Febrifugine (FF) is the active component of the Chinese herb Chang Shan (Dichroa febrifuga Lour)1,2, which has been used for treating malaria-induced fever for about 2000 years. Halofuginone (HF), the halogenated derivative of FF, has had clinical trials for potential therapeutic applications in cancer and fibrotic disease 3-6. Recently, HF was reported to inhibit TH17 cell differentiation by activating the amino acid response (AAR) pathway7, through inhibiting human prolyl-tRNA synthetase (ProRS) to cause intracellular accumulation of uncharged tRNA8,9. Curiously, inhibition requires un-hydrolyzed ATP. Here we report an unusual 2.0 Å structure showing that ATP directly locks onto and orients two parts of HF onto human ProRS, so that one part of HF mimics bound proline and the other mimics the 3′-end of bound tRNA. Thus, HF is a novel ATP-dependent inhibitor that simultaneously occupies two different substrate binding sites. Moreover, our structure suggests that the efficacy of FF for malaria treatment might work by a similar mechanism. Finally, the elucidation here of a two-site modular targeting activity of HF raises the possibility that substrate-directed capture of similar inhibitors might be generalized to other synthetases.
ProRS is a member of the aminoacyl-tRNA synthetase (aaRSs) family of enzymes that activate amino acids for protein synthesis through formation of aminoacyl adenylates (AA-AMP) and subsequent transfer of the activated amino acids to the 3′-ends of the cognate tRNAs. Because of their crucial role in protein synthesis, aaRS inhibition halts the growth and suppresses viability of all cell types. Consequently, aaRSs are attractive targets for discovering drugs, like antibiotics and suppressors of cell hyperproliferation10,11. Many tRNA synthetase inhibitors mimic AA-AMPs, and thereby directly block the active site. Because the AA-AMP mimics occupy the active site for ATP and amino acid, they compete out the substrate ATP. In contrast, ATP was reported to be essential for HF binding to ProRS8. This ATP dependence suggested HF binds to ProRS through a novel mechanism and not as a mimetic of Pro-AMP. Thus, understanding how HF inhibits human ProRS is of great interest.
In its simplest description, HF is comprised of a hydroxypiperidine ring joined by bridging atoms to a double ring halogenated 4-quinazolinone (Fig. 1a). To clarify the mechanism of inhibition of HF, we cloned and expressed human ProRS and were able to obtain co-crystals with a non-hydrolyzable ATP analog ((adenosine 5′-(β,γ-imido)triphosphate)(ATPa)) and HF, which diffracted to 2.0 Å resolution (Supplementary Table 1). The structure was solved by molecular replacement using T. thermophilus ProRS as template. The asymmetric unit contained two ProRS molecules that formed a homodimer (Fig. 1b), as also seen in gel filtration experiments. Each subunit encoded an N-terminal catalytic domain characteristic of a class II tRNA synthetase, an anticodon-binding domain, and a C-terminal zinc-binding domain (Fig. 1b). In the two catalytic domains of the ProRS homodimer, HF and ATPa were bound to the active site for Pro-AMP formation (Fig. 1b, c).
Figure 1. Structure of human ProRS and of bound ligands.
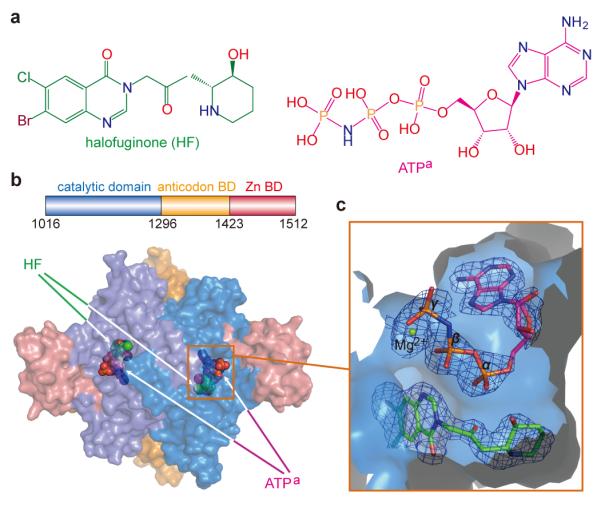
a, Chemical structures of halofuginone (HF) and the ATP analog (ATPa). b, Two ProRS monomers form an asymmetric unit that is a homodimer. HF and ATPa are shown as spherical models at the active site of both subunits. Here and throughout, ATPa and HF are colored as in Fig.1a. c, HF is buried at the bottom of the pocket and covered by co-bound ATPa. A simulated annealing omit map was calculated with Fourier coefficients 2Fo-Fc, and contoured at 1.5σ.
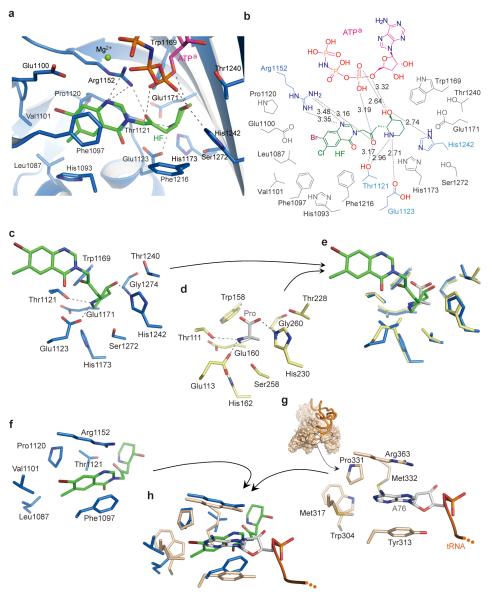
ATPa was located at the canonical ATP binding pocket of class II aaRSs12. Three hydrogen bonds contributed by Thr1164 and Thr1276 and hydrophobic stacking of Phe1167 stabilized the adenosine group (Supplementary Fig. 2). The ribose moiety was fixed by three hydrogen bonds with Gln1237, Thr1240 and Arg1278. The hydrogen bonds from Arg1152 and Arg1163 contributed to stabilizing the α-, β- and γ-phosphate groups of ATPa (Supplementary Fig. 2), which form a cap over the HF binding pocket (Fig. 1c). These extensive hydrophilic and hydrophobic interactions fixed the ATP in a “U”-like bent conformation with the α-phosphate group exposed at the bottom of the U. Superposition of the catalytic domains of human ProRS with that of the previously determined structure of T. thermophilus ProRS showed the conformation and location of ATP was the same (Supplementary Fig. 3), with the α-phosphate group poised for proline activation. In the human ProRS:HF:ATPa ternary complex, the α-phosphate group is proximal to the hydroxypiperidine ring of HF and forms two hydrogen bonds with its hydroxyl group (Fig. 2a, b). It also forms an additional hydrogen bond with the keto group in the bridge between the piperidine ring and the quinazolinone moiety of HF (Fig. 2a, b). These direct hydrogen-bond interactions enable ATP to lock onto and orient HF.
Figure 2. Mechanistic basis for ATP-dependent inhibition of ProRS by halofuginone.
a, HF forms extensive hydrophobic contacts and hydrogen-bonding interactions with ProRS and with ATPa. b, Two-dimensional presentation of HF binding. The HF, ATPa and hydrogen-bonded residues are colored as previously, and other residues within 4 Å of HF are colored in gray. c, A stick model showing the binding of HF’s piperidine ring to human ProRS. d, The proline binding pocket of T. thermophilus ProRS in complex with proline (PDB 1H4T, protein is colored as yellow and proline as gray-white). e, Structure superposition of catalytic domain of human ProRS with T. thermophilus ProRS reveals the piperidine ring of HF directly occupies the proline binding pocket. f, The stick model showing the binding of the halogenated 4-quinazolinone group of HF to human ProRS. g, Structure of E. coli ThrRS:tRNA complex, with the pocket for the adenosine group for A76 (PDB 1QF6). h, Overlay of the catalytic domain of human ProRS onto E. coli ThrRS (brown) reveals that HF uses the binding pocket for A76 of the CCA76-3′ end for binding the quinazolinone moiety.
Beside the interactions from ATP, ProRS itself forms seven hydrogen bonds and several hydrophobic contacts with each moiety of HF (Fig. 2a, b). Thus, the pocket for the HF piperidine ring, built by Thr1121, Glu1123, Trp1169, Glu1171, His1173, Thr1240, His1242 and Ser1272 (Fig. 2c), has both hydrophobic and hydrophilic character. These residues are absolutely conserved among eukaryotic ProRS (Supplementary Fig. 4), and were found to be also important for proline recognition in the structure of T. thermophilus eukaryotic-like ProRS with proline (Fig. 2d)13. Overlay of the catalytic domains of the human and T. thermophilus ProRS structures showed the piperidine ring of HF bound to the same site as proline (Fig. 2e). In particular, the orientations of the proline pyrrolidine ring and of the HF piperidine ring, and the locations of nitrogen and oxygen atoms, are similar. Thus, the HF piperidine ring appears as a proline-like group, and would directly compete with proline and block proline activation. The proline mimic mechanism is consistent with HF/FF derivatives with modifications on piperidine ring being unfit for binding to the proline-binding pocket, and, therefore, unable to inhibit or bind to ProRS or affect cell function (e.g. MAZ1310)8. In contrast, derivatives that replaced the piperidine ring by the pyrrolidine ring of proline retained the similar or superior biological activity that was tested14.
The halogenated 4-quinazolinone group is buried in a pocket mainly comprised of Leu1087, Phe1097, Val1101, Pro1120, Thr1121 and Arg1152 (Fig. 2a, f). Beside the hydrogen bond between Arg1152 and nitrogen atom, the quinazolinone group was mainly stabilized by hydrophobic contacts, particularly by the hydrophobic stack effect from Phe1097 (Fig. 2a, f). ThrRS also belongs to class II aminoacyl-tRNA synthetases, and it is the closest homolog to ProRS. Arg1152 and Phe1097 of ProRS are conserved in class II synthetases. Their corresponding residues Arg363 and Tyr313 in the structure of E. coli threonyl-tRNA synthetase (ThrRS) were found to flank the two sides of the adenosine of A76 of tRNAThr and thus play crucial roles in CCA 3′-end binding (Fig. 2g)15. Beside these two residues, other parts of the A76 adenosine pocket are also quite similar to the quinazolinone group binding pocket in the ProRS structure (Fig. 2g). Overlay of the ProRS and ThrRS structures by aligning the conserved 7-strand β-sheet core of their catalytic domains showed that the quinazolinone group of HF bound to the same position as bound A76, and with a similar orientation and similar side chain contacts (Fig. 2h). This structural similarity suggests that the quinazolinone group of HF binds to ProRS by employing a strategy of mimicking the terminal adenosine of the tRNA substrate. Thus, in contrast to the single-site and conventional AA-AMP mimics, HF is a dual site inhibitor by virtue of simultaneously occupying two active site pockets (Supplementary Fig. 1).
We used our crystal structure of the ProRS:ATP:HF complex to further dissect the mechanism of action of HF. Prior work by others showed that HF specifically inhibits overall aminoacylation of tRNAPro and that binding of HF to ProRS is greatly enhanced by ATP8. (Not known was how much weaker HF binds in the absence of ATP.) According to our structure, HF is ‘two-headed’ and, with bound ATP, uses the piperidine ring to block the proline binding site and the quinazolinone group to block the site for docking the 3′-end of tRNAPro. We designed experiments to probe the function of each site, i.e., the site for adenylate formation/binding and that for docking the 3′-end of tRNA. In addition to HF, we used another inhibitor, Pro-SA (5′-O-(N-(L-prolyl)-sulfamoyl-adenosine), as a comparator. Pro-SA is a tight-binding non-reactive adenylate analog of Pro-AMP16.
We first determined that the thermal melting of ProRS was barely affected by either proline or ATPa. In contrast, in the presence of HF, the melting curve was shifted to higher temperature by about 10 oC by HF alone, and by about 18 oC with HF plus ATPa (Fig 3a). These data show that HF binds even in the absence of ATP, and also confirm that HF binds much tighter in the presence of ATP. Not surprisingly, the thermal shift obtained with Pro-SA was the largest.
Figure 3. HF interacts with both the site for amino acid activation and the site for docking the 3′-end of tRNA.
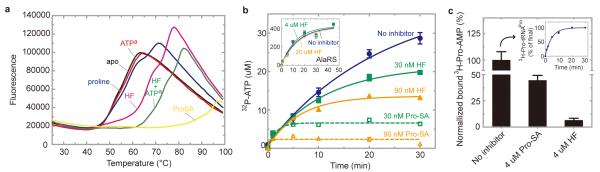
a, Thermal melting19 of ProRS in the presence of different ligands. HF binds in the absence of ATPa, and binds more strongly in the presence of ATPa. b, HF and the comparator Pro-SA block formation of Pro-AMP, in the proline-dependent ATP-PPi exchange reaction. The inset shows that HF had no effect on the alanine-dependent ATP-PPi exchange reaction with AlaRS. c, HF mobilizes the release of Pro-AMP from ProRS. The ProRS:3H-Pro-AMP complex was prepared on ice and then isolated on a column and for 10 min was left untreated, or exposed to HF, or exposed to Pro-SA, respectively. The complex was then re-run on the column and the amount of bound 3H-Pro-AMP was determined. The inset shows that the isolated 3H-Pro was activated (as 3H-Pro-AMP) and could be transferred to tRNA. Error bars are s.e.m. (n=2).
To probe the adenylate-formation site, we studied the first step of aminoacylation, which is the activation of proline by ProRS to form a tightly bound Pro-AMP adenylate complex and release of PPi.
The conventional proline-dependent ATP-PPi exchange reaction was used. As expected, both Pro-SA and HF were potent inhibitors of this reaction in a dose-dependent fashion, with Pro-SA being more effective (Fig. 3b). This result is consistent with the thermal melting data of Figure 3a showing the tighter binding of Pro-SA, and with our x-ray crystal structure showing the docking of one ‘head’ of HF at the site for the prolyl moiety of Pro-AMP.
Next, we investigated the ability of HF to mimic docking of A76 of tRNAPro to ProRS. For this purpose we studied the effect of HF on the relatively stable preformed ProRS:Pro-AMP complex, which was isolated on a column. Because binding of tRNA to tRNA synthetases is generally associated with conformational changes that mobilize the active site for transfer of the activated amino acid to A76, we reasoned that binding of the A76-mimicking quinazolinone group of HF would result in an abortive transfer and thereby release Pro-AMP from ProRS. Accordingly, the ProRS:Pro-AMP complex was incubated separately with Pro-SA (which should not have access to the A76 site) and with HF (without ATP). As expected, Pro-SA caused some reduction in the bound Pro-AMP, which is attributed to an exchange on the enzyme between bound Pro-AMP and Pro-SA. In contrast, free HF, while having a much lower affinity than Pro-SA for ProRS, completely released Pro-AMP from the complex (Fig. 3c).This result supports the conclusion (from our x-ray structure) that HF also mimics A76 of tRNA.
HF is a halogenated derivative of febrifugine, which is the bioactive constituent in the Chang Shan herb that has long been used to treat malaria. The human and malaria parasite P. falciparum ProRS binding sites are highly conversed (Supplementary Fig. 4). With that in mind, using our ATP-dependent docking of HF to human ProRS as a template, we were able to show the potential for docking of FF to P. falcipaurm ProRS (Fig. 4). Thus, our analysis suggests malarial ProRS may also be the target of FF. This possibility could also explain why the killing effect of FF on P. falciparum in culture is reversed by proline8.
Figure 4. FF blocks ProRS.
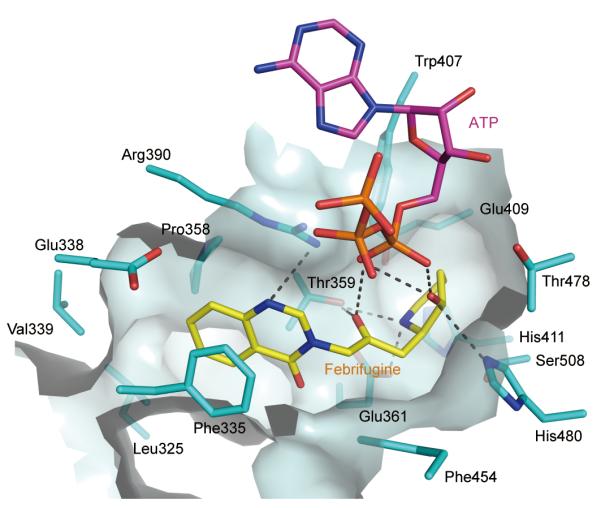
Febrifugine (FF) docked to the active site of P. falcipaurm ProRS (UniProtKB code Q8I5R7). ProRS and FF are colored as cyan and yellow, respectively. The hydrogen bonds are indicated with dashes. The P. falciparum ProRS structure was generated by the protein structure homology-modeling sever SWISS-MODEL20. FF was docked into the P. falciparum ProRS active site based on the structure of ProRS:halofuginone:ATPa complex, while ATP was docked based on the structure of the T. thermophilus ProRS:tRNA:ATP complex (PDB code 1H4Q).
Interestingly, in addition to the inhibition of amino acid activation by mimics of AA-AMP (including the “operational” mimic pseudomonic acid (mupirocin)17), the ATP-dependent two-site HF inhibition mechanism reported here, and the single-site tRNA-dependent editing-site-trapping mechanism reported earlier18, illustrate two entirely different and unusual mechanisms for substrate participation in capture of an inhibitor (Supplementary Fig. 5). In addition, by substitution of the piperidine ring with other amino acid mimics, dual site inhibitors could in principle be designed for some other aaRSs.
METHODS
Protein expression and purification
The cDNA fragment encoding human prolyl-tRNA synthetase (ProRS) was cloned into E.coli plasmid pET21a (Novagen, Darmstadt, Germany), with an additional N-terminal His6-tag. The plasmid encoding ProRS was transformed into E.coli strain BL21(DE3) pLysS (Life Technologies, San Diego, CA) and was induced overnight for overexpression with 0.5 mM IPTG at room temperature. The E.coli cell lysate was firstly loaded onto a Ni-NTA column (Qiagen, Valencia, CA), and the elution fraction was further purified with mono Q ion-exchange chromatography (GE Healthcare, 5/50 GL, Piscataway, NJ) to a single band as indicated on SDS-PAGE with Coomassie brilliant blue staining. The purified protein was found as a single peak with the elution volume consistent with a homogeneous ProRS homodimer on the Superdex 200 analytical gel filtration column (GE Healthcare, 10/300 GL). The purified ProRS was finally concentrated to 50 mg/mL and stored at −80°C in a buffer of 50 mM NaCl, 2 mM Tris (pH 8.0).
Crystallization and data collection
ProRS was crystallized with the sitting-drop vapor-diffusion method using a Mosquito robot (TTP Labtech, Cambridge, MA). Prior to crystallization, ProRS was diluted to 40 mg/mL with 3 mM halofuginone (racemic mixture) (Sigma-Aldrich, Saint Louis, MO), 5 mM ATP analog adenosine 5′-(β,γ-imido)triphosphate (Sigma-Aldrich), 5 mM MgCl2 and 5 mM β-mercaptoethanol, and then incubated on ice for 30 min. Next, sitting drops were set up by mixing 100 nL of protein (40 mg/mL) and 100 nL of reservoir solution containing 20% (w/v) PEG 3350, 0.6 M CaCl2 and 50 mM HEPES (pH 7.5), and equilibrated against a 70 μL of reservoir solution at 30°C for 2–3 days before data collection. The X-ray diffraction data were collected using a single crystal at a wavelength of 1.000 Å and temperature of 100 oK at beamline 7-1 of Stanford Synchrotron Radiation Lightsource (SSRL). The complete data set contains 500 images with the oscillation angle of 0.5° for each image. The ProRS:HF:ATPa crystal belonged to space group P21. Data were integrated and scaled with the program HKL200022.
Structure determination and refinement
The initial model was determined by molecular replacement using the program MOLREP23. The crystal structure of T. thermophilus ProRS (PDB code 1HC7), which has 43% sequence identity to human ProRS, was used as the searching model. The structure was further refined by iterative cycles of positional refinement and TLS refinement with Refmac524 and Phenix25 and model building with COOT26, and the final model was refined to 2.0 Å resolution with Rwork/Rfree of 20.4%/22.7%. The model has good geometry quality, and 98.9%/99.8% residues are in favored/allowed regions of the MolProbity27 Ramachandran plot. Statistics of data collection and structure refinement are given in Supplementary Table 1. The atomic coordinates and structural factors have been deposited into Protein Data Bank under the code 4HVC.
Sequence analysis and Structure Presentation
Protein sequences were aligned using the program Multalin28. The alignment result was submitted to ESPript29 for figure generation. All protein structure illustrations were prepared with the program PyMol (www.pymol.org).
Thermal Shift Assay
ProRS (3 μg) was diluted in 30 μL buffer containing 500 mM NaCl, 20 mM Tris pH 8.0, 1/2500 SYPRO Orange dye (Life Technologies) and different ligands (5 mM ATPa, 5 mM proline, 1 mM HF, 1 mM HF + 5 mM ATPa, or 1 mM Pro-SA, respectively), and incubated at room temperature for 10 min. Then, ProRS samples were heated from 25 to 98°C at a rate of 1°C/min, and the fluorescence signals were monitored by the StepOnePlus real-time PCR system (Life Technologies). Each curve is an average of three measurements.
ATP-pyrophosphate exchange assays
The reaction buffer contained 100 mM HEPES pH 7.5, 20 mM KCl, 10 mM MgCl2, 1 mM DTT, 2 mM ATP, 1 mM Na pyrophosphate (NaPPi), 20 μCi/ml 32P-PPi and 0.5 mM proline. Inhibitor HF and Pro-SA (5′-O-(N-(L-prolyl)-sulfamoyl)adenosine (kindly provided by Prof. Karin Musier-Forsyth, The Ohio State University) were added at different concentrations (0, 30 or 90 nM). The reactions were started by adding ProRS into buffer at the final concentration of 10 nM, and then incubated at 20°C. 20 μL of each of the reactions was quenched at different time points in 200 μL quench buffer (1 M HCl, 200 mM NaPPi, 4% charcoal). The free 32P-PPi was removed by flowing through the 96-well PVDF filter plates, and the residual 32P-PPi was washed five times using 200 μL washing buffer (1 M HCl and 200 mM NaPPi). Then, the produced 32P-ATP remaining in filter plates was transferred to scintillation vials and counted. The data were analyzed with GraphPad Prism (GraphPad Software, La Jolla, CA). The experiments here and below were independently repeated three times with duplicated measurements each time.
HF - Pro-AMP competition assay
The isolation of ProRS:Pro-AMP was slightly modified from that of a previous report30. In brief, 2 μM ProRS was added to the buffer containing 50 mM HEPES (pH 7.5), 20 mM KCl, 5 mM MgCl2, 2 mM ATP, 2 mM DTT, 20 μM proline and 1 μM 3H-proline, and then incubated at 4°C for 10 min to create ProRS:3H-Pro-AMP. The reaction containing ProRS:3H-Pro-AMP was flowed through a desalting column (SpinTrap G-25, GE healthcare) to isolate the complex and remove the extra ATP and proline. HF and Pro-SA were added at the concentration of 4 μM to the complex to compete for 10 min at 20°C. The reaction was passed through desalting column again to remove dissociated 3H-Pro-AMP. The eluted 3H-Pro-AMP still bound to ProRS was then measured.
Supplementary Material
Acknowledgement
We thank Prof. Karin Musier-Forsyth (The Ohio State University) for providing the ProRS gene and Pro-SA, staff at beamline 7-1 of Stanford Synchrotron Radiation Lightsource (SSRL) for assistance in X-ray diffraction data collection, and Prof. Min Guo (The Florida Scripps Research Institute) for comments. This work was supported by grants GM15539, 23562 and 88278 from the NIH and by a fellowship from the National Foundation for Cancer Research.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions H.Z. L.S., X.-L.Y. and P.S. designed the experiments. H.Z. and L.S. performed the experiments and all four authors analyzed the data. All authors discussed the results and H.Z. and P.S. wrote the manuscript.
Author Information Atomic coordinates and structure factors for the reported crystal structure have been deposited in the Protein Data Bank under accession code 4HVC Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
REFERENCES
- 1.Koepfli JB, Mead JF, Brockman JA., Jr. An alkaloid with high antimalarial activity from Dichroa febrifuga. J. Am. Chem. Soc. 1947;69:1837. doi: 10.1021/ja01199a513. [DOI] [PubMed] [Google Scholar]
- 2.Coatney GR, Cooper WC, Culwell WB, White WC, Imboden CA., Jr. Studies in human malaria. XXV. Trial of febrifugine, an alkaloid obtained from Dichroa febrifuga lour., against the Chesson strain of Plasmodium vivax. J. Natl. Malar. Soc. 1950;9:183–6. [PubMed] [Google Scholar]
- 3.Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol. Blood. Marrow Transplant. 2003;9:417–25. doi: 10.1016/s1083-8791(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 4.Pines M, Nagler A. Halofuginone: a novel antifibrotic therapy. Gen. Pharmacol. 1998;30:445–50. doi: 10.1016/s0306-3623(97)00307-8. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge MJ, et al. Phase I and pharmacokinetic study of halofuginone, an oral quinazolinone derivative in patients with advanced solid tumours. Eur. J. Cancer. 2006;42:1768–74. doi: 10.1016/j.ejca.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Koon HB, et al. Phase II AIDS Malignancy Consortium trial of topical halofuginone in AIDS-related Kaposi sarcoma. J. Acquir. Immune. Defic. Syndr. 2011;56:64–8. doi: 10.1097/QAI.0b013e3181fc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundrud MS, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–8. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller TL, et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012;8:311–7. doi: 10.1038/nchembio.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu. Rev. Nutr. 2005;25:59–85. doi: 10.1146/annurev.nutr.24.012003.132145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schimmel P, Tao J, Hill J. Aminoacyl tRNA synthetases as targets for new anti-infectives. FASEB J. 1998;12:1599–609. [PubMed] [Google Scholar]
- 11.Hill J. Aminoacyl sulfamides for the treatment of hyperproliferative disorders Cubist Pharmaceuticals, Inc. 5,824,657 U.S. Patent. 1998
- 12.Carter CW., Jr. Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 1993;62:715–48. doi: 10.1146/annurev.bi.62.070193.003435. [DOI] [PubMed] [Google Scholar]
- 13.Yaremchuk A, Tukalo M, Grotli M, Cusack S. A succession of substrate induced conformational changes ensures the amino acid specificity of Thermus thermophilus prolyl-tRNA synthetase: comparison with histidyl-tRNA synthetase. J. Mol. Biol. 2001;309:989–1002. doi: 10.1006/jmbi.2001.4712. [DOI] [PubMed] [Google Scholar]
- 14.Zhu S, et al. Synthesis and biological evaluation of febrifugine analogues as potential antimalarial agents. Bioorg. Med. Chem. 2009;17:4496–502. doi: 10.1016/j.bmc.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, et al. The structure of threonyl-tRNA synthetase-tRNA(Thr) complex enlightens its repressor activity and reveals an essential zinc ion in the active site. Cell. 1999;97:371–81. doi: 10.1016/s0092-8674(00)80746-1. [DOI] [PubMed] [Google Scholar]
- 16.Heacock D, Forsyth CJ, Shiba K, Musier-Forsyth K. Synthesis and aminoacyl-tRNA synthetase inhibitory activity of prolyl adenylate analogs. Bioorg. Chem. 1996;24:273–89. [Google Scholar]
- 17.Nakama T, Nureki O, Yokoyama S. Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-tRNA synthetase. J. Biol. Chem. 2001;276:47387–93. doi: 10.1074/jbc.M109089200. [DOI] [PubMed] [Google Scholar]
- 18.Rock FL, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–61. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 19.Pantoliano MW, et al. High-density miniaturized thermal shift assays as general strategy for drug discovery. J. Biomol. Screen. 2001;6:429–40. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- 20.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 21.Beebe K, et al. A universal plate format for increased throughput of assays that monitor multiple aminoacyl transfer RNA synthetase activities. Anal. Biochem. 2007;368:111–21. doi: 10.1016/j.ab.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–26. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 23.Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. J. Appl. Cryst. 1997;30:1022–25. [Google Scholar]
- 24.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta. Crystallogr. D. Biol. Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 25.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta. Crystallogr. D. Biol. Crystallogr. 2010;66:213–21. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta. Crystallogr. D. Biol. Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 27.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta. Crystallogr. D. Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–90. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–8. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- 30.Shi JP, Schimmel P. Aminoacylation of alanine minihelices. “Discriminator” base modulates transition state of single turnover reaction. J. Biol. Chem. 1991;266:2705–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.