Abstract
Capsaicin, a transient receptor potential vanilloid 1 (TRPV1) agonist, has recently been shown to provide neuroprotection against brain injury in experimental adult models of cerebral ischemia. Accordingly, in this study, we investigated the way in which capsaicin-mediated TRPV1 modulation could attenuate damage in an experimental hypoxic-ischemic (HI) neonatal brain injury model. The Rice-Vannucci method was used in 10-day-old rat pups by performing unilateral carotid artery ligation followed by 2 h of hypoxia (8% O2 at 37°C). Capsaicin was administered intraperitoneally (0.2 mg/kg or 2.0 mg/kg) at 3 h pre-HI or 1 h post-HI. Post assessment included measurement of infarction volume at 24 and 72 h in addition to an assessment of the vascular dynamics of the middle cerebral artery (MCA) at 6 h post-HI. The results indicated that pre-treatment with capsaicin reduced infarction volume significantly with either low-dose or high-dose treatment. Pre-treatment also improved myogenic tone and decreased apoptotic changes in the distal MCA. We concluded that capsaicin pre-treatment may provide neurovascular protection against neonatal HI.
Keywords: Transient receptor potential vanilloid 1 (TRPV1), Capsaicin, Neonatal hypoxia ischemia
Introduction
Perinatal brain injury can occur because of a wide variety of events, including hypoxia-ischemia (HI), intrauterine infections, and cerebral hemorrhage. Of these three, HI is the most common cause of perinatal brain injury, and is responsible for a large number of morbidities and mortalities [1]. In the past, many studies have been aimed at determining the cause of brain damage in an effort to reduce the major consequences of injury, including cerebral palsy, seizure disorders and motor/cognitive disabilities [2]. To date, various mechanisms including excitotoxicity have been implicated as a key component of brain damage in HI. Unfortunately, the lack of effective therapeutic options to combat excitotoxic cell death and prevent further damage has driven the need for additional research in this field.
Transient receptor potential vanilloid 1 (TRPV1, or capsaicin receptor) is a nonspecific cation channel belonging to the TRP superfamily [3]. It is highly expressed in spinal and peripheral nerve terminals and has an important role in nociception as well as analgesia [3, 4]. In order for TRPV1 to be activated, it must be exposed to various noxious and painful stimuli, such as low pH (<5.9), heat (>43°C), capsaicin and endocannabinoids. In doing so, activated TRPV1 causes an increase in calcium influx, thereby promoting excitotoxic cell death mechanisms in neurons [3]. Recently, experimental adult models have shown that capsaicin administration may provide neuroprotection against excitotoxic and ischemic brain injury by desensitizing the TRPV1 receptor [5].
Accordingly, in the present study we investigated whether modulation of TRPV1 with capsaicin provides neuroprotection after HI brain injury using the established Rice-Vannucci neonatal rat model. Specifically, we investigated the effects of capsaicin treatment on infarction volume and vascular dynamics in our brain-injured neonatal rat pups.
Materials and Methods
Animal Groups
All procedures for this study were approved by the Animal Care and Use Committee at Loma Linda University and complied with the NIH Guide for the Care and Use of Laboratory Animals. Ninety-five 10-day-old rat pups were randomly divided into the following groups: sham, HI (n = 39), HI + 0.2 mg/kg capsaicin pre-treatment (n = 14), HI + 2 mg/kg capsaicin pre-treatment (n = 14), HI + 0.2 mg/kg capsaicin post-treatment (n = 14) and HI + 0.2 mg/kg capsaicin post-treatment (n = 14).
Operative Procedure
Timed pregnant female Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN) and housed in individual cages. The day of birth was considered day 0. After birth, pups were housed with their dam under a 12:12-h light-dark cycle, with food and water available ad libitum throughout the study. A modified Rice-Vannucci model was adopted as follows [6]: 10-day-old postnatal pups were anesthetized with isoflurane. The right common carotid artery of each pup was permanently ligated with 5-0 surgical silk through a near-midline incision. After recovering with their dams for 2 h, the pups were then placed in a jar perfused with a humidified and pre-warmed gas mixture (8% oxygen balanced with nitrogen) for 2 h. A constant temperature of 37°C was maintained throughout all the procedures. After hypoxia, the animals were returned to their dams, and the ambient temperature was maintained at 37°C for 24 h. Sham animals underwent anesthesia, and the common carotid artery was exposed without ligation and hypoxia.
Treatment Method
Capsaicin (Tocris Bioscience, MO), a TRPV1 agonist, was administered intraperitoneally as a single treatment in two doses of 0.2 mg/kg and 2.0 mg/kg based on previous studies [7] either as pretreatment (3 h prior to HI) or post-treatment (1 h after HI). The drugs were constituted in 1% dimethyl sulfoxide (DMSO).
Immunohistochemistry
Naïve pups (10 days old) were transcardially perfused under deep anesthesia with PBS followed by 4% paraformaldehyde. The brains were then removed and post-fixed in formalin. Paraffin-embedded brains were sectioned into 10-μm-thick slices by cryostat (CM3050S; Leica Microsystems). Double immunofluorescent staining was performed using the following primary antibodies: rabbit polyclonal anti-TRPV1 antibody (Tocris Biosciences, 1:200), goat polyclonal anti-vWF (Santa Cruz Biotechnology, 1:200) and mouse anti-NeuN antibody (Chemicon, MAB377, 1:200) as described before [8].
Infarct Volume Measurement
2, 3, 5-Triphenyltetrazolium chloride monohydrate (TTC) staining was used to measure infarct volume as previously described [9]. Briefly, at 24 h and 72 h after HI, animals were perfused transcardially with PBS under deep anesthesia. The brains were removed and sectioned into 2-mm slices, then immersed into 2% TTC solution at 37°C for 5 min, followed by 10% formaldehyde. The infarction volume was traced and analyzed by Adobe Photoshop 6.0 by an investigator blinded to the treatment groups.
Vascular Dynamics
These experiments were carried out on distal MCA segments. All methods used for tissue dissection and mounting in the organ were performed as described previously [10]. Briefly, rat brains were rapidly removed from the cranial cavity after decapitation and placed in ice cold PBS at a pH level of 7.4 via stepwise addition of 1 M NaOH. Distal MCA was dissected from the brain surface, cut into lengths of 5 mm and mounted on cannulas in an organ chamber (Living Systems, Burlington, VT). In the organ chamber, the proximal cannula was connected in parallel to a pressure transducer, a reservoir of PSS, and a servo-controlled pump system used to set transmural pressure. The distal cannula was connected to a luer-lock valve that was open to gently flush the lumen during the initial cannulation. After cannulation, the distal valve was closed, and all measurements were conducted under no-flow conditions. Arterial diameter was recorded with the SoftEdge Acquisition Subsystem (Ion-Optix, Milton, MA). The percent myogenic tone at each pressure (20, 40, 60 and 80 mmHg) was calculated as passive diameter (diameter in zero Ca2+ PSS) minus active diameter (diameter in PSS) divided by passive diameter times 100 [10].
Statistics
All the data were expressed as mean ± SEM. Statistical differences between groups were analyzed by using one-way ANOVA followed by Holm-Sidak post-hoc analysis.
Results
Ubiquitous expression of TRPV1 in the neonatal brain
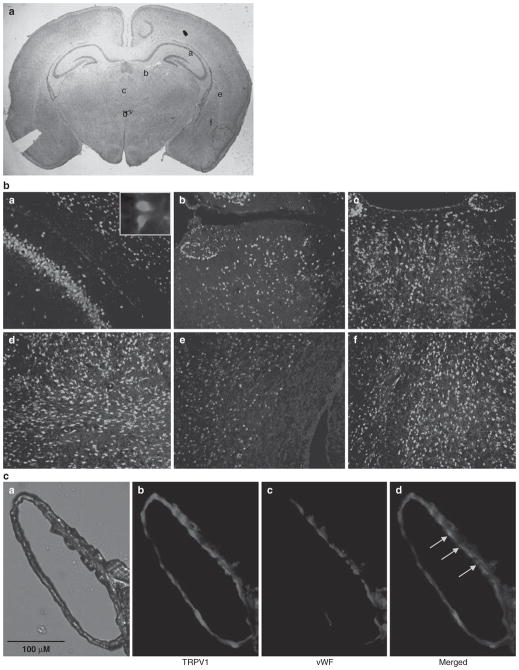
The distribution of TRPV1 in the neonatal brain using double-labeling fluorescent immunostaining was examined. We found that the receptor was widely distributed in the neonatal brain, including the cortex, hippocampus, striatum and sub-ventricular zone, as shown in Fig. 1, similar to the distribution in the adult brain, as reported by others [7, 11]. TRPV1 immunoreactivites (blue color) were expressed in both neuronal as well as non-neuronal cell types as indicated by the partial co-localization with a neuronal marker (NeuN, red immunoreactivites) in the merged images shown in Fig. 1b. TRPV1 immunoreactivites were also expressed in the cerebral vasculature (distal MCA). There was partial co-localization with vWF, an endothelial marker indicating presence of TRPV1 on the endothelial as well as non-endothelial cells in the vasculature (Fig. 1c).
Fig. 1.
Cell-specific distribution of TRPV1. (b) The double immuno-fluorescent images in panels a–f show the TRPV1 immunoreactivites (blue color) and neuronal marker, NeuN (red color), in different areas of the neonatal brain as indicated in Fig. 1a. (c) Panels a–d show the presence of TRPV1 immunoreactivities (panel b, red color) and endothelial marker, vWF (panel c, blue color), merged at some areas (panel d, indicated by arrows) in the middle cerebral artery (panel a) from neonatal rat brain
Infarction volume after neonatal hypoxia ischemia
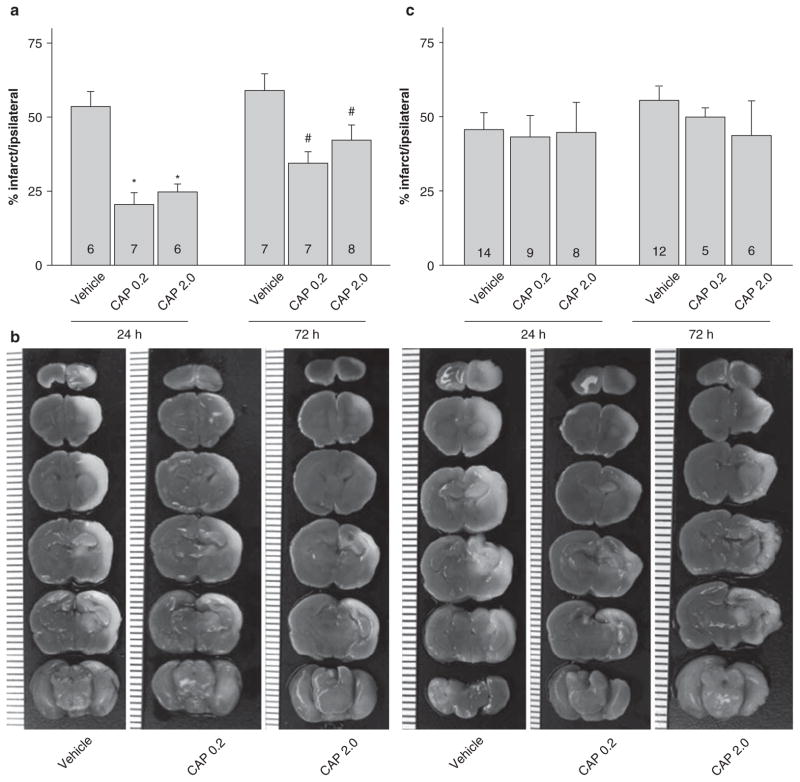
Based on existing reports in adult models [5], TRPV1 modulation was done using an agonist (capsaicin, 0.2 and 2 mg/kg, i.p) at 3 h before HI and at 1 h after HI. Both doses of capsaicin pre-treatment significantly reduced the infarction volume at 24 h and 72 h after neonatal HI (Fig. 2a, b). However, when capsaicin was administered as a post-treatment, there were no effects on infarction size at either 24 h or 72 h (Fig. 2c).
Fig. 2.
Infarction volume with capsaicin pre/post treatment. (a) Bar graph showing the quantified results of infarction volume calculated from TTC-stained brain coronal slices from pre-treatment groups (3 h pre-HI): vehicle, capsaicin 0.2 mg/kg, capsaicin 2 mg/kg at 24 h and 72 h post-HI. The data indicated that both capsaicin pretreated groups significantly decreased infarction volume as compared to respective vehicle-treated groups at 24 h (*p < 0.05) and 72 h (#p < 0.05) post-HI. (b) Represented brain slices stained with TTC. The vertical scale on the left side of the brain slices in the lower panel has a count of 1 mm. (c) Bar graph showing similar tests as in 2a, however with 1 h post-treatment. The data indicated that capsaicin post-treated groups did not have any effects on the infarction volume as compared to respective vehicle-treated groups at 24 h and 72 h (p < 0.05)
Vascular dynamics after neonatal HI
Distal MCA segments were obtained from sham (n = 3), vehicle (n = 6) and capsaicin pre-treated (0.2 mg/kg, n = 6) pups at 6 h post-HI, i.e., during the development of infarction. This time point was chosen specifically to allow for studying the vascular dynamics of the MCA during the progression of infarction. There was significant loss of myogenic tone (Fig. 3a) and increased diameter (Fig. 3b) (loss of the ability to contract and maintain tone) in the arterial segments obtained from vehicle-treated animals. The capsaicin pretreatment group significantly improved the myogenic tone in the MCA segments at 60 and 80 mmHg pressure as compared to the vehicle-treated group. Capsaicin pretreatment showed slightly improved vascular diameter dynamics; however, the values were not significant as compared to the vehicle-treated group.
Fig. 3.
Vascular dynamics after neonatal HI. Effects of changes in myogenic tone (a) and diameter (b) of distal MCA post-HI. Sequential pressure steps from 20 to 80 mmHg were applied after exposing the arteries to normal PSS or zero calcium PSS (3 mM EDTA), respectively. The arterial segments showed significant loss of myogenic tone and increased diameter in both capsaicin pretreated (n = 6) and vehicle-treated (n = 6) groups compared to sham (n = 3). The capsaicin-pre-treated group showed significant improvement in myogenic tone at 60 and 80 mmHg as compared to the vehicle-treated group (Fig. 3a). (p < 0.05, denoted by *v/s sham and #v/s vehicle)
Discussion
Neonatal HI brain injury is a major cause of morbidity and mortality in infants and children. Even if newborns are able to survive the initial attack, the damage to the brain is so severe that most individuals are left with chronic neurobehavioral disabilities and limited growth capabilities. In this study, we investigated the effects of capsaicin treatment on HI brain injury, specifically investigating its potential to reduce the acute changes that occur after injury. Our study suggests that for the first time, pre-treatment with capsaicin may provide neurovascular protection in neonatal HI by reducing the volume of infarction and improving overall vascular dynamics of major arteries in the brain.
TRPV1 receptors are calcium cation channels responsible for transducing the nociceptive effects of sensory neurons in the body as well as in the brain. In various adult species, immunohistochemistry markers have demonstrated TRPV1’s expression in various regions of the brain including the cortex, hippocampus, dentate gyrus, central amygdala, striatum, hypothalamus, thalamus and cerebellum [7]. Where it has not been confirmed is in the immature, neonatal brain. Nevertheless, in our study, we found a similar degree of distribution of TRPV1 receptors in the neonatal rat brain through the use of double immunofluorescence. Specifically, we found the ubiquitous presence of TRPV1 in neuronal and endothelial cells within the cerebral vasculature. As a result of their widespread distribution in the brain, we felt that by inhibiting TRPV1, we could potentially reduce the damages induced by HI injury.
Many of the damaging initial occurrences after an HI event are a result of the body’s attempt to cope with the surrounding environment. With an inadequate amount of oxygen reaching the brain, anaerobic glycolysis is quickly initiated, leading to an insufficient supply of energy. As a result, key processes in the brain responsible for maintaining cellular survival shut down, such as energy-dependent ion pumps [12]. This effect can occur from activation of TRPV1 receptors by pathophysiologic mediators, such as cell membrane breakdown products, endocannabinoids and pH changes, which occur after HI [3]. This leads to an overstimulation of glutamate-dependent NMDA receptors and an abnormal rise in intracellular calcium levels. Consequently, a high production of reactive oxygenated species (ROS) and activation of nitric oxide synthase occurs, ultimately resulting in neuronal excitotoxicty. In previous works, capsaicin treatment has been suggested to desensitize the TRPV1 receptor [5], and thus lead to a decrease in Ca++ influx post-ischemia and a significant reduction in excitotoxic damage. In our study we found similar results. Both the low- and high-dose capsaicin pre-treatment groups were found to decrease the volume of infarction in our neonatal rat population. This may be due to the reduction in excitotoxic damage caused by TRPV1 inhibition by capsaicin.
The brain area supplied by the MCA is known to include the penumbra after the hypoxic ischemic insult. Without intervention, the infarction after neonatal HI has been shown to progress and increase in size over 72 h, but the earliest irreversible changes, such as activation of caspase-3 in the penumbra, were seen as early as 3 h after neonatal HI [13]. The myogenic response is critical in autoregulation, which is a hallmark of cerebral circulation. In recent studies, it was suggested that the activation of TRPV1 can regulate the myogenic tone via release of vasoactive neuropeptides [14]. To examine the effects of capsaicin on the cerebral vasculature and the vascular dynamics during this acute and critical phase, we harvested the distal MCA from the ipsilateral brain at 6 h after neonatal HI to study the vascular dynamics in the vessel. We found that there was a loss in myogenic tone in the distal MCA after injury, which was attenuated with capsaicin pre-treatment. The results of this study suggest that TRPV1 modulation by capsaicin provides vascular protection.
Conclusion
In summary, this study provides evidence that capsaicin, a TRPV1 agonist, provides neurovascular protection against neonatal HI brain damage. This effect may be partly exerted by improvement of vascular dynamics and inhibition of TRPV1-mediated excitotoxic cell damage. Further studies will need to be conducted to determine its potential in the clinical setting.
Acknowledgments
This study is partially supported by NIH NS043338 to J.H. Zhang, and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Nikan H. Khatibi, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA
Vikram Jadhav, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Shelton Charles, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Jeffrey Chiu, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
John Buchholz, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Jiping Tang, Email: jtang@llu.edu, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Loma Linda, CA, 92354, USA.
John H. Zhang, Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA and Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda Medical Center, Loma Linda, CA, USA
References
- 1.Bracci R, Perrone S, Buonocore G. The timing of neonatal brain damage. Biol Neonate. 2006;90:145–155. doi: 10.1159/000092517. [DOI] [PubMed] [Google Scholar]
- 2.Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia–ischemia is programmed cell death. Dev Neurosci. 2001;23:186–191. doi: 10.1159/000046141. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38(3–4):233–252. doi: 10.1016/j.ceca.2005.06.028. Review. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R64–R76. doi: 10.1152/ajpregu.00446.2006. Epub 2006 Sep 14. Review. [DOI] [PubMed] [Google Scholar]
- 5.Pegorini S, Braida D, Verzoni C, Guerini-Rocco C, Consalez GG, Croci L, Sala M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br J Pharmacol. 2005;144(5):727–735. doi: 10.1038/sj.bjp.0706115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis. 2008;31(3):433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97(7):3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88(2):205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhong B, Wang DH. N-oleoyldopamine, a novel endogenous capsaicin-like lipid, protects the heart against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol. 2008;295(2):H728–H735. doi: 10.1152/ajpheart.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles SM, Zhang L, Longo LD, Buchholz JN, Pearce WJ. Postnatal maturation attenuates pressure-evoked myogenic tone and stretch-induced increases in Ca2+ in rat cerebral. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R737–R744. doi: 10.1152/ajpregu.00869.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139(4):1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. Epub 2006 Apr 17. [DOI] [PubMed] [Google Scholar]
- 12.Kaur C, Ling EA. Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog Neurobiol. 2009;87(4):264–280. doi: 10.1016/j.pneurobio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34(1):207–213. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res. 2004;95(10):1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]