ? | − | + | + | + | 7 | L | U | L | L | ? |
| Buttagat et al70 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Buttagat et al71 | + | − | − | − | − | + | + | ? | + | + | + | + | 7 | L | U | U | L | ? |
| Chatchawan et al72 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Farasyn et al82 | + | − | − | − | − | + | + | ? | + | + | + | + | 7 | L | U | U | L | ? |
| Farasyn and Meeusen73 | + | − | − | − | − | + | + | ? | + | + | + | + | 7 | U | U | U | L | ? |
| Fernández-de-las-Peñas et al74 (2006) | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Fernandez-Lao et al75 (2012) | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Frey Law et al29 | + | − | − | − | + | + | − | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Fryer and Hodgson76 | + | + | − | − | − | + | + | ? | + | + | + | + | 7 | L | U | L | L | ? |
| Gemmell and Allen77 (2008a) | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Gemmell et al83 (2008b) | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Hamilton et al84 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Hou et al88 (2002) | + | − | − | − | − | + | + | ? | + | + | + | + | 7 | L | U | U | L | ? |
| Lewis et al85 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Mancinelli et al78 (2006) | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Meseguer et al86 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Oliveira-Campelo et al87 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Saiz-Llamosas et al79 | + | − | − | − | + | + | + | ? | + | + | + | + | 8 | L | U | L | L | ? |
| Toro-Velasco et al80 | + | + | − | − | + | + | + | ? | + | + | + | + | 9 | L | U | L | L | ? |
| Across all studies | L | U | L | L | ? | |||||||||||||
Notes: (+) met criteria, (−) did not meet criteria, (?) unsure, (L) low risk of bias, (U) unclear risk of bias, (H) high risk of bias. Criteria: 1 – was the method of randomization adequate?; 2 – was the treatment allocation concealed?; 3 – was the patient blinded to the intervention?; 6 – was the dropout rate described and adequate?; 7 – were all randomized participants analyzed in the group which they were allocated?; 8 – are reports of the study free of suggestion of selective outcome reporting?; 9 – were the groups similar at baseline regarding the most important prognostic indicators?; 10 – were con-interventions avoided or similar?; 11 – was the compliance acceptable in all groups?; 12 – was the timing of the outcome assessment similar in all groups?. A, selection bias; B, performance bias; C, detection bias; D, attrition bias; E, reporting bias. *1 point for each item meeting criteria.
Meta-analysis results
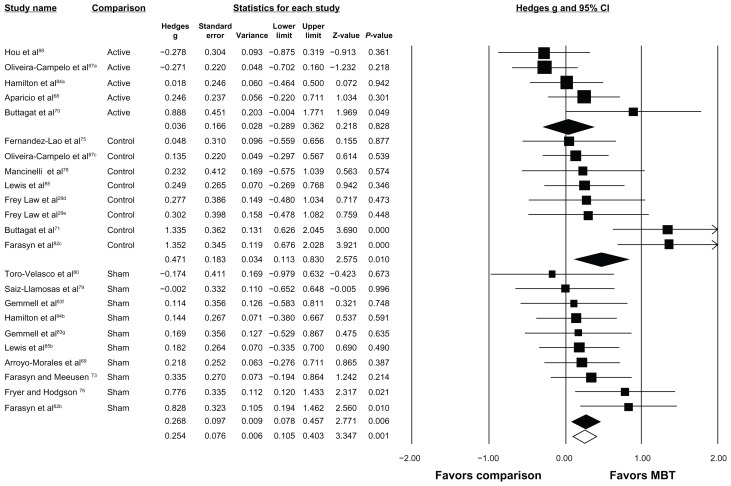
Seventeen of the 24 studies were eligible for meta-analysis.29,70–76,78–80,82–84,87,88 All 17 studies examined an immediate effect of MBT on pressure pain thresholds. The overall effect estimate generated from the random effects model (I2 = 44.0%, P < 0.05) suggested a small, favorable effect of MBT on increasing pressure pain threshold response as compared with all other interventions (Hedges g = 0.254 [95% CI 0.105–0.403], P < 0.05, Figure 2).
Figure 2.
Forest plot depicting overall effect of MBT across all studies and subgroup effect of MBT based on type of comparison group.
Notes: Open diamond depicts overall effect size across all studies. Colored diamonds depict subgroup effect size based on type of comparison group. For example, top colored diamond is effect size for MBT studies with active comparison groups. The width of diamond corresponds to its 95% CI (listed in figure). Individual study effect sizes (and 95% CI) are depicted with boxes (whiskers). Box and whisker size and thickness for each individual study illustrate the weighted contribution of that study to the overall effect size (eg, larger boxes/thicker lines contribute more to overall effect size). aActive comparison; bsham comparison; ccontrol comparison; dMBT arm received superficial pressure; eMBT arm received deep pressure; fMBT arm received ischemic compression; gMBT arm received trigger point therapy.
Abbreviations: CI, confidence interval; MBT, muscle-biased therapy.
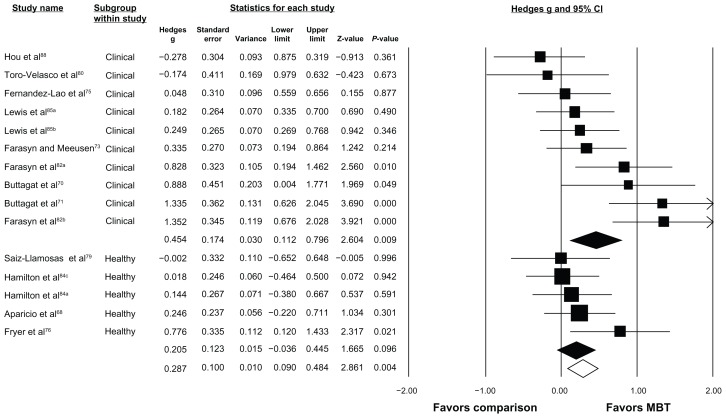
The same 17 studies were able to be stratified by comparison group and the results are depicted in Figure 2. Five studies included comparisons of MBT with active treatment (I2 = 45.2%, P = 0.12), ten with sham treatment (I2 = 0.0%, P = 0.55), and eight with no-treatment controls (I2 = 60.4%, P < 0.05). A small, favorable effect on increasing pressure pain thresholds was observed when MBT was compared with sham treatments (Hedges g = 0.268 [95% CI 0.078–0.457], P < 0.05) and a small-to-moderate, favorable effect when compared with no-treatment controls (Hedges g = 0.471 [95% CI 0.113–0.830], P < 0.05). No favorable effect was observed when MBT was compared with other active interventions (Hedges g = 0.036 [95% CI −0.289–0.362], P = 0.83). Twelve of the 17 studies were stratified based on sample population and the results are depicted in Figure 3. Five studies were removed because they included individuals with induced pain (eg, delayed-onset muscle soreness) or nonclinical trigger points. Ten studies examined the effect of MBT on pressure pain threshold responses in clinical participants (I2 = 66.0%, P < 0.05), while five studies examined these effects in healthy participants (I2 = 0.0%, P = 0.41). A small-to-moderate, favorable effect on increasing pressure pain thresholds following MBT was seen in studies with clinical participants (Hedges g = 0.454 [95% CI 0.112–0.796], P < 0.05). A small, favorable effect was seen in studies with healthy participants (Hedges g = 0.205 [95% CI −0.036–0.445], P = 0.10), but was not statistically significant.
Figure 3.
Forest plot depicting subgroup effect of MBT based on type of sample population.
Notes: Open diamond depicts overall effect size across all studies included in this subgroup analysis. Colored diamonds depict subgroup effect size based on type of comparison group. For example, top colored diamond is effect size for MBT studies with clinical samples. The width of diamond corresponds to its 95% CI (listed in figure). Individual study effect sizes (and 95% CI) are depicted with boxes (whiskers). Box and whisker size and thickness for each individual study illustrate the weighted contribution of that study to the overall effect size (eg, larger boxes/thicker lines contribute more to overall effect size). aSham comparison; bcontrol comparison; cactive comparison.
Abbreviations: CI, confidence interval; MBT, muscle-biased therapy.
Discussion
We conducted a comprehensive systematic review of the literature to address the three key questions of whether MBT affects pain sensitivity, whether MBT intensity and/or duration influences pain sensitivity, and whether short-term changes in pain sensitivity are associated with changes in clinical pain over similar time points. Our results suggest that a single session of MBT has a favorable effect on measures of pain sensitivity. Further, the degree of pressure intensity used during treatment seems to influence the effect because a significantly greater proportion of studies reported a favorable effect on pain sensitivity when the intervention induced tolerable amounts of pain during the treatment compared with interventions that did not induce tolerable amounts of pain. Finally, the changes in pain sensitivity reported in the studies were related to changes in clinical pain intensity, thus highlighting the clinical relevance of pain sensitivity.
Prior reviews of the clinical effectiveness of MBT to relieve pain have been inconclusive, and the administration of inadequate treatments may account for the lack of effect in some studies.7,17 One of the issues that may be related to the lack of effect is that optimal treatment parameters for MBT in treatment of musculoskeletal pain conditions are not known. The review by Lewis et al reported variation in duration and dosage parameters of massage interventions and suggested that the dosage may be inadequate.7 The review herein also found variation across the duration of a single MBT session; however, interventions that were excluded in the previous review were included herein, which could account for additional variability. This review expands upon prior work and reports on variation in the ability of some interventions to reduce pain sensitivity, and suggests the perceived intensity of pressure applied during MBT may be an important parameter. Our results confirm that heterogeneity exists across MBT interventions, and there is a need to establish optimal MBT parameters. Further, our results suggest that pain sensitivity may be a viable intermediate physiologic endpoint in determining adequate treatment protocols.
The findings of this review have implications for future mechanistic MBT research. The gate control theory of pain is often implicated as the neurophysiologic mechanism underlying symptomatic pain relief.12 Through activation of non-noxious afferents, ongoing nociceptive transmission is inhibited.7 Our finding that inducing a tolerable amount of pain during a treatment session may preferentially affect pain sensitivity suggests that additional mechanisms may be involved, such as conditioned pain modulation, also known as diffuse noxious inhibitory control. This lends further support for a recent model of the mechanisms of all manual therapies, including muscle, joint, and nerve biased techniques, that suggests changes in pain are related to an interaction of neurophysiologic responses in the peripheral and central nervous system that may involve several analgesic pathways.26
This review has clinical implications for future studies. First, these findings support that short-term reductions in pain sensitivity are a potential mechanism underlying short-term clinical benefit. Increased pain sensitivity is associated with a number of clinical pain conditions and is offered as a mechanistic explanation of pain persistence and a therapeutic target.89 Thus, MBT is a viable treatment option to reduce pain sensitivity. Future investigations will need to explore the link between short-term changes and long-term clinical improvement. Second, clinical pain is multidimensional (ie, affective-motivational, sensory-discriminative) and evidence suggests different dimensions may reflect diverse underlying mechanisms.90–92 The evidence also suggests that treatments may preferentially improve some dimensions over others.21,93,94 Reductions in measures of pain sensitivity are associated with improvements in movement-related pain to a greater extent than improvements in spontaneous pain.21 The primary outcome in many MBT clinical trials is commonly single-dimension measures (eg, visual analog scale scores, numerical rating scale scores, and verbal rating scale scores) of pain intensity, potentially making assessment of treatment effects for MBT challenging. Future clinical studies of the effectiveness of MBT may consider assessing movement-related and spontaneous pain separately, in addition to recommended single-dimension measures.
Limitations
There are several limitations to this review. We did not account for the type of practitioner applying the intervention, the years of experience of the practitioner delivering the intervention, the duration of pain in the clinical populations (ie, acute versus chronic), differences across the MBT treatments in their intended aim (short-term pain relief versus long-term improvement in condition), and changes in other physiologic measures. Thus, caution is recommended when applying the results of this review to clinical practice and for developing optimal therapeutic MBT protocols. Only one of several proposed mechanisms underlying clinical improvement was included, ie, pain sensitivity. Other systematic reviews have reported that MBT decreases psychologic measures of anxiety and tension, promotes feelings of well-being and relaxation, and induces changes in physiologic indicators of relaxation, such as heart rate and blood pressure.95,96 Interactions between the patient’s perceived intensity of MBT and other intermediate physiologic endpoints are not known. Additional consideration should be given to the patient’s perceptions regarding the effectiveness of MBT (ie, beliefs) and the patient’s preference for using amounts of perceived pressure that may induce levels of pain. Future research into developing optimal MBT protocols should consider several potential physiologic intermediate endpoints simultaneously, treatment expectations, and patient preference.
While relatively few studies had a high risk of bias for any one domain, all of the studies presented herein had unclear performance bias, which raises some doubt about the results being only attributable to exposure to the intervention and not other factors. Adequate blinding of participants, practitioners, and outcome assessors is difficult for all studies, and the increased difficulty has been well documented for many complementary and alternative medicine approaches, especially manipulative and body-based therapies.97 Nonetheless, performance bias is likely to be introduced when blinding is not adequate. Further, the inclusion of a “sham” treatment arm in a study does not alone adequately control for potential systematic differences between groups that could account for the results. Post-randomization checks should be included and reported for such trials. The simple questions of “what do you expect from this treatment?” and “which group do you believe to belong to?” have been offered as additional ways to help maintain internal validity.98 An additional limitation is the lack of consistent reporting of pain sensitivity outcome measures and descriptions of the MBT intervention. When baseline and post-treatment means and standard deviations are not reported by authors, it is difficult to conduct meta-analyses. The duration of MBT interventions are generally well described. However, the intensity of treatment and whether subjects experienced pain during the treatment is not easily discernible. For example, using only descriptive terms such as deep and/or light pressure requires assumptions to be made regarding how the subjects perceived the pressure. An alternative approach would be to standardize the amount of pressure and pain to a general amount that is experienced by the subjects during treatment.
Without a post-randomization or post-treatment measurement of whether or not the patients believed they received the active or inactive treatment, or if they felt they were receiving a treatment that might benefit them (ie, expectation), it is difficult to assess if differences existed between groups. Even for studies that attempted to blind the patients where “sham” techniques were applied, no measure of effectiveness of the blinding process was reported; thus a true “sham” or placebo treatment cannot be assumed. Previous research has shown that patient expectations affect pain sensitivity, which was the primary outcome measure of this review. Therefore patient expectations represent a relevant risk of bias. Further differences in expectation have been shown to be better predictors of short-term and long-term clinical improvement than group assignment to either genuine or “sham” acupuncture.99 Moreover, manipulations of expectation have shown the ability to enhance and diminish the analgesic effects of a potent synthetic μ-opioid agonist and forms of manual therapy.100,101 Unfortunately, assessing for potential contributions of group assignment based on positive or negative expectations is not routinely done in this area of research and contributes to plausible biases that raise concern over the internal validity of results.
Conclusion
MBT demonstrates the ability to modulate pain sensitivity in a fairly consistent and favorable manner. It appears the intensity at which the MBT is delivered during the session but not the duration, which may preferentially affect the magnitude of reduction, and further research is needed to validate these findings. The evidence herein supports the use of pain sensitivity measures in future research as an intermediate physiologic marker to help elucidate optimal therapeutic parameters.
Acknowledgments
This manuscript was written while CWG received support from the University of Florida Alumni Fellowship and the National Chiropractic Mutual Insurance Company Foundation. MJA and RAC acknowledge support from a National Institutes of Health T32 Interdisciplinary Training in Rehabilitation and Neuromuscular Plasticity grant (5T32 HD043730). MJA also acknowledges support from The Foundation for Physical Therapy (Promotion of Doctoral Studies I scholarship). MDB and MEH received support from the National Center of Complementary and Alternative Medicine (R01AT006334).
Footnotes
Disclosure
The authors of this manuscript attest that there are no conflicts of interest to report in this work.
References
- 1.Lund I. Massage as a pain relieving method. Physiotherapy. 2000;86:638–639. [Google Scholar]
- 2.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 3.Bercovitz A, Sengupta M, Jones A, Harris-Kojetin L. Complementary and Alternative Therapies in Hospice: The National Home and Hospice Care Survey: United States, 2007. Hyattsville, MD: National Center for Health Statistics; [Accessed November 13, 2012]. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr033.pdf. [PubMed] [Google Scholar]
- 4.Kanodia AK, Legedza AT, Davis RB, Eisenberg DM, Phillips RS. Perceived benefit of complementary and alternative medicine (CAM) for back pain: a national survey. J Am Board Fam Med. 2010;23:354–362. doi: 10.3122/jabfm.2010.03.080252. [DOI] [PubMed] [Google Scholar]
- 5.Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1–14. [PubMed] [Google Scholar]
- 6.Frass M, Strassl RP, Friehs H, Mullner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12:45–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis M, Johnson MI. The clinical effectiveness of therapeutic massage for musculoskeletal pain: a systematic review. Physiotherapy. 2006;92:146–158. [Google Scholar]
- 8.Ernst E. Manual therapies for pain control: chiropractic and massage. Clin J Pain. 2004;20:8–12. doi: 10.1097/00002508-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cuadrado ML, Miangolarra JC, Barriga FJ, Pareja JA. Are manual therapies effective in reducing pain from tension-type headache? a systematic review. Clin J Pain. 2006;22:278–285. doi: 10.1097/01.ajp.0000173017.64741.86. [DOI] [PubMed] [Google Scholar]
- 10.Ezzo J, Haraldsson BG, Gross AR, et al. Massage for mechanical neck disorders: a systematic review. Spine. 2007;32:353–362. doi: 10.1097/01.brs.0000254099.07294.21. [DOI] [PubMed] [Google Scholar]
- 11.Ho CY, Sole G, Munn J. The effectiveness of manual therapy in the management of musculoskeletal disorders of the shoulder: a systematic review. Man Ther. 2009;14:463–474. doi: 10.1016/j.math.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Furlan AD, Imamura M, Dryden T, Irvin E. Massage for low back pain: an updated systematic review within the framework of the Cochrane Back Review Group. Spine. 2009;34:1669–1684. doi: 10.1097/BRS.0b013e3181ad7bd6. [DOI] [PubMed] [Google Scholar]
- 13.Ernst E. Massage therapy for low back pain: a systematic review. J Pain Symptom Manage. 1999;17:65–69. doi: 10.1016/s0885-3924(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 14.Furlan AD, Brosseau L, Imamura M, Irvin E. Massage for low-back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2002;27:1896–1910. doi: 10.1097/00007632-200209010-00017. [DOI] [PubMed] [Google Scholar]
- 15.Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol. 2006;24:5457–5464. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- 16.Ernst E. Does post-exercise massage treatment reduce delayed onset muscle soreness? A systematic review. Br J Sports Med. 1998;32:212–214. doi: 10.1136/bjsm.32.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis M, Johnson MI. Response to Drs Brown, Hay-Smith, Dean and Taylor regarding “The clinical effectiveness of therapeutic massage for musculoskeletal pain: a systematic review”. Physiotherapy. 2007;93:79–80. [Google Scholar]
- 18.Brown M, Hay-Smith J, Dean S, Taylor W. Comments on article by Lewis and Johnson: “The clinical effectiveness of therapeutic massage for musculoskeletal pain: a systematic review”. Physiotherapy. 2007;93:78–79. [Google Scholar]
- 19.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003;4:109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 20.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 22.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 23.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Khalsa PS, Eberhart A, Cotler A, Nahin R. The 2005 conference on the biology of manual therapies. J Manipulative Physiol Ther. 2006;29:341–346. doi: 10.1016/j.jmpt.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22:752–767. doi: 10.1016/j.jelekin.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blikstad A, Gemmell H. Immediate effect of activator trigger point therapy and myofascial band therapy on non-specific neck pain in patients with upper trapezius trigger points compared to sham ultrasound: a randomised controlled trial. Clin Chiropr. 2008;11:23–29. [Google Scholar]
- 29.Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9:714–721. doi: 10.1016/j.jpain.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated Sep 2009] The Cochrane Collaboration. 2009. [Accessed October 27, 2010]. Available from: http://www.cochrane-handbook.org.
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 32.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed. Upper Saddle River, NJ: Pearson/Prentice Hall; 2009. [Google Scholar]
- 33.Borenstein M. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2009. [Google Scholar]
- 34.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988. [Google Scholar]
- 35.Robb A, Pajaczkowski J. Immediate effect on pain thresholds using active release technique on adductor strains: pilot study. J Bodyw Mov Ther. 2011;15:57–62. doi: 10.1016/j.jbmt.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Burke J, Buchberger DJ, Carey-Loghmani MT, Dougherty PE, Greco DS, Dishman JD. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. J Manipulative Physiol Ther. 2007;30:50–61. doi: 10.1016/j.jmpt.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 37.De Laat A, Stappaerts K, Papy S. Counseling and physical therapy as treatment for myofascial pain of the masticatory system. J Orofac Pain. 2003;17:42–49. [PubMed] [Google Scholar]
- 38.Ekici G, Bakar Y, Akbayrak T, Yuksel I. Comparison of manual lymph drainage therapy and connective tissue massage in women with fibromyalgia: a randomized controlled trial. J Manipulative Physiol Ther. 2009;32:127–133. doi: 10.1016/j.jmpt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Ghanbari A, Rahimijaberi A, Mohamadi M, Abbasi L, Sarvestani FK. The effect of trigger point management by positional release therapy on tension type headache. Neuro Rehabilitation. 2012;30:333–339. doi: 10.3233/NRE-2012-0764. [DOI] [PubMed] [Google Scholar]
- 40.Godfrey CM, Morgan PP, Schatzker J. A randomized trial of manipulation for low-back pain in a medical setting. Spine. 1984;9:301–304. doi: 10.1097/00007632-198404000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Hasson D, Arnetz B, Jelveus L, Edelstam B. A randomized clinical trial of the treatment effects of massage compared to relaxation tape recordings on diffuse long-term pain. Psychother Psychosom. 2004;73:17–24. doi: 10.1159/000074436. [DOI] [PubMed] [Google Scholar]
- 42.Ibáñez-García J, Alburquerque-Sendín F, Rodríguez-Blanco C, et al. Changes in masseter muscle trigger points following strain-counterstrain or neuro-muscular technique. J Bodyw Mov Ther. 2009;13:2–10. doi: 10.1016/j.jbmt.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001;322:1574–1578. doi: 10.1136/bmj.322.7302.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jönhagen S, Ackermann P, Eriksson T, Saartok T, Renström PAF. Sports massage after eccentric exercise. Am J Sports Med. 2004;32:1499–1503. doi: 10.1177/0363546503262196. [DOI] [PubMed] [Google Scholar]
- 45.Kumnerddee W. Effectiveness comparison between Thai traditional massage and Chinese acupuncture for myofascial back pain in Thai military personnel: a preliminary report. J Med Assoc Thai. 2009;92( Suppl 1):S117–S123. [PubMed] [Google Scholar]
- 46.Senbursa G, Baltaci G, Atay A. Comparison of conservative treatment with and without manual physical therapy for patients with shoulder impingement syndrome: a prospective, randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:915–921. doi: 10.1007/s00167-007-0288-x. [DOI] [PubMed] [Google Scholar]
- 47.von Piekartz H, Lüdtke K. Effect of treatment of temporomandibular disorders (TMD) in patients with cervicogenic headache: a single-blind, randomized controlled study. Cranio. 2011;29:43–56. doi: 10.1179/crn.2011.008. [DOI] [PubMed] [Google Scholar]
- 48.Craane B, Dijkstra PU, Stappaerts K, De Laat A. Randomized controlled trial on physical therapy for TMJ closed lock. J Dent Res. 2012;91:364–369. doi: 10.1177/0022034512438275. [DOI] [PubMed] [Google Scholar]
- 49.Anaya-Terroba L, Arroyo-Morales M, Fernandez-de-Las-Penas C, Diaz-Rodriguez L, Cleland JA. Effects of ice massage on pressure pain thresholds and electromyography activity postexercise: a randomized controlled crossover study. J Manipulative Physiol Ther. 2010;33:212–219. doi: 10.1016/j.jmpt.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Lao C, Cantarero-Villanueva I, Fernandez-de-Las-Penas C, del Moral-Avila R, Castro-Sanchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28:113–121. doi: 10.1097/AJP.0b013e318225dc02. [DOI] [PubMed] [Google Scholar]
- 51.Gulick DT, Kimura IF, Sitler M, Paolone A, Kelly JI. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31:145–152. [PMC free article] [PubMed] [Google Scholar]
- 52.Howatson G, Van Someren KA. Ice massage: effects on exercise-induced muscle damage. J Sports Med Phys Fitness. 2003;43:500–505. [PubMed] [Google Scholar]
- 53.Collins N, Teys P, Vicenzino B. The initial effects of a Mulligan’s mobilization with movement technique on dorsiflexion and pain in subacute ankle sprains. Man Ther. 2004;9:77–82. doi: 10.1016/S1356-689X(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 54.Farasyn A, Meeusen R. Pressure pain thresholds in healthy subjects: influence of physical activity, history of lower back pain factors and the use of endermology as a placebo-like treatment. J Bodyw Mov Ther. 2003;7:53–61. [Google Scholar]
- 55.Lucas KR, Polus BI, Rich PA. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8:160–166. [Google Scholar]
- 56.Slater H, Arendt-Nielsen L, Wright A, Graven-Nielsen T. Effects of a manual therapy technique in experimental lateral epicondylalgia. Man Ther. 2006;11:107–117. doi: 10.1016/j.math.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6:205–212. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- 58.Albertin A, Kerppers II, Amorim CF, Costa RV, Ferrari Correa JC, Oliveira CS. The effect of manual therapy on masseter muscle pain and spasm. Electromyogr Clin Neurophysiol. 2010;50:107–112. [PubMed] [Google Scholar]
- 59.Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4:119ra113. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- 60.Hart JM, Swanik CB, Tierney RT. Effects of sport massage on limb girth and discomfort associated with eccentric exercise. J Athl Train. 2005;40:181–185. [PMC free article] [PubMed] [Google Scholar]
- 61.Kimber L, McNabb M, McCourt C, Haines A, Brocklehurst P. Massage or music for pain relief in labour: a pilot randomised placebo controlled trial. Eur J Pain. 2008;12:961–969. doi: 10.1016/j.ejpain.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Lightfoot JT, Char D, McDermott J, Goya C. Immediate postexercise massage does not attenuate delayed onset muscle soreness. J Athl Train. 1997;11:119–124. [Google Scholar]
- 63.Micklewright D. The effect of soft tissue release on delayed onset muscle soreness: a pilot study. Phys Ther Sport. 2009;10:19–24. doi: 10.1016/j.ptsp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Smith LL, Keating MN, Holbert D, et al. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preliminary report. J Orthop Sports Phys Ther. 1994;19:93–99. doi: 10.2519/jospt.1994.19.2.93. [DOI] [PubMed] [Google Scholar]
- 65.Taylor AG, Galper DI, Taylor P, et al. Effects of adjunctive Swedish massage and vibration therapy on short-term postoperative outcomes: a randomized, controlled trial. J Altern Complement Med. 2003;9:77–89. doi: 10.1089/107555303321222964. [DOI] [PubMed] [Google Scholar]
- 66.Weber MD, Servedio FJ, Woodall WR. The effects of three modalities on delayed onset muscle soreness. J Orthop Sports Phys Ther. 1994;20:236–242. doi: 10.2519/jospt.1994.20.5.236. [DOI] [PubMed] [Google Scholar]
- 67.Zainuddin Z, Newton M, Sacco P, Nosaka K. Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J Athl Train. 2005;40:174–180. [PMC free article] [PubMed] [Google Scholar]
- 68.Aparicio EQ, Quirante LB, Blanco CR, Sendín FA. Immediate effects of the suboccipital muscle inhibition technique in subjects with short hamstring syndrome. J Manipulative Physiol Ther. 2009;32:262–269. doi: 10.1016/j.jmpt.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Arroyo-Morales M, Olea N, Martínez MM, Hidalgo-Lozano A, Ruiz-Rodríguez C, Díaz-Rodríguez L. Psychophysiological effects of massage-myofascial release after exercise: a randomized sham-control study. J Altern Complement Med. 2008;14:1223–1229. doi: 10.1089/acm.2008.0253. [DOI] [PubMed] [Google Scholar]
- 70.Buttagat V, Eungpinichpong W, Chatchawan U, Arayawichanon P. Therapeutic effects of traditional Thai massage on pain, muscle tension and anxiety in patients with scapulocostal syndrome: a randomized single-blinded pilot study. J Bodyw Mov Ther. 2012;16:57–63. doi: 10.1016/j.jbmt.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Buttagat V, Eungpinichpong W, Chatchawan U, Kharmwan S. The immediate effects of traditional Thai massage on heart rate variability and stress-related parameters in patients with back pain associated with myofascial trigger points. J Bodyw Mov Ther. 2011;15:15–23. doi: 10.1016/j.jbmt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Chatchawan U, Thinkhamrop B, Kharmwan S, Knowles J, Eungpinichpong W. Effectiveness of traditional Thai massage versus Swedish massage among patients with back pain associated with myofascial trigger points. J Bodyw Mov Ther. 2005;9:298–309. [Google Scholar]
- 73.Farasyn A, Meeusen R. Effect of roptrotherapy on pressure-pain thresholds in patients with subacute nonspecific low back pain. J Musculoskelet Pain. 2007;15:41–53. [Google Scholar]
- 74.Fernández-de-las-Peñas C, Alonso-Blanco C, Fernández-Carnero J, Miangolarra-Page JC. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. J Bodyw Mov Ther. 2006;10:3–9. [Google Scholar]
- 75.Fernandez-Lao C, Cantarero-Villanueva I, Diaz-Rodriguez L, Fernandez-de-las-Penas C, Sanchez-Salado C, Arroyo-Morales M. The influence of patient attitude toward massage on pressure pain sensitivity and immune system after application of myofascial release in breast cancer survivors: a randomized, controlled crossover study. J Manipulative Physiol Ther. 2012;35:94–100. doi: 10.1016/j.jmpt.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Fryer G, Hodgson L. The effect of manual pressure release on myofascial trigger points in the upper trapezius muscle. J Bodyw Mov Ther. 2005;9:248–255. [Google Scholar]
- 77.Gemmell H, Allen A. Relative immediate effect of ischaemic compression and activator trigger point therapy on active upper trapezius trigger points: a randomised trial. Clin Chiropract. 2008;11:175–181. [Google Scholar]
- 78.Mancinelli CA, Davis DS, Aboulhosn L, Brady M, Eisenhofer J, Foutty S. The effects of massage on delayed onset muscle soreness and physical performance in female collegiate athletes. Phys Ther Sport. 2006;7:5–13. [Google Scholar]
- 79.Saiz-Llamosas JR, Fernandez-Perez AM, Fajardo-Rodriguez MF, Pilat A, Valenza-Demet G, Fernandez-de-Las-Penas C. Changes in neck mobility and pressure pain threshold levels following a cervical myofascial induction technique in pain-free healthy subjects. J Manipulative Physiol Ther. 2009;32:352–357. doi: 10.1016/j.jmpt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Toro-Velasco C, Arroyo-Morales M, Fernández-de-las-Peñas C, Cleland JA, Barrero-Hernández FJ. Short-term effects of manual therapy on heart rate variability, mood state, and pressure pain sensitivity in patients with chronic tension-type headache: a pilot study. J Manipulative Physiol Ther. 2009;32:527–535. doi: 10.1016/j.jmpt.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Aguilera FJM, Martín DP, Masanet RA, Botella AC, Soler LB, Morell FB. Immediate effect of ultrasound and ischemic compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: a randomized controlled study. J Manipulative Physiol Ther. 2009;32:515–520. doi: 10.1016/j.jmpt.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Farasyn A, Meeusen R, Nijs J. A pilot randomized placebo-controlled trial of roptrotherapy in patients with subacute non-specific low back pain. J Back Musculoskelet. 2006;19:111–117. [Google Scholar]
- 83.Gemmell H, Miller P, Nordstrom H. Immediate effect of ischaemic compression and trigger point pressure release on neck pain and upper trapezius trigger points: a randomised controlled trial. Clin Chiropract. 2008;11:30–36. [Google Scholar]
- 84.Hamilton L, Boswell C, Fryer G. The effects of high-velocity, low-amplitude manipulation and muscle energy technique on suboccipital tenderness. International Journal of Osteopathic Medicine. 2007;10:42–49. [Google Scholar]
- 85.Lewis C, Khan A, Souvlis T, Sterling M. A randomised controlled study examining the short-term effects of strain-counterstrain treatment on quantitative sensory measures at digitally tender points in the low back. Man Ther. 2010;15:536–541. doi: 10.1016/j.math.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 86.Meseguer AA, Fernández-de-las-Peñas C, Navarro-Poza JL, Rodríguez-Blanco C, Gandia JJB. Immediate effects of the strain/counterstrain technique in local pain evoked by tender points in the upper trapezius muscle. Clin Chiropract. 2006;9:112–118. [Google Scholar]
- 87.Oliveira-Campelo NM, Rubens-Rebelatto J, Marti NVFJ, Alburquerque-Sendi NF, Fernandez-de-Las-Penas C. The immediate effects of atlanto-occipital joint manipulation and suboccipital muscle inhibition technique on active mouth opening and pressure pain sensitivity over latent myofascial trigger points in the masticatory muscles. J Orthop Sports Phys Ther. 2010;40:310–317. doi: 10.2519/jospt.2010.3257. [DOI] [PubMed] [Google Scholar]
- 88.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil. 2002;83:1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 89.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(Suppl 3):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trost Z. All pain is not created equal: differentiating between pain during movement versus pain at rest following total knee arthroplasty. Pain. 2012;153:2161–2162. doi: 10.1016/j.pain.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 91.Rakel BA, Blodgett NP, Bridget Zimmerman M, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain. 2012;153:2192–2203. doi: 10.1016/j.pain.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treede R-D, Kenshalo DR, Gracely RH, Jones AKP. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 93.Arendt-Nielsen L, Mansikka H, Staahl C, et al. A translational study of the effects of ketamine and pregabalin on temporal summation of experimental pain. Reg Anesth Pain Med. 2011;36:585–591. doi: 10.1097/AAP.0b013e31822b0db0. [DOI] [PubMed] [Google Scholar]
- 94.Gilron I, Tu D, Holden RR. Sensory and affective pain descriptors respond differentially to pharmacological interventions in neuropathic conditions. Clin J Pain. doi: 10.1097/AJP.0b013e31824ce65c. Epub 2012 Jun 28. [DOI] [PubMed] [Google Scholar]
- 95.Richards KC, Gibson R, Overton-McCoy AL. Effects of massage in acute and critical care. AACN Clin Issues. 2000;11:77–96. doi: 10.1097/00044067-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 96.Harris M, Richards KC. The physiological and psychological effects of slow-stroke back massage and hand massage on relaxation in older people. J Clin Nurs. 2010;19:917–926. doi: 10.1111/j.1365-2702.2009.03165.x. [DOI] [PubMed] [Google Scholar]
- 97.Miller FG, Emanuel EJ, Rosenstein DL, Straus SE. Ethical issues concerning research in complementary and alternative medicine. JAMA. 2004;291:599–604. doi: 10.1001/jama.291.5.599. [DOI] [PubMed] [Google Scholar]
- 98.Benedetti F. What do you expect from this treatment? Changing our mind about clinical trials. Pain. 2007;128:193–194. doi: 10.1016/j.pain.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128:264–271. doi: 10.1016/j.pain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 101.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]