Abstract
We investigated the effect of the heat shock protein inducer geldanamycin on the development of secondary brain injury after ICH in mice. The effect of the drug at two different concentrations was evaluated at two time points: 24 and 72 h after ICH induction. In the first part of this study, a total of 30 male CD-1 mice were randomly divided into four groups: one sham group and three ICH groups. ICH animals received either an intraperitoneal injection of vehicle or geldanamycin (1 or 10 mg/kg). Neurological deficits and brain water content were evaluated 24 h after ICH. In the second part of this study, the effect of a high concentration of geldanamycin was evaluated 72 h after ICH. Neurological deficits were evaluated by the Garcia neuroscoring, wire hanging and beam balance tests. For estimation of brain water content, the “wet/dry weight” method was used. We demonstrated that administration of geldanamycin (10 mg/kg) ameliorated ICH-induced increase of brain water content significantly in both parts of the study. Geldanamycin improved the neurological outcome according to performance on Garcia and beam balance tests in the 72 h part of this study. Geldanamycin-induced induction of heat shock protein after ICH has a neuroprotective effect and may be a therapeutic target for ICH.
Keywords: Geldanamycin, HSP, ICH Neuroprotection
Introduction
Intracerebral hemorrhage (ICH) is a devastating clinical event with greater than 40% initial mortality, leaving many of the survivors permanently disabled. Currently, there is neither an effective therapy to increase survival after intracerebral hemorrhage nor a treatment to improve the quality of life of survivors [1]. There are biphasic effects of intracerebral hemorrhage upon brain tissue. Initial injury is in response to the expanding hematoma imposing sheer forces and mass effect upon the cerebral tissues [2]. Intracerebral bleeding leads to increased intracranial pressures and could lead to transtentorial herniation secondary to the mass effect [3]. A later phase involves hematoma-induced neuronal and glial apoptotic cell death at the surrounding parenchymal rim [4], inflammation and progressive rupture of the blood-brain-barrier, and rising brain edema [5]. Geldanamycin belongs to the family of ansamycin antibiotics. Biochemical and structural studies have demonstrated that geldanamycin binds specifically to the ATP pocket of the constitutively induced heat shock protein (HSP) 90 and inhibits its chaperone activity and its ability to bind to target proteins [6]. HSP 90 affects multiple signal transduction pathways [7] and has been shown to regulate more than 100 proteins involved in cellular signaling [7]. HSP 90 is a negative regulator of heat shock factor 1 (HSF 1). The binding between HSP 90 and geldanamycin makes HSP 90 unable to associate with HSF 1 [8]. The free HSF 1 initiates production of other HSPs, specifically HSP 70 [9]. A neuroprotective effect of geldanamycin-induced HSP70 upregulation was demonstrated previously [10, 11]. To date, no study has assessed the effects of geldanamycin on the outcomes of intracerebral hemorrhage. In the present study we aimed to investigate if post-treatment with the HSP modulator geldanamycin will result in decreased brain edema and improvement in neurological outcomes
Methods
Experimental animals
All procedures and methods for these studies were approved by the Animal Care and Use Committee at Loma Linda University and complied with the Guide for the Care and Use of Laboratory Animals (http://research.llu.edu/forms/appendixb.doc). A total of 30 male CD-1 mice were divided into four groups: sham, ICH treated with vehicle, ICH treated with low-dose geldanamycin (1 mg/kg), and ICH treated with high-dose geldanamycin (10 mg/kg). Geldanamycin was dissolved in 2.5% of DMSO and administered intraperitoneally 1 h after ICH induction. Animals were tested for neurological function and euthanized for brain edema measurements at 24 and 72 h after ICH.
Intracerebral hemorrhage induction
We adopted the collagenase-induced intracerebral hemorrhage model as previously described in mice [12]. Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg, intraperitoneal injection) and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL). An electronic thermostat-controlled warming blanket was used to maintain the core temperature at 37°C. The calvarium was exposed by a midline scalp incision from the nasion to the superior nuchal line, and the skin was retracted laterally. With a variable speed drill (Fine Scientific Tools, Foster City, CA) a 1.0-mm burr hole was made 0.9 mm posterior to bregma and 1.45 mm to the right of the midline. A 26-gauge needle on a Hamilton syringe was inserted with stereotaxic guidance 4.0 mm into the right deep cortex/basal ganglia at a rate 1 mm/ min. The collagenase (0.075 units in 0.5 μL saline, VII-S; Sigma, St Louis, MO) in the syringe was infused into the brain at a rate of 0.25 μL/min over 2 min with an infusion pump (Stoelting, Wood Dale, IL). The needle was left in place for an additional 10 min after injection to prevent the possible leakage of collagenase solution. After removal of the needle, the incision was closed, and the mice were allowed to recover. A sham operation was performed with needle insertion only.
Brain water content
The brain water content was measured as previously described [12]. Briefly, mice were euthanized under deep anesthesia. Brains were removed immediately and divided into five parts: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex, and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were weighed on an electronic analytical balance (model AE 100; Mettler Instrument Co., Columbus, OH) to the nearest 0.1 mg to obtain the wet weight. The tissue was then dried at 100°C for 24 h to determine the dry weight. Brain water content (%) was calculated as [(wet weight – dry weight)/wet weight] × 100.
Neurological deficits
Neurological scores were assessed by an independent researcher blinded to the procedure 24 and 72 h after ICH using a modification of scoring (21 points) (maximum 3 = healthy animal) [13]. In addition, beam balance and wire hang tests were performed.
Statistical analysis
Quantitative data are expressed as the mean ± SEM. Statistical significance was verified by analysis of variance (ANOVA; Bonferroni test) for analysis of acute effect (24 h) and by ANOVA (Tukey test) for analysis of delayed effect (72 h). P < 0.05 was considered statistically significant.
Results
Geldanamycin reduced brain edema
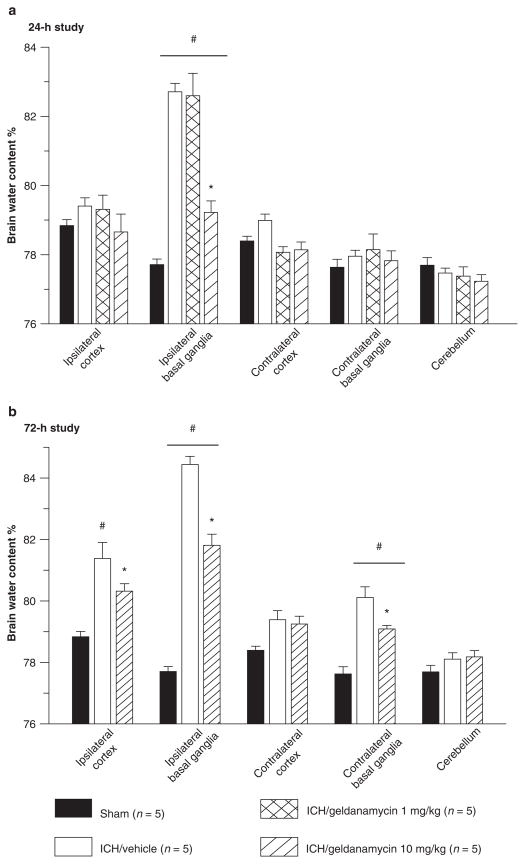
In the 24-h study, collagenase injection caused a significant rise in brain water content in the ipsilateral basal ganglia of all ICH animals (p < 0.001, ANOVA, Bonferroni test). The high dose of geldanamycin treatment significantly reduced brain edema in the ipsilateral basal ganglia when compared with the vehicle-treated and low-dose-treated ICH groups (p < 0.001, ANOVA, Bonferroni test). Low-dose geldanamycin did not reduce brain water content (Fig. 1a). In the 72-h study, we observed that injection of collagenase caused an increase of brain water content in both basal ganglias and in the ipsilateral cortex. High-dose geldanamycin treatment reduced brain water content significantly (p < 0.001, ANOVA, Bonferroni test) (Fig. 1b).
Fig. 1.
(a) The 24-h study: Brain edema detected in ipsilateral basal ganglia was significantly higher among ICH groups when compared with sham-operated animals (#p < 0.001 vs. sham, ANOVA). Geldanamycin (10 mg/kg) reduced the brain water content of ipsilaterally basal ganglia significantly (*p < 0.001 vs. vehicle, ANOVA). A low concentration of drug remains ineffective. (b) The 72-h study: Brain edema spread into other brain compartment of vehicle-treated mice compared with sham-operated animals (#p < 0.001 vs. sham, ANOVA). Geldanamycin (10 mg/kg) reduced brain water content significantly (*p < 0.001 vs. vehicle, ANOVA)
High-dose geldanamycin reduced neurological deficits at 72 h after ICH
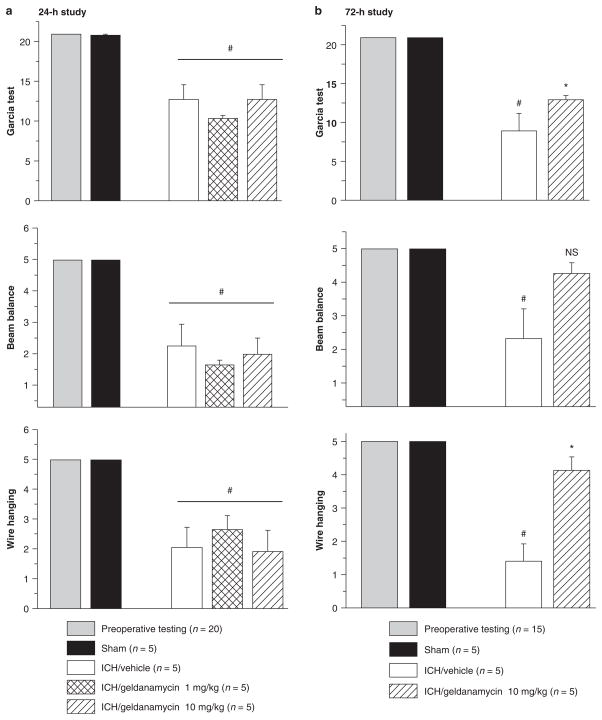
Neurological deficits were present in all animals after collagenase injection. Neither the low dose nor high dose of geldanamycin led to any improvement of neurological function when compared with the vehicle-treated intracerebral hemorrhage group at 24 after ICH (Fig. 2a). In the 72-h study, high-dose geldanamycin treatment significantly reduced neurological deficits that were indicated by improvements in the Garcia test (*p < 0.05, ANOVA, Dunn’s method) and wire hanging test (*p < 0.05 ANOVA, Bonferroni t-test) (Fig. 2b). Beam balance test results tended to improve after high-dose geldanamycin treatment; however, this change did not reach statistical significance (p = 0.072).
Fig 2.
Neurological function tests. In the 24-h study (a), significant neurological deficits were observed in all animals receiving collagenase (*p < 0.05, vs. sham). However, no differences were observed between vehicle- and geldanamycin-treated groups. The data of wire hanging and beam balance tests are represented as the average of three trials per animal. (b) In the 72-h study, a high concentration of geldanamycin leads to significant improvement of the neurological deficit according to performance on Garcia and wire hanging tests. Tendency to improvement in the beam balance test remains insignificant (NS p = 0.072)
Discussion
Our study demonstrated that (1) geldanamycin at a dose of 10 mg/kg significantly reduced brain edema at 24 and 72 h after ICH; (2) geldanamycin at a dose of 10 mg/kg significantly improved neurological function at 72 h after ICH; (3) geldanamycin at a dose of 1 mg/kg did not produce a significant effect on ICH-induced brain edema and neurological deficits.
The neuroprotective effect of geldanamycin has been demonstrated in several in vitro and in vivo models of neuronal injury. It has been shown that administration of geldanamycin after middle cerebral artery occlusion in rats reduced infarct volume and brain swelling, and significantly improved the neurological function [10, 11]. In an animal model of hemorrhage, intraperitoneal administration of geldanamycin 16 h before hemorrhage preserved energy loss by amelioration of hemorrhage-induced ATP breakdown [14]. Moreover, geldanamycin pre-treatment affects caspase-3 activity in the brain in an animal model of hemorrhage [15]. The mechanism of geldanamycin-induced neuroprotection appears to be due to its ability to affect HSP 70 [9]. HSP 70 is an inducible form of heat shock protein. It is the major inducible stress protein and has long been thought to contribute to cell survival following potentially lethal stresses. The neuroprotective effect of HSP 70 upregulation has been described in several models of brain injury [16]. Transgenic animals overexpressing HSP 70 have fewer apoptotic cells compared with wild-type animals after permanent focal ischemia [17]. Similar results were demonstrated by Matsumori et al. by studying the activation of mitochondrial apoptotic pathways in mice overexpressing HSP 70 in a model of neonatal hypoxia-ischemia. They showed that high constitutive expression of HSP 70 protects the brain from hypoxia-ischemia in the neonatal period and affects the apoptotic pathways [18].
This study provides the first evidence that geldanamycin treatment protects the brain against intracerebral hemorrhage. Geldanamycin decreases brain water content in acute (24 h after ICH induction) and delayed (72 h after ICH induction) phases. Moreover, the administration of geldanamycin improved the neurological deficit in the delayed phase.
Acknowledgments
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.D’Ambrosio AL, Sughrue ME, Yorgason JG, Mocco JD, Kreiter KT, Mayer SA, McKhann GM, II, Connolly ES., Jr Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: clinical outcome and quality of life assessment. Neurosurgery. 2005;56:12–19. doi: 10.1227/01.neu.0000144820.38439.63. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 3.Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist. 2005;11:311–324. doi: 10.1097/01.nrl.0000178757.68551.26. [DOI] [PubMed] [Google Scholar]
- 4.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, Hopkins LN. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med. 2001;29:152–157. doi: 10.1097/00003246-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 7.Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–872. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp 60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HM, Kim Y, Yang SI, Kim YJ, Lee SH, Yoon BW. Geldanamycin protects rat brain through overexpression of HSP70 and reducing brain edema after cerebral focal ischemia. Neurol Res. 2008;30:740–745. doi: 10.1179/174313208X289615. [DOI] [PubMed] [Google Scholar]
- 11.Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 14.Kiang JG, Bowman PD, Lu X, Li Y, Ding XZ, Zhao B, Juang YT, Atkins JL, Tsokos GC. Geldanamycin prevents hemorrhage-induced ATP loss by overexpressing inducible HSP70 and activating pyruvate dehydrogenase. Am J Physiol Gastrointest Liver Physiol. 2006;291:G117–G127. doi: 10.1152/ajpgi.00397.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kiang JG, Bowman PD, Lu X, Li Y, Wu BW, Loh HH, Tsen KT, Tsokos GC. Geldanamycin inhibits hemorrhage-induced increases in caspase-3 activity: role of inducible nitric oxide synthase. J Appl Physiol. 2007;103:1045–1055. doi: 10.1152/japplphysiol.00100.2007. [DOI] [PubMed] [Google Scholar]
- 16.Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab. 2003;23:718–727. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- 18.Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. HSP 70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25(7):899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]