Abstract
Intracerebral hemorrhage (ICH) accounts for 20% of all strokes and is the most devastating form across all stroke types. Lymphocytes have been shown to potentiate cerebral inflammation and brain injury after stroke. FTY720 (Fingolimod) is an immune-modulating drug that prevents the egress of peripheral lymphocytes from peripheral stores. We hypothesized that FTY720 would reduce peripheral circulating lymphocytes, resulting in reduced brain injury and improved functional outcomes. CD-1 mice were anesthetized and then injected with collagenase into the right basal ganglia. Animals were divided into three groups: sham, ICH + Vehicle, and ICH + FTY720, by the intra-peritoneal route at 1 h after ICH induction. Brain water content was measured at 24 and 72 h. Neurobehavioral tests included corner test, forelimb use asymmetry, paw placement, wire-hang test, beam balance test, and a Neuroscore. FTY720 significantly reduced brain edema and improved neurological function at all time points tested. Lymphocyte modulation with FTY720 is an effective neuroprotective strategy to reduce brain injury and promote functional recovery after ICH.
Keywords: Intracerebral hemorrhage, FTY720, Fingolimod, Lymphocytes, Neuroprotection, Brain edema, Neurological function
Introduction
Intracerebral hemorrhage (ICH) is a spontaneous bleeding event in the brain for which there are no effective therapies. ICH accounts for about 20% of all strokes and affects 1 in 6,000 people each year [1]. ICH results in a higher mortality rate than ischemic stroke, and 40–50% of patients in the United States die within the first 30 days [2].
The inflammatory response after ICH is a highly complex process that includes acute and delayed events requiring local and peripheral cellular cross-talk. Significant orchestrators of this inflammatory process are blood-derived immune cells, trafficking from the periphery into the brain parenchyma [3]. Monocyte and neutrophil infiltration, along with microglia and macrophage activation, occur in and around the hematoma within hours after ictus and peak within a few days [3, 4], leading to the production of proinflammatory mediators, such as TNF-α, IL-1β, and proteolytic enzymes, such as MMP-9. While the myeloid branch of the immune system includes the above cell types involved in inflammation, lymphoid cells also interact with resident and infiltrated cells in the CNS, potentiating the inflammatory response. T-cells have been shown to play a major role in the numerous neurological diseases, such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis [5–7], as well as the deleterious events following stroke [8]. It has been demonstrated that T-cells are significantly increased in the brain as early as 24 h after stroke [9]. These lymphocytes are responsible for further driving the local inflammatory reaction, leading to increased blood-brain barrier (BBB) permeability, edema formation, cell death, and neurological deficits following stroke.
FTY720 (Fingolimod), a sphingosine-1-phosphate(S1P) analog that is an agonist for S1P receptors (S1P1, 3, 4, and 5), has a half life of 20 h [10, 11]. FTY720’s immunosuppressive activity results from inhibition of S1P1 receptor-dependent lymphocyte egress mediated by downregulating S1P1 receptor on T-cells [12]. Therefore, a single administration of FTY720 causes peripheral lymphopenia. It has been shown to be effective in several phase II clinical trials at reducing the incidence of relapsing/remitting MS [13, 14] and for renal transplantation [15]. In a recent study in our laboratory, FTY720 administration was shown to be neuroprotective following 2 h middle cerebral artery occlusion in rats [16].
In the present study, we hypothesized that FTY720 administration following ICH would reduce brain edema and improve neurological function in mice. To test this hypothesis, we administered FTY720 to reduce circulating peripheral lymphocytes, and then evaluated brain edema and neurological deficits 24 and 72 h following collagenase-induced intracerebral hemorrhage.
Materials and Methods
Experimental Animal Preparation
All procedures for this study were approved by the Animal Care and Use Committee at Loma Linda University, and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Eight-week-old CD-1 mice were used in our study and were housed on a 12:12 light/dark cycle in a pathogen-free facility with controlled temperature and humidity, and were given food and water ad libitum.
Experimental Protocol
In total, 30 mice were used in this study. Mice were divided into three groups: sham (needle insertion), ICH + vehicle (ICH), and ICH + FTY720 treatment (1 mg/kg, i.p). FTY720 was dissolved in 2% DMSO and given 1 h after ICH induction. Both sham and vehicle animals received the same volume of i.p. 2% DMSO in saline. All animals were neurologically tested, and brains were harvested 24 and 72 h after ICH induction. Evaluation of neurological deficits was carried out by a blinded investigator. Brain samples were collected for measurement of brain edema.
Intracerebral Hemorrhage Model in Mice
ICH was induced by intrastriatal collagenase injection as described by Rosenberg et al. [17, 18]. Briefly, mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) (2:1 v/v), and positioned prone in a stereotactic head frame (Kopf Instruments, Tujunga, CA). A borehole (1 mm) was drilled near the right coronal suture 1.7 mm lateral to the midline. A 27-gauge needle was inserted stereotactically into the right basal ganglia according to the coordinates: 0.9 mm posterior to the bregma, 1.7 mm lateral to the midline, and 3.7 mm below the surface of the calvaria. Clostridal collagenase (VII-S, Sigma; 0.1 U in 0.5 µL of saline) was infused into the brain over 2 min at a rate of 0.25 µL/min with a microinfusion pump (Harvard Apparatus, Holliston, MA). Sham-operated mice were subjected to needle insertion only. The needle was left in place for an additional 10 min after injection to prevent possible leakage or backflow of the collagenase solution. After removing the needle, the bore hole was closed with bone wax, the incision closed with sutures, and mice were allowed to recover. To avoid postsurgical dehydration, 0.5 ml of normal saline was given subcutaneously to each mouse after surgery.
Testing Neurological Function
All neurological tests were performed during the light cycle. Neurological deficits were evaluated by using a modified Garcia test [19], beam balance and wire-hanging tests [20]. In the modified Garcia test, seven items, including spontaneous activity, side stroke, vibrissae touch, limb symmetry, lateral turning, forelimb walking, and climbing were tested (the total possible neurological score was 21 for a healthy mouse). For both the modified beam balance and wire-hanging test, the total possible score for healthy mice was five. The behavior testing was conducted at 24 and 72 h after ICH induction by a blinded investigator.
Measurement of Brain Edema
Brain water content was measured as previously described [18]. Briefly, mice were decapitated under deep anesthesia. Brains were immediately removed and cut into 4-mm sections. Each section was divided into four parts: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex. The cerebellum was used as an internal control. Tissue samples were weighed to obtain the wet weight (WW) and then dried at 100°C for 24 h to determine the dry weight (DW). Brain water content (%) was calculated as [(WW − DW)/WW] × 100.
Statistical Analysis
All the data were expressed as Mean ± SEM. Statistical differences were analyzed by one way-ANOVA Tukey test or Dunn’s test on rank. A P value of < 0.05 was considered statistically significant.
Results
Brain Water Content
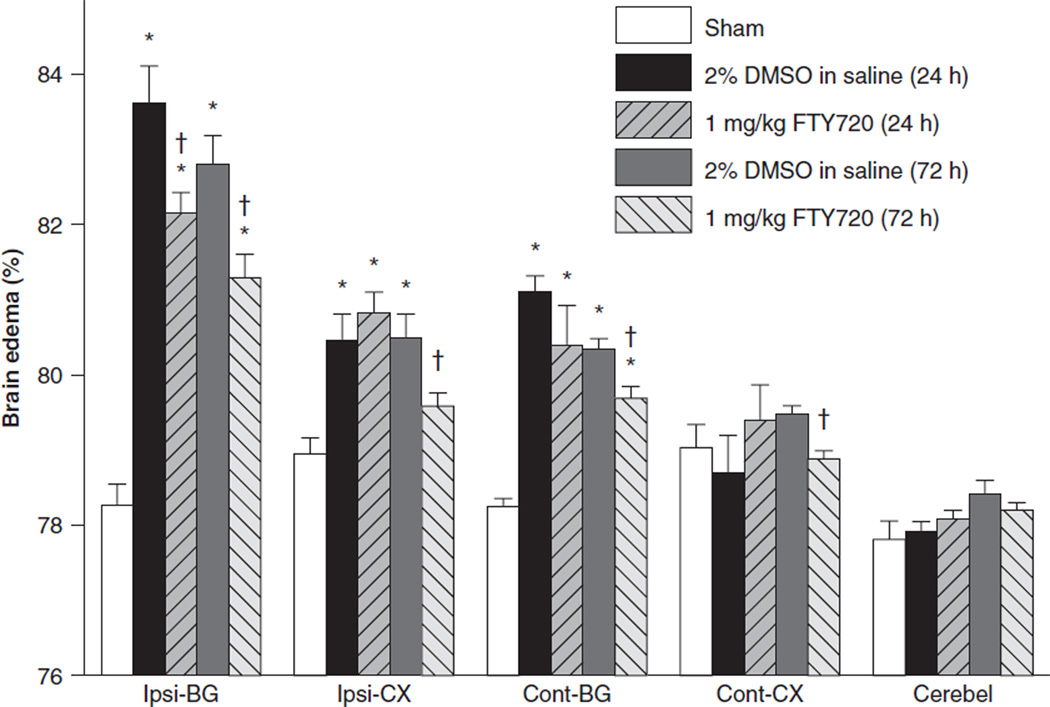
Brain water content was tested at 24 and 72 h after ICH (Fig. 1). There was a very significant increase in brain water content for the ICH + Vehicle group at both 24 and 72 h, respectively, in the ipsilateral basal ganglia (ICH + Vehicle[24 h], 83.62 ± 0.48 & ICH + Vehicle[72 h], 82.79 ± 0.41 vs. sham, 78.27 ± 0.24, P < 0.05) and cortex (ICH + Vehicle[24 h], 80.46 ± 0.36 & ICH + Vehicle[72 h], 80.48 ± 0.34 vs. sham, 78.96 ± 0.22, P < 0.05) compared to the sham group. Brain edema was also significantly increased in the contralateral basal ganglia for ICH + Vehicle at 24 and 72 h (ICH + Vehicle[24 h], 81.12 ± 0.20 and ICH + Vehicle[72 h], 80.34 ± 0.13 vs. Sham, 78.25 ± 0.11, P < 0.05) compared to the sham group. FTY720 treatment (1 mg/kg) significantly reduced brain edema in the ipsilateral basal ganglia at 24 and 72 h (ICH + FTY720[24 h], 82.16 ± 0.28 vs. ICH + Vehicle [24 h], 83.62 ± 0.48 and ICH + FTY720[72 h], 81.28±.33 vs. ICH + Vehicle[72 h], 82.79 ± 0.41, P < 0.05) and in the contralateral basal ganglia at 72 h after ICH (ICH + FTY720 [72 h], 79.70±.15 vs. ICH + Vehicle[72 h], 80.34±.13). FTY720 treatment also significantly reduced brain edema in the ipsilateral cortex (ICH + FTY720[72 h], 79.59 ± 0.17 vs. ICH + Vehicle[72 h], 80.48 ± 0.34, P < 0.05) and contralateral cortex at 72 h after ICH (ICH + FTY720[72 h], 78.89 ± 0.09 vs. ICH + Vehicle[72 h], 79.± 0.11).
Fig. 1.
FTY720 treatment improved brain edema at 24 and 72 h after ICH. Cont-CX, contralateral cortex; Ipsi-CX, ipsilateral cortex; Cont-BG, contralateral basal ganglia; Ipsi-BG, ipsilateral basal ganglia; Cerebel, cerebellum. Values are expressed as mean ± SEM. *P < 0.01 vs. Sham; †P < 0.05 vs. Vehicle
Neurobehavioral Testing and Neurological Function
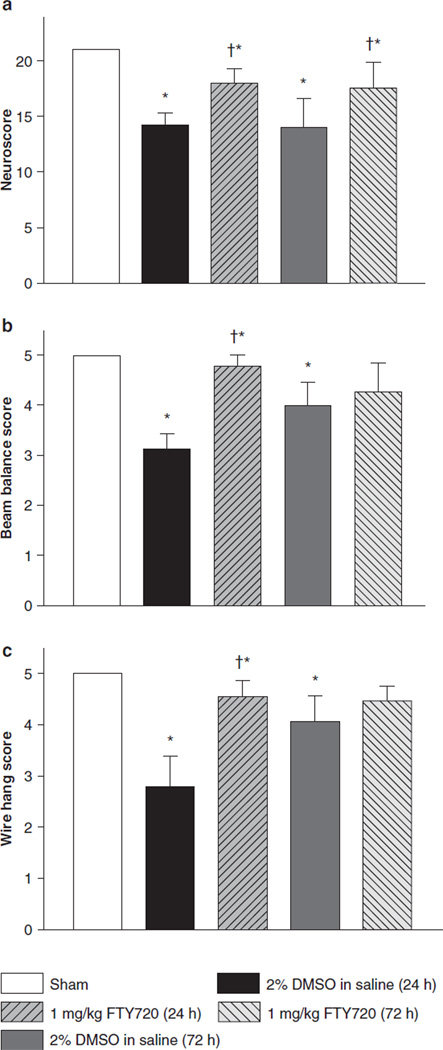
Neurobehavioral evaluations were completed at 24 and 72 h after ICH induction by using the modified Garcia test, wire-hanging test, and beam balance test (Fig. 2a–c). At 24 and 72 h after ICH, the ICH + Vehicle group demonstrated significant deficits compared to the sham group for the modified Garcia test, wire-hanging test, and beam walking test (P < 0.05). After FTY720 treatment, the Garcia neurological score was significantly improved at both time points (P < 0.05). FTY720 treatment significantly improved performance on the beam balance and wire-hanging tests at 24 h after ICH (P < 0.05) and showed a trend to improve performance at 72 h after ICH; however, we did not find significant results.
Fig. 2.
FTY720 treatment improved neurological function at 24 and 72 h after ICH. (a) Modified Garcia test; (b) wire-hanging test; (c) beam balance test. Values are expressed as mean ± SEM. *P < 0.01 vs. Sham; †P < 0.05 vs. Vehicle
Discussion
Hemorrhagic stroke is the most fatal stroke type, and currently there is no effective treatment. The extravasated blood and blood components trigger a complex series of events leading to the production of inflammatory mediators and the infiltration of a wide variety of immune cells, resulting in edema formation, cell death, and permanent neurological deficits. The present study used a clinically relevant immunosuppressant that is already in clinical trials and has been submitted for regulatory approval in the US and EU for oral administration to treat MS patients. We found that administration of FTY720 reduced brain edema, improved neurological function, and offered neuroprotection after ICH. This is the first study to demonstrate the neuroprotective effect of FTY720 in a mouse intracerebral hemorrhage model.
Although T-cells are not the largest population of immune cells in the brain after stroke, their presence is certainly important. Inhibition of T-cell activation in rats with FK-506 and knockout mice for CD18 (a lymphocyte-associated adhesion molecule) leads to decreased cell death and improved functional outcome in the days and weeks following ICH [21, 22]. FTY720 binds to four of the five known S1P receptors and has been repeatedly shown to induce peripheral lymphopenia when administered at doses around 0.1 mg/kg [10, 23]. Our dosage of 1 mg/kg can be safely assumed to be causing the same effect in mice, which will reduce the amount of T-cells available for trafficking into the brain after ICH. This reduction in T-cells available for trafficking in effect reduces the driving force behind inflammation, leading to reduced brain edema and neurological deficits.
FTY720 has been shown to have an endothelial barrier enhancement capacity by inhibiting VEGF induced vascular permeability in vitro and in vivo [24]. Moreover, FTY720 has been shown to induce the translocation of vascular endothelial cadherin and β-catenin, via S1P1 or S1P3 activation, to the focal contacts between endothelial cells, promoting adherens junction assembly [25]. Lastly, it has been demonstrated that activation of S1P1 with FTY720 induces anti-apoptotic signaling, rescuing cells from the brink of death. In a recent study in our laboratory, FTY720 treatment was shown to increase the levels of phosphor-Akt, phospho-Erk, and Bcl-2 as well as reduced cleaved caspase-3 after middle cerebral artery occlusion in rats.
In summary, FTY720 post-treatment reduced the brain edema and improved neurological function after intracerebral hemorrhage in mice. This result may be explained by its anti-inflammatory effect.
Acknowledgement
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
William B. Rolland, II, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Anatol Manaenko, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Tim Lekic, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Yu Hasegawa, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Robert Ostrowski, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Jiping Tang, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
John H. Zhang, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Physiology and Pharmacology, Loma Linda University, School of Medicine, Risley Hall, Room 219, Loma Linda, CA 92350, USA and Department of Anesthesiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA jhzhang@llu.edu
References
- 1.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 2.James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2008;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 4.Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. discussion 2477–2478. [DOI] [PubMed] [Google Scholar]
- 5.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray- Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith D. Interferon-beta therapy for multiple sclerosis–is the injection site the relevant action site? J Neuroimmunol. 2009;215:117–121. doi: 10.1016/j.jneuroim.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 8.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 9.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 11.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(−4-octylphenyl)ethyl]propane-1, 3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–1487. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- 12.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 15.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, Vanrenterghem Y. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2004;77:1826–1833. [PubMed] [Google Scholar]
- 16.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 19.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 20.Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeling J, Yan HJ, Corbett D, Xue M, Del Bigio MR. Effect of FK-506 on inflammation and behavioral outcome following intracerebral hemorrhage in rat. Exp Neurol. 2001;167:341–347. doi: 10.1006/exnr.2000.7564. [DOI] [PubMed] [Google Scholar]
- 22.Titova E, Ostrowski RP, Kevil CG, Tong W, Rojas H, Sowers LC, Zhang JH, Tang J. Reduced brain injury in CD18-deficient mice after experimental intracerebral hemorrhage. J Neurosci Res. 2008;86:3240–3245. doi: 10.1002/jnr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in ratsIFTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 24.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]