Abstract
Background
Germinal matrix hemorrhage (GMH) is a potentially devastating neurological disease of very low birth weight premature infants. This leads to post-hemorrhagic hydrocephalus, cerebral palsy, and mental retardation. Hyperbaric oxygen (HBO) treatment is a broad neuroprotectant after brain injury. This study investigated the therapeutic effect of HBO after neonatal GMH.
Methods
Neonatal rats underwent stereotaxic infusion of clostridial collagenase into the right germinal matrix (anterior caudate) brain region. Cognitive function was assessed at 3 weeks, and then sensorimotor, cerebral, cardiac, and splenic growths were measured 1 week thereafter.
Results
Hyperbaric oxygen (HBO) treatment markedly improved upon the mental retardation and cerebral palsy outcome measurements in rats at the juvenile developmental stage. The administration of HBO early after neonatal GMH also normalized brain atrophy, splenomegaly, and cardiac hypertrophy 1 month after injury.
Conclusion
This study supports the role of hyperbaric oxygen (HBO) treatment in the early period after neonatal GMH. HBO is an effective strategy to help protect the infant’s brain from the post-hemorrhagic consequences of brain atrophy, mental retardation, and cerebral palsy. Further studies are necessary to determine the mechanistic basis of these neuroprotective effects.
Keywords: Hyperbaric oxygenation; Neurological deficits; Stroke, experimental
Introduction
Germinal matrix hemorrhage (GMH) is a potentially devastating clinical condition with life-long consequences. This occurs when immature blood vessels rupture within the subventricular (anterior caudate) region during the first 7 days of life [1, 2]. Approximately 20–25% of very low birth weight (VLBW ≤ 1,500 g) premature infants will be affected, accounting for around 3.5 per 1,000 births in the United States every year [3–6]. The long-term consequences of GMH are hydrocephalus (post-hemorrhagic ventricular dilation), developmental delay, cerebral palsy, and mental retardation [4, 7]. This is an important clinical problem for which experimental studies investigating thearapeutic modalities are generally lacking [8].
Hyperbaric oxygen (HBO) treatment will induce multiple neuroprotective pathways across a wide domain of brain injury [9]. HBO has been shown in randomized controlled trials to improve cognition and overall functioning in autistic children [10], and may ameliorate outcomes after cerebral palsy as well [11]. Emerging evidence shows that both HBO and normobaric oxygen (NBO) treatments exert similar neuroprotective mechanisms to improve outcomes after adult ischemic stroke [12], and perhaps after neonatal hypoxiaischemia as well [13].
In light of this evidence, we hypothesized that HBO therapy can be a therapeutic strategy to ameliorate brain injury mechanisms, to thereby improve cognitive and sensorimotor outcomes after germinal matrix hemorrhage in neonatal rats.
Methods and Materials
Animal Groups and General Procedures
This study was in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Timed pregnant Sprague-Dawley rats were housed with food and water available ad libitum. Treatment consisted of 1 h normobaric oxygen (NBO, 0 atm) or hyperbaric oxygen (HBO, 2.5 atm) administration beginning 1 h after collagenase infusion. Postnatal day 7 (P7) pups were blindly assigned to the following (n = 8/group): sham (naive), needle (control), GMH (collagenase-infusion), GMH + NBO, and GMH + HBO. All groups were evenly divided within each litter.
Experimental Model of GMH
Using aseptic technique, rat pups were gently anesthetized with 3% isoflurane (in mixed air and oxygen) while placed prone onto a stereotaxic frame. Betadine sterilized the surgical scalp area, which was incised in the longitudinal plane to expose the skull and reveal the bregma. The following stereotactic coordinates were determined: 1 mm (anterior), 1.5 mm (lateral), and 3.5 mm (ventral) from the bregma. A bore hole (1 mm) was drilled, into which a 27-gauge needle was inserted at a rate of 1 mm/min. A microinfusion pump (Harvard Apparatus, Holliston, MA) infused 0.3 units of clostridial collagenase VII-S (Sigma, St Louis, MO) through a Hamilton syringe. The needle remained in place for an additional 10 min after injection to prevent back-leakage. After needle removal, the burr hole was sealed with bone wax, the incision sutured closed, and the animals allowed to recover. The entire surgery took on average 20 min. Upon recovering from anesthesia, the animals were returned to their dams. Needle controls consisted of needle insertion alone without collagenase infusion, while naïve animals did not receive any surgery.
Cognitive Measures
Higher order brain function was assessed during the third week after collagenase infusion. The T-maze assessed short-term (working) memory [14]. Rats were placed into the stem (40 cm × 10 cm) of a maze and allowed to explore until one arm (46 cm × 10 cm) was chosen. From the sequence of ten trials, of left and right arm choices, the rate of spontaneous alternation (0% = none and 100% = complete, alternations/trial) was calculated, as routinely performed [15, 16]. The Morris water maze assessed spatial learning and memory on four daily blocks, as described previously in detail [17, 18]. The apparatus consisted of a metal pool (110 cm diameter) filled to within 15 cm of the upper edge, with a platform (11 cm diameter) for the animal to escape onto, which changed location for each block (maximum = 60 s/trial), and data were digitally analyzed by Noldus Ethovision tracking software. Cued trials measured place learning with the escape platform visible above water. Spatial trials measured spatial learning with the platform submerged, and probe trials measured spatial memory once the platform was removed. For the locomotor activity, in an open field, the path length in open-topped plastic boxes (49 cm long, 35.5 cm wide, 44.5 cm tall) was digitally recorded for 30 min and analyzed by Noldus Ethovision tracking software [18].
Sensorimotor Function
At 4 weeks after collagenase infusion, the animals were tested for functional ability. The neurodeficit was quantified using a summation of scores (maximum = 12) given for (1) postural reflex, (2) proprioceptive limb placing, (3) back pressure towards the edge, (4) lateral pressure towards the edge, (5) forelimb placement, and (6) lateral limb placement (2 = severe, 1 = moderate, 0 = none), as routinely performed [15]. For the rotarod, striatal ability was assessed using an apparatus consisting of a horizontal, accelerated (2 rpm/5 s), rotating cylinder (7 cm-diameter × 9.5 cm-wide), requiring continuous walking to avoid falling, recorded by photo-beam circuit (Columbus Instruments) [17, 18]. For foot fault, the number of complete limb missteps through the openings while exploring over an elevated wire (3 mm) grid (20 cm × 40 cm) floor was counted over 2 min [16].
Assessment of Treatment upon Cerebral and Somatic Growth
At the completion of experiments, the brains were removed and hemispheres separated by midline incision (loss of brain weight has been used as the primary variable to estimate brain damage in juvenile animals after neonatal brain injury [19]). For organ weights, the spleen and heart were separated from surrounding tissue and vessels. The quantification was performed using an analytical microbalance (model AE 100; Mettler Instrument Co., Columbus, OH) capable of 1.0 µg precision.
Statistical Analysis
Significance was considered at p < 0.05. Data were analyzed using analysis of variance (ANOVA) with repeated measures (RM-ANOVA) for long-term neurobehavior. Significant interactions were explored with conservative Scheffé post hoc and Mann-Whitney rank sum when appropriate.
Results
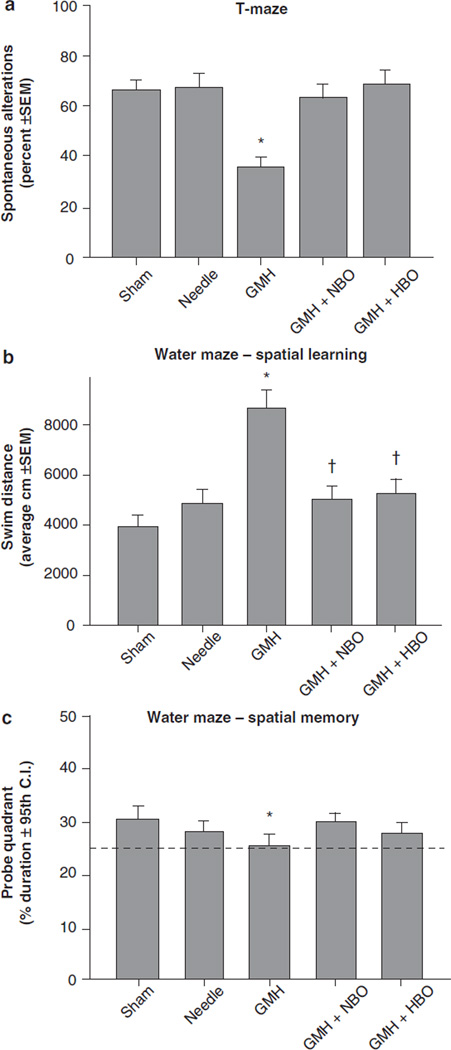
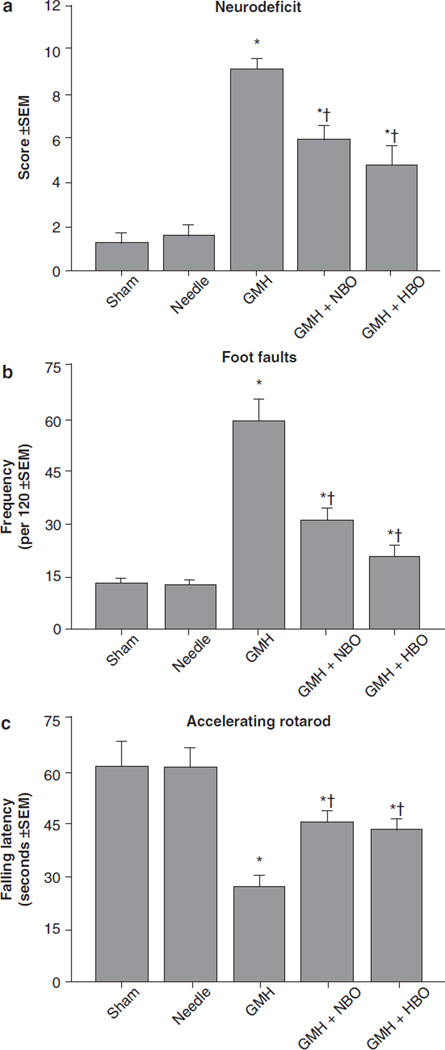
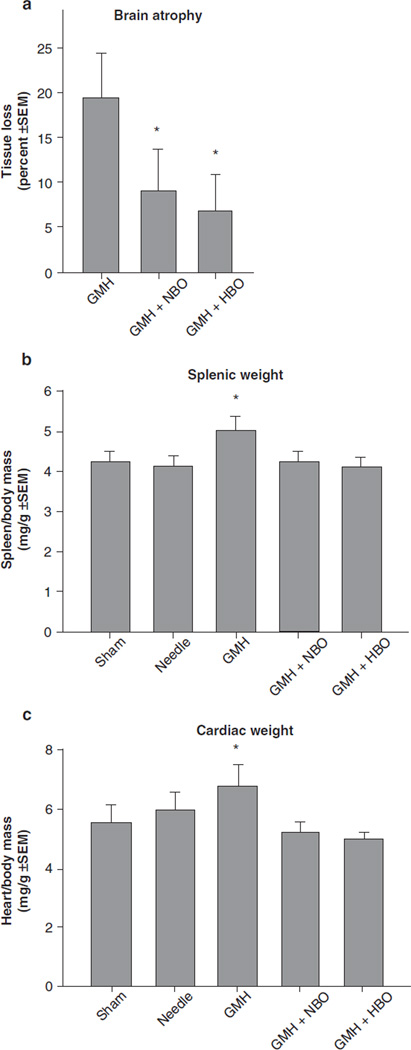
HBO and NBO treatment normalized T-maze deficits (Fig. 1a, p > 0.05 compared to controls) and water maze (spatial) learning deficits (Fig. 1b, p < 0.05 compared to GMH), without improving spatial memory (Fig. 1c, p > 0.05). Oxygenation treatment also normalized (p < 0.05) sensorimotor dysfunction (compared to juvenile GMH animals) in the neurodeficit score, the number of foot faults, and accelerating rotarod falling latency (Fig. 2a–c, p < 0.05). This was confirmed with normalization of brain atrophy (Fig. 3a, p < 0.05 compared to GMH), splenomegaly, and cardiomegaly (Fig. 3b, c, p > 0.05 compared to controls).
Fig. 1.
Cognitive function normalization in juvenile rats by HBO/ NBO after neonatal GMH. Higher order function was measured at the 3rd week after collagenase infusion: (a) T-maze, (b) spatial learning water maze, (c) spatial memory (probe) water maze. Values expressed as mean ± 95th CI (probe quadrant) or mean ± SEM (all others), n = 8 (per group), *p < 0.05 compared with controls (sham and needle trauma), and †p < 0.05 compared with GMH
Fig. 2.
Sensorimotor function normalization in juvenile rats by HBO/ NBO after neonatal GMH. Cerebral palsy measurements were performed in the juveniles at 1 month after collagenase infusion: (a) neurodeficit score, (b) foot faults and (c) rotarod. Values expressed as mean ± SEM, n = 8 (per group), *p < 0.05 compared with controls (sham and needle trauma), and †p < 0.05 compared with GMH
Fig. 3.
Cerebral and somatic growth normalization in juvenile rats by HBO/NBO after GMH. (a) Brain atrophy (percent tissue loss), (b) splenic weight, and (c) cardiac weight. Values expressed as mean ± SEM, n = 8 (per group), *p < 0.05 compared with controls (sham and needle trauma)
Discussion
These results indicate that hyperbaric oxygen (HBO) treatment after neonatal GMH can reduce long-term brain atrophy and return sensorimotor and cognitive functional deficits back to near-normal levels in juvenile animals. In support of the findings from others, this provides preliminary evidence about the importance of HBO-mediated mechanisms upon improvement of outcomes after neonatal injury [10–13].
Hyperbaric oxygen (HBO) treatment can induce multiple neuroprotective pathways across a wide domain of cerebral pathophysiology [9]. Our results support descriptive clinical studies using HBO to improve cognition and overall functioning in autistic and cerebral palsy children [10, 11]. In agreement with our findings, recent evidence shows that both HBO and normobaric oxygen (NBO) therapy can upregulate neuroprotective mechanisms and improve outcomes in adult ischemic stroke models [12]. Although hyper-oxygenation treatment has been shown to be protective after neonatal hypoxia-ischemia [13], our study demonstrates the first preliminary evidence, in this patient sub-population, advocating the use of oxygenation after hemorrhagic brain injury as well.
This study supports the notion that HBO treatment has no adverse affects in neonatal rats and can be applied as a strategy to improve functional outcomes after brain injury from hemorrhagic stroke in premature infants. Further investigation is needed to determine the mechanistic basis of these neuroprotective effects.
Acknowledgements
This study was partially supported by a grant (NS053407) from the National Institutes of Health to J.H.Z.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Tim Lekic, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Anatol Manaenko, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
William Rolland, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Robert P. Ostrowski, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA
Kelly Virbel, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Jiping Tang, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
John H. Zhang, Email: johnzhang3910@yahoo.com, Departments of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and; Departments of Anesthesiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and; Departments of Neurosurgery, Loma Linda University, School of Medicine, Loma Linda, CA, USA and; Department of Physiology, Loma Linda University, School of Medicine, Risley Hall, Room 223, Loma Linda, CA 92354, USA.
References
- 1.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. doi:10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–1090. doi: 10.1007/s00381-006-0050-6. doi:10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile LA, Kaplan MD. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 4.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–F41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermont-Oxford. The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Investigators of the Vermont- Oxford Trials Network Database Project. Pediatrics. 1993;91:540–545. [PubMed] [Google Scholar]
- 6.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. doi:peds.2009-2416 [pii], 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 7.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. doi:10.1016/j.nbd.2003.12.016, S0969996103002833 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21:365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JH, Lo T, Mychaskiw G, Colohan A. Mechanisms of hyperbaric oxygen and neuroprotection in stroke. Pathophysiology. 2005;12:63–77. doi: 10.1016/j.pathophys.2005.01.003. doi:S0928-4680(05)00013-1 [pii], 10.1016/j.pathophys.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Rossignol DA, Rossignol LW, Smith S, Schneider C, Logerquist S, Usman A, Neubrander J, Madren EM, Hintz G, Grushkin B, Mumper EA. Hyperbaric treatment for children with autism: a multicenter, randomized, double-blind, controlled trial. BMC Pediatr. 2009;9:21. doi: 10.1186/1471-2431-9-21. doi:1471-2431-9-21 [pii], 10.1186/1471-2431-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy P, Collet JP, Goldberg J, Ducruet T, Vanasse M, Lambert J, Marois P, Amar M, Montgomery DL, Lecomte JM, Johnston KM, Lassonde M. Neuropsychological effects of hyperbaric oxygen therapy in cerebral palsy. Dev Med Child Neurol. 2002;44:436–446. doi: 10.1017/s0012162201002341. [DOI] [PubMed] [Google Scholar]
- 12.Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke – experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9:227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- 13.Calvert JW, Zhang JH. Oxygen treatment restores energy status following experimental neonatal hypoxia-ischemia. Pediatr Crit Care Med. 2007;8:165–173. doi: 10.1097/01.PCC.0000257113.75488.84. doi:10.1097/01.PCC.0000257113.75488.84. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. doi:S0149-7634(04)00073-9[pii], 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. doi:10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. doi:S0006-8993(09)00520-4 [pii],10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27:627–637. doi: 10.1089/neu.2009.1163. doi:10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. doi:S0006-8993(09)00957-3 [pii], 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]