Abstract
Surgically induced brain injury (SBI) is a common concern after a neurosurgical procedure. Current treatments aimed at reducing the postoperative sequela are limited. Granulocyte-colony stimulating factor (G-CSF), a hematopoietic growth factor involved in the inflammatory process, has been shown in various animal models to be neuroprotective. Consequently, in this study, we investigated the use of G-CSF as a treatment modality to reduce cell death and brain edema, while improving neurobehavioral deficits following an SBI in mice. Eleven-week-old C57 black mice (n = 76) were randomly placed into four groups: sham (n = 19), SBI (n = 21), SBI with G-CSF pre-treatment (n = 15) and SBI with G-CSF pre/post-treatment (n = 21). Treated groups received a single dose of G-CSF intraperitoneally at 24, 12 and 1 h pre-surgery and/or 6 and 12 h post-surgery. Postoperative assessment occurred at 24 h and included neurobehavioral testing and measurement for both cell death and brain edema. Results indicated that pre-treatment with G-CSF reduced both cell death and brain edema, while post-treatment reduced neurobehavioral deficits. This study implies that the morphological changes in the brain are effected by pre-treatment; however, in order to activate and/ or amplify targets involved in the recovery process, more dosing regimens may be needed.
Keywords: Granulocyte colony-stimulating factor (G-CSF), Surgically induced brain injury (SBI), Neurosurgery
Introduction
Surgically induced brain injury (SBI) refers to the wide range of unintentional injuries that take place during routine neurosurgical procedures. These injuries occur more commonly along the edge of tissue resection because of direct surgical trauma, electrocautery burn, stretch damage from tissue retraction and intraoperative bleeding [1, 2]. The concern with SBI is the heightened inflammatory response that mounts in an attempt to orchestrate the effects of tissue damage. Side effects of this response include disruption of blood-brain-barrier (BBB) integrity, apoptotic cell death and subsequent neurobehavioral deterioration. Unfortunately, current therapies aimed at battling the effects of SBI are limited. Consequently, discovering direct anti-inflammatory therapies that could potentially decrease brain edema and cell death while improving neurobehavioral function would be of great benefit.
Granulocyte colony-stimulating factor (G-CSF) is a hematopoietic growth factor, cytokine, and glycoprotein responsible for the proliferation, survival, and maturation of neutrophil precursors [3]. It stimulates the bone marrow to produce and release neutrophilic precursors as well as hematopoietic stem cells (HSC) into the circulation. It has been discovered that G-CSF plays a key role in the central nervous system (CNS), where it provides a level of neuroprotection [3]. The underlying mechanism involved in the G-CSF induced protection is unknown; however, in various stroke models G-CSF has been shown to exert is neuroprotective actions through the inhibition of apoptosis, inflammation and the stimulation of both neurogenesis and angiogenesis [4].
Accordingly, in this study, we investigated the effects of G-CSF pre- and post-treatment on cerebral edema, neuronal cell death and neurobehavioral functioning in SBI mice. Two dosing regimens were used to evaluate these outcomes.
Materials and Methods
Animal Groups
All procedures for this study were approved by the Animal Care and Use Committee at Loma Linda University and complied with the NIH Guide for the Care and Use of Laboratory Animals. Seventy-six Sprague-Dawley mice weighing between 35 and 45 g were randomly divided into the following four groups: sham surgery (n = 19), SBI (n = 21), SBI with G-CSF pre-treatment (n = 15) and SBI with G-CSF pre-/post-treatment (n = 21).
Operative Procedure
The SBI model was adapted as previously described in mice [5]. Briefly, mice were anesthetized with a ketamine (100 mg/ kg)/xylazine (10 mg/kg) combination intraperitoneal injection and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL). A 3 mm by 3 mm cranial window was created 1 mm anterior and 2 mm lateral to the coronal and sagittal sutures, respectively. Using a flat blade (6 mm × 1.5 mm), two incisions were made along the sagittal and coronal planes leading away from the bregma and extending to the edge of the craniotomy window. The sectioned brains were weighed and were not significantly different between animals. Electrocautery was utilized for 2 s along the medial coronal and posterior sagittal borders at a power level consistent with the coagulation setting used in the operating room. Sham surgery included only a craniotomy window and replacement of the bone flap without any dural incisions.
Treatment Method
A 100 ug/kg G-CSF (Neupogen, Amgen, Thousand Oaks, CA) intraperitoneal injection was given at 24, 12 and 1 h pre-surgery in the pre-treatment group, while the post-treatment group received an additional treatment at 6 and 12 h post-surgery. Normal saline injections in similar volumes were given to the sham and vehicle groups on the same dosing schedule as G-CSF.
Assessment of Neurobehavioral Deficits
Prior to sacrificing at 24 h, neurological outcomes were assessed by a blind observer using the Modified Garcia Score [6], beam balance test and modified wire hanging test [7].
The Modified Garcia Score is a 21-point sensorimotor assessment system consisting of seven tests with scores of 0–3 for each test (maximum score = 21). These seven tests included: (1) spontaneous activity, (2) side stroking, (3) vibrios touch, (4) limb symmetry, (5) climbing, (6) lateral turning and (7) forelimb walking.
Additionally, beam balance and wire hanging testing were performed. Both the beam (590 cm in length by 51 cm in width) and wire (550 cm in length by 51 mm in width) were constructed and held in place by two platforms on each side. Mice were observed for both their time and behavior until they reached one platform and scored according to six grades. The test was repeated three times, and an average score was taken [minimum score 0; maximum score (healthy rat) 5].
Brain Water Content
Brain water content was measured as previously described [8]. Briefly, mice were killed 24 h post-SBI, and the brains were immediately removed and divided into three parts: ipsilateral frontal, contralateral frontal and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were then weighed on an electronic analytical balance (APX-60, Denver Instrument; Arvada, CO) to the nearest 0.1 mg to obtain the wet weight (WW). The tissue was then dried at 105°C for 48 h to determine the dry weight (DW). The percent brain water content was calculated as [(WW – DW)/ WW] × 100.
Assessing Cell Death
Cell Death Detection ELISA kit (Roche Applied Science) was used to quantify cell death in the ipsilateral frontal cortex 24 h after SBI. For quantification of DNA fragmentation, which indicates apoptotic cell death, we used a commercial enzyme immunoassay to determine cytoplasmic histone-associated DNA fragments (Roche Molecular Biochemicals).
Statistical Analysis
Quantitative data were expressed as the mean ± SEM. One-way ANOVA and Tukey test were used to determine significance in differences between the means. Neurological scores were evaluated using the Dunn method. A p-value < 0.05 was considered statistically significant.
Results
G-CSF Pre-Treatment Reduces Brain Edema
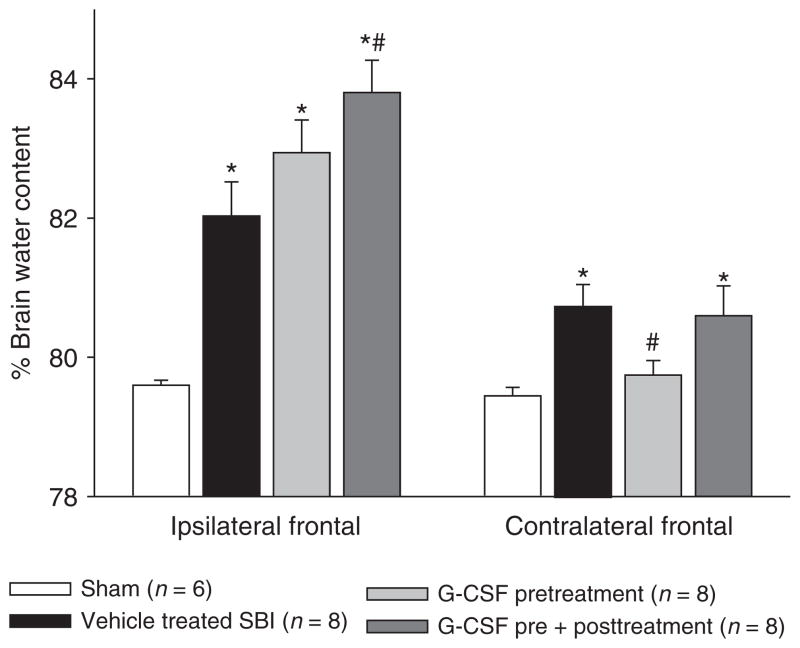
Brain water content was measured 24 h post-SBI (Fig. 1). The results showed that vehicle mice presented with significantly worse brain edema compared to sham mice. After pre-treatment with G-CSF, brain edema reduced significantly in the contralateral frontal cortex compared to vehicle group. The ipsilateral cortex increased in brain edema, which is attributed to the direct BBB disruption from frontal lobe removal.
Fig. 1.
Brain water content. The results showed that vehicle mice presented with significantly worse brain edema compared to sham mice. After pre-treatment with G-CSF, brain edema reduced significantly in the contralateral frontal cortex compared to vehicle group. The ipsilateral cortex increased in brain edema, which is attributed to the direct BBB disruption from frontal lobe removal. *Significant difference vs. sham (p < 0.05)
G-CSF Pre-Treatment Reduces Cell Death
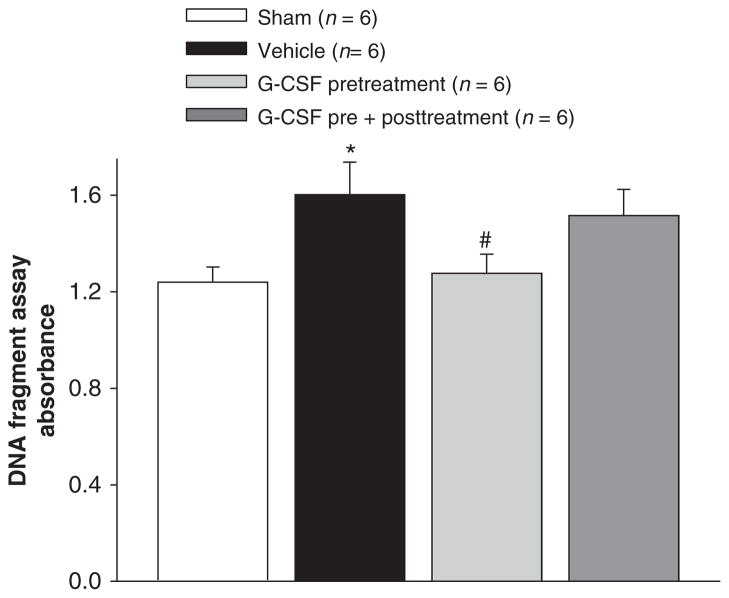
Cell death was also measured via an absorbance assay at 24 h post-SBI (Fig. 2). The results showed that vehicle mice presented with a significant increase in the number of cell deaths compared to sham mice. After pre-treatment with G-CSF, the number of cell deaths reduced significantly.
Fig. 2.
Cell death measurement 24 h post SBI. The results showed that vehicle mice presented with a significant increase in the number of cell deaths compared to sham mice. After pre-treatment with G-CSF, the number of cell deaths reduced significantly. *Significant difference vs. sham (p < 0.05); #significant difference vs. vehicle (p < 0.05)
Post-Treatment with G-CSF Improves Neurobehavioral Deficits
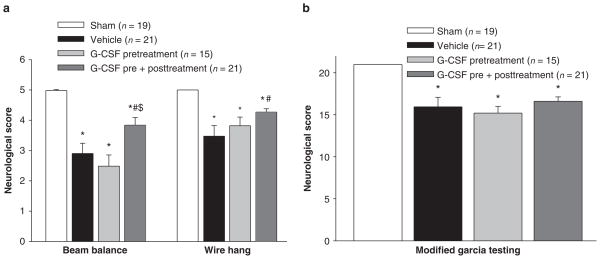
To evaluate the sensorimotor deficits after SBI, the modified Garcia test, wire hanging test and beam balance test were conducted 24 h post-SBI. The results showed that vehicle mice presented with severe neurobehavioral deficits compared to sham mice. After both pre- and post-treatment with G-CSF, a significant improvement in neurobehavioral function was seen with the beam balance and wire hanging neurological tests (Fig. 3a); there was no improvement with the modified Garcia test (Fig. 3b).
Fig. 3.
Neurobehavioral deficits. The results showed that vehicle mice presented with severe neurobehavioral deficits compared to sham mice. After both pre- and post-treatment with G-CSF, a significant improvement in neurobehavioral function was seen with the beam balance and wire hanging neurological tests (a); there was no improvement with the modified Garcia test (b). *Significant difference vs. sham (p < 0.05); #significant difference vs. vehicle (p < 0.05)
Discussion
The aim of this study was to determine the effects of G-CSF treatment after SBI. We showed for the first time that pre-treatment with G-CSF could reduce cell death and attenuate brain edema following a SBI in mice. Additionally, at 24 h post-injury, we observed a marked improvement in neurobehavioral deficits after pre- and post-treatment with G-CSF. Consequently, this study suggests that the morphologic changes in the brain are effected by G-CSF pre-treatment; however, in order to activate and/or amplify targets involved in the plasticity process and improve neurobehavioral functioning, more dosing regimens may be needed after injury.
Cell death following a SBI is not an uncommon phenomenon. Previous works in SBI have shown massive neuronal degeneration and death in the area of injury [9], specifically, finding increased expression of pro-apoptotic BAX and active caspase-3 in cortical neurons. In particular, studies in transient focal ischemic models in mice found that G-CSF exerted a protective effect by upregulating anti-apoptotic proteins, such as STAT3 and Bcl-2. This was confirmed by Schabitz [10], who also found an elevation of STAT3 expression in the infarction penumbra in ischemic injured rats treated with G-CSF. In our study, we found that the vehicle group had a significant increase in neuronal cell deaths – an effect partially reduced by pre-treatment with G-CSF – thus suggesting that G-CSF may play a role in decreasing neuronal cell death after SBI.
In addition to its effects on neuronal cell death, SBI has been implicated in increasing the formation of brain edema by disrupting the BBB. According to Jadhav [11], infiltration of inflammatory markers, such as matrix metalloproteinases, to the site of injury is responsible for disrupting the BBB and subsequently causing an influx of edema. In a focal ischemic mouse model, G-CSF treatment was shown to decrease the amount of edema accumulated in the penumbral brain tissue [12]. G-CSF has also been found to reduce brain edema and inflammatory cell infiltration in a rat intracerebral hemorrhage model [13]. These studies are in line with our results, which showed a significant reduction in brain edema in the contralateral brain hemisphere, corresponding to the penumbral region. This implies that G-CSF pre-treatment may reduce damage associated with inflammatory infiltration caused by SBI. Although our results did show an increase in brain edema in the ipsilateral frontal cortex, this increase is most likely due to the direct BBB disruption from performing the model. Consequently, the contralateral side was evaluated, investigating the potential of G-CSF to reduce penumbral edema following a SBI.
Although there were morphological improvements with G-CSF pre-treatment, it took additional treatments after the injury to reduce neurobehavioral deficits. In our mouse population, we found a significant improvement with motor and strength testing after both pre- and post-G-CSF treatments compared to vehicle. This was confirmed using the beam balance and wire hanging tests. Previous works have found similar results. In a focal ischemic rat model, G-CSF was found to improve lesion repair and improve neuronal plasticity and vascularization [14], while in a MCAO model, a post-treatment dose of G-CSF improved long-term functional and cognitive outcomes [15].
Conclusion
This study implies that the morphologic changes in the brain are effected by G-CSF pre-treatment; however, in order to activate and/or amplify targets involved in the plasticity process and improve neurobehavioral deficits, more dosing regimens may be needed after the initial injury. Consequently, further studies will be needed to explore the use of G-CSF in the clinical setting of SBI.
Acknowledgments
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Nikan H. Khatibi, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA
Vikram Jadhav, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Mehdi Saidi, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Wanqiu Chen, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Robert Martin, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA.
Gary Stier, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA.
Jiping Tang, Email: jtang@llu.edu, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA 92354, USA.
John H. Zhang, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA and Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda Medical Center, Loma Linda, CA, USA
References
- 1.Andrews RJ, Muto RP. Retraction brain ischaemia: cerebral blood flow, evoked potentials, hypotension and hyperventilation in a new animal model. Neurol Res. 1992;14(1):12–18. doi: 10.1080/01616412.1992.11740004. [DOI] [PubMed] [Google Scholar]
- 2.Hernesniemi J, Leivo S. Management outcome in third ventricular colloid cysts in a defined population: a series of 40 patients treated mainly by transcallosal microsurgery. Surg Neurol. 1996;45(1):2–14. doi: 10.1016/0090-3019(95)00379-7. [DOI] [PubMed] [Google Scholar]
- 3.Schneider A, Hans-George K. A role for G-CSF in the central nervous system. Cell Cycle. 2005;4(12):1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 4.Lu CZ, Xiao G. G-CSF and neuroprotection: a therapeutic perspective in cerebral ischaemia. Biochem Soc Trans. 2006;34:6. doi: 10.1042/BST0341327. [DOI] [PubMed] [Google Scholar]
- 5.Jadhav V, et al. Neuroprotection against surgically induced brain injury. Surg Neurol. 2007;67(1):15–20. doi: 10.1016/j.surneu.2006.07.014. discussion 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia JH, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, et al. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61(5):1067–1075. doi: 10.1227/01.neu.0000303203.07866.18. discussion 1075–1076. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 9.Sulejczak D, Grieb P, Walski M. Apoptotic death of cortical neurons following surgical brain injury. Folia Neuropathol. 2008;46(3):213–219. [PubMed] [Google Scholar]
- 10.Schabitz WR, Kollmar R, Schwaninger M. Neuroprotective effect of G-CSF after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 11.Jadhav V, Yamaguchi M, Obenaus A, Zhang JH. Matrix metalloproteinase inhibition attenuates brain edema after surgical brain injury. Acta Neurochir Suppl. 2008;102:357–361. doi: 10.1007/978-3-211-85578-2_68. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CL, Jones NC, Prior MJ. G-CSF suppresses edema formation and reduces interleukin-1b expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005;64:763–769. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- 13.Park HK, Chu K, Lee ST. Granulocyte colony-stimulating factor induces sensorimotor recovery in intracerebral hemorrhage. Brain Res. 2005;1041:125–131. doi: 10.1016/j.brainres.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 14.Shyu WC, Lin SZ, Yang HI. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25(4):431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]