Abstract
Background
Germinal matrix hemorrhage (GMH) is a devastating neurological disorder of very low birth weight premature infants that leads to post-hemorrhagic hydrocephalus, cerebral palsy, and mental retardation. Melatonin is a potent antioxidant known to reverse free-radical mediated injury in the brain. This study investigated the effect of melatonin treatment after GMH injury.
Methods
Clostridial collagenase was infused into the right germinal matrix region of neonatal rats with stereotaxic technique. Cognitive function, sensorimotor ability, cerebral, cardiac and splenic growths were measured in juvenile animals.
Results
Systemic melatonin treatment ameliorated cognitive and sensorimotor dysfunction at the juvenile developmental stage. This hormone also normalized brain atrophy, splenomegaly, and cardiac hypertrophy consequences at 1 month after injury.
Conclusion
This study supports the role of free radicals in acute neonatal hemorrhagic brain injury. Melatonin is an effective antioxidant that can protect the infant’s brain from the post-hemorrhagic consequences of mental retardation and cerebral palsy. Further mechanistic studies are warranted to determine the mechanisms behind these neuroprotective effects.
Keywords: Melatonin, Neurological deficits, Stroke, Experimental
Introduction
Germinal matrix hemorrhage (GMH) is a clinical condition of very low birth weight (VLBW ≤ 1,500 g) premature neonates in which immature blood vessels rupture within the anterior caudate (sub-ventricular) brain region during the first 7 days of life [1, 2]. This affects approximately 3.5/1,000 births in the United States each year [3]. The consequences of this brain injury are hydrocephalus (post-hemorrhagic ventricular dilation), developmental delay, and a lifetime of cerebral palsy and mental retardation [4, 5]. Although this is an important disease, experimental studies investigating thearapeutic modalities are lacking [6].
Interventions targeting free-radical mechanisms have been shown to be neuroprotective after brain hemorrhage in adult rats [7-11]. Thrombin is released from the clot, and erythrocytes undergo lysis to release the neurotoxins hemoglobin, heme, and iron [12-14]. These will, in turn, diffusely oxidatively damage proteins, lipid, and DNA within the first day after brain hemorrhage [15-21]. Melatonin is a potent antioxidant and free-radical scavenger [22-24] shown to inhibit free-radical-associated red blood cell lysis [25], hemoglobin degradation [26], neuronal cell death [27], and hippocampal and nigrostriatal degeneration [28].
In light of this evidence, we hypothesized that melatonin can be a reasonable therapeutic modality for the amelioration of hemorrhage-mediated free-radical brain injury mechanisms in neonatal rats. This intervention could improve juvenile cognitive and sensorimotor outcomes after neonatal germinal matrix hemorrhage.
Methods and Materials
Animal Groups and General Procedures
This study was in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Timed pregnant Sprague-Dawley rats were housed with food and water available ad libitum. Treatment consisted of melatonin (Sigma Aldrich) dissolved in 10% ethanol and diluted with 0.9% normal saline. This was administered (I.P.) at 60 min after collagenase infusion using treatment dosages of 5 mg/kg and 10 mg/kg. Postnatal day 7 (P7) pups were blindly assigned to the following (n = 8/group): sham (naive), needle (control), GMH (collagenase-infusion), GMH + 5 mg/kg melatonin, and GMH + 10 mg/kg melatonin. All groups were evenly divided within each litter.
Experimental Model of GMH
Using an aseptic technique, rat pups were gently anaesthetized with 3% isoflurane (in mixed air and oxygen) while placed prone on a stereotaxic frame. Betadine sterilized the surgical scalp area, which was incised in the longitudinal plane to expose the skull and reveal the bregma. The following stereotactic coordinates were determined: 1 mm (anterior), 1.5 mm (lateral), and 3.5 mm (ventral) from the bregma. A bore hole (1 mm) was drilled, into which a 27-gauge needle was inserted at a rate of 1 mm/min. A microinfusion pump (Harvard Apparatus, Holliston, MA) infused 0.3 units of clostridial collagenase VII-S (Sigma, St Louis, MO) through a Hamilton syringe. The needle remained in place for an additional 10 min after injection to prevent “back-leakage.” After needle removal, the burr hole was sealed with bone wax, the incision sutured closed, and the animals allowed to recover. The entire surgery took on average 20 min. Upon recovering from anesthesia, the animals were returned to their dams. Needle controls consisted of needle insertion alone without collagenase infusion, while naïve animals did not receive any surgery.
Cognitive Measures
Higher order brain function was assessed during the third week after collagenase infusion. The T-Maze assessed short-term (working) memory [29]. Rats were placed into the stem (40 cm × 10 cm) of a maze and allowed to explore until one arm (46 cm × 10 cm) was chosen. From the sequence of ten trials, of left and right arm choices, the rate of spontaneous alternation (0% = none and 100% = complete, alternations/trial) was calculated, as routinely performed [30, 31]. The Morris water maze assessed spatial learning and memory on four daily blocks, as described previously in detail [11, 32]. The apparatus consisted of a metal pool (110 cm diameter), filled to within 15 cm of the upper edge, with a platform (11 cm diameter) for the animal to escape onto, which changed location for each block (maximum = 60 s/trial), and data were digitally analyzed by Noldus Ethovision tracking software. Cued trials measured place learning with the escape platform visible above water. Spatial trials measured spatial learning with the platform submerged, and probe trials measured spatial memory once the platform was removed. For the locomotor activity, in an open field, the path length in open-topped plastic boxes (49 cm long, 35.5 cm wide, 44.5 cm tall) was digitally recorded for 30 min and analyzed by Noldus Ethovision tracking software [32].
Sensorimotor Function
At 4 weeks after collagenase infusion, the animals were tested for functional ability. Neurodeficit was quantified using a summation of scores (maximum = 12) given for (1) postural reflex, (2) proprioceptive limb placing, (3) back pressure towards the edge, (4) lateral pressure towards the edge, (5) forelimb placement, and (6) lateral limb placement (2 = severe, 1 = moderate, 0 = none), as routinely performed [30]. For the rotarod, striatal ability was assessed using an apparatus consisting of a horizontal, accelerated (2 rpm/5 s), rotating cylinder (7 cm-diameter × 9.5 cm-wide) requiring continuous walking to avoid falling recorded by the photo-beam circuit (Columbus Instruments) [11, 32]. For foot fault, the number of complete limb missteps through the openings was counted over 2 min while exploring over an elevated wire (3 mm) grid (20 cm × 40 cm) floor [31].
Assessment of Treatment upon Cerebral and Somatic Growth
At the completion of experiments, the brains were removed and hemispheres separated by midline incision (loss of brain weight has been used as the primary variable to estimate brain damage in juvenile animals after neonatal brain injury [33]). For organ weights, the spleen and heart were separated from surrounding tissue and vessels. The quantification was performed using an analytical microbalance (model AE 100; Mettler Instrument Co., Columbus, OH) capable of 1.0 μg precision.
Statistical Analysis
Significance was considered at P < 0.05. Data were analyzed using analysis of variance (ANOVA) with repeated measures (RM-ANOVA) for long-term neurobehavior. Significant interactions were explored with conservative Scheffé post hoc and Mann-Whitney rank sum test when appropriate.
Results
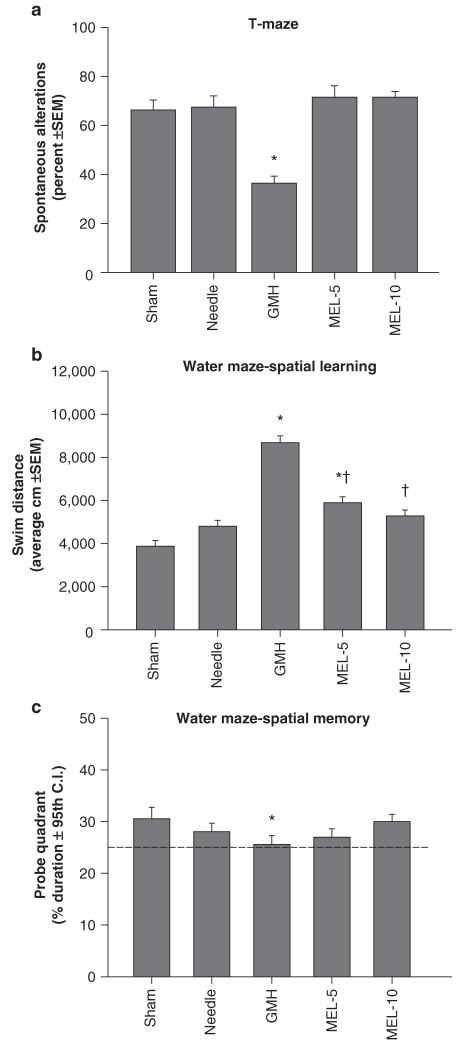
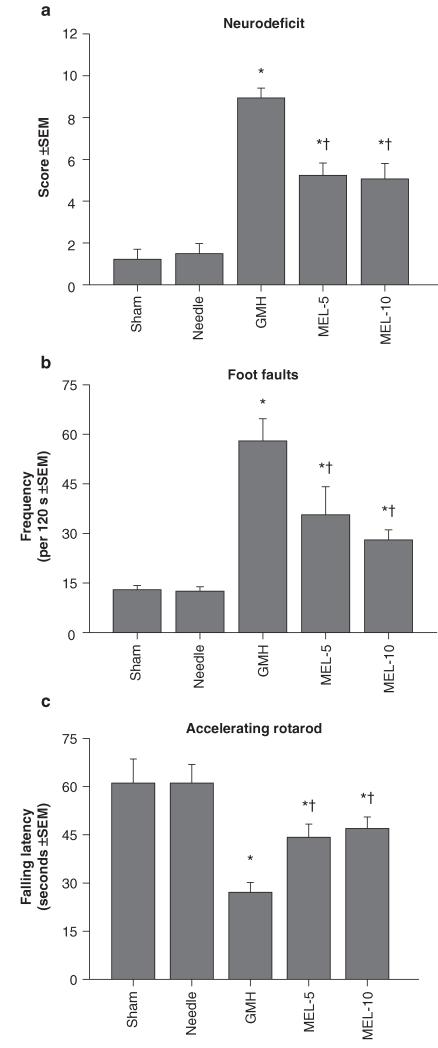
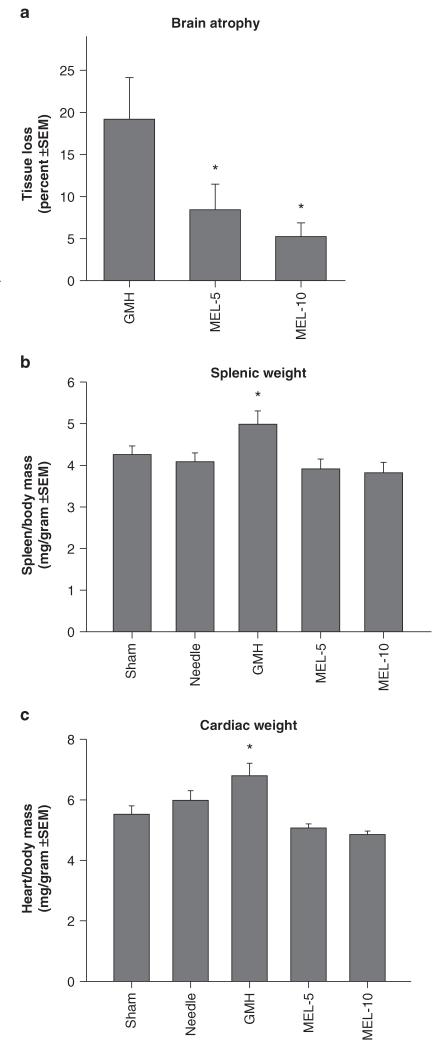
Collagenase infusion led to significant cognitive dysfunction in the T-maze (working) memory and water maze (spatial) learning and memory (Figs. 1a–c, P < 0.05). Melatonin treatment normalized T-maze deficits (Fig. 1a, P > 0.05 compared to controls) and water maze (spatial) learning deficits (Fig. 1b, P < 0.05 compared to GMH), without improving spatial memory (Fig. 1c, P > 0.05). Both doses of melatonin also normalized (P < 0.05) the significant sensorimotor dysfunction (compared to juvenile GMH animals), demonstrated by the neurodeficit score, number of foot faults, and accelerating rotarod falling latency (Figs. 2a–c, P < 0.05). Broad cytoprotection by melatonin was confirmed by the improvement upon brain atrophy (Fig. 3a, P < 0.05 compared to GMH), and normalization of peripheral splenomegaly and cardiomegaly (Fig. 3b and c, P > 0.05 compared to controls) at 4 weeks after injury.
Fig. 1.
Cognitive function normalization in juvenile rats by melatonin (MEL) after neonatal GMH. Higher order function was measured at the third week after collagenase infusion: (a) T-maze, (b) spatial learning water maze, (c) spatial memory (Probe) water maze. Values expressed as mean ± 95th CI (probe quadrant) or mean ± SEM (all others), n = 8 (per group), *P < 0.05 compared with controls (sham and needle trauma), and †P < 0.05 compared with GMH
Fig. 2.
Sensorimotor function normalization in juvenile rats by melatonin (MEL) after neonatal GMH. Cerebral palsy measurements were performed in the juveniles at 1 month after collagenase infusion: (a) neurodeficit score, (b) foot faults and (c) rotarod. Values expressed as mean ± SEM, n = 8 (per group), *P < 0.05 compared with controls (sham and needle trauma), and †P < 0.05 compared with GMH
Fig. 3.
Cerebral and somatic growth normalization in juvenile rats by melatonin (MEL) after GMH. (a) Brain atrophy (percent tissue loss), (b) splenic weight, and (c) cardiac weight were measured at 4 weeks after injury. Values expressed as mean ± SEM, n = 8 (per group), *P < 0.05 compared with controls (sham and needle trauma)
Discussion
These results indicate that systemic melatonin treatment after neonatal injury can reduce long-term brain atrophy, and return sensorimotor and cognitive function to near-normal levels in juvenile animals. In support of the findings from others, these outcomes provide preliminary evidence about the importance of oxidative stress mechanisms on outcomes after neonatal GMH [7, 8, 10].
Beyond the improvements in sensorimotor function and brain atrophy, the cognitive normalization by melatonin could have mechanistic benefits beyond reductions of periventricular free radical injury. Hippocampal neurons have receptors for melatonin [34, 35], upon which can be modulated excitability, synaptic transmission, and plasticity [35-38]. These targets could augment melatonin’s neuroprotective effects beyond a reduction of oxidative stress alone [38-44]. Mechanistic studies can investigate these processes further, as a window of opportunity for lasting neuroprotection after neonatal GMH.
Melatonin is a widely tested neuroprotectant shown to ameliorate brain injury in adult animal models of cerebral ischemia [45] and hemorrhage [11]. This study supports the notion that the application of melatonin has no adverse affects in neonatal rats and can lead to improvements in functional outcomes after brain injury from hemorrhagic stroke in premature infants as well.
Acknowledgements and Funding
This study was partially supported by a grant (NS053407) from the National Institutes of Health to J.H. Zhang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Tim Lekic, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Anatol Manaenko, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
William Rolland, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Kelly Virbel, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Richard Hartman, Department of Psychology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
Jiping Tang, Department of Physiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA.
John H. Zhang, Department of Anesthesiology, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda University, School of Medicine, Loma Linda, CA, USA and Department of Physiology, Loma Linda University, School of Medicine, Risley Hall, Room 223, 92354 Loma Linda, CA, USA
References
- 1.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. doi:10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–1090. doi: 10.1007/s00381-006-0050-6. doi:10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 3.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. doi:peds.2009-2416 [pii] 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. doi:10.1016/j.nbd.2003.12.016 S0969996103002833 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21:365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 7.Peeling J, Del Bigio MR, Corbett D, Green AR, Jackson DM. Efficacy of disodium 4-[(tert-butylimino)methyl]benzene-1, 3-disulfonate N-oxide (NXY-059), a free radical trapping agent, in a rat model of hemorrhagic stroke. Neuropharmacology. 2001;40:433–439. doi: 10.1016/s0028-3908(00)00170-2. doi:S0028390800001702 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Peeling J, Yan HJ, Chen SG, Campbell M, Del Bigio MR. Protective effects of free radical inhibitors in intracerebral hemorrhage in rat. Brain Res. 1998;795:63–70. doi: 10.1016/s0006-8993(98)00253-4. doi:S0006-8993(98)00253-4 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Peeling J, Yan HJ, Corbett D, Xue M, Bigio MR. Effect of FK-506 on inflammation and behavioral outcome following intracerebral hemorrhage in rat. Exp Neurol. 2001;167:341–347. doi: 10.1006/exnr.2000.7564. doi:10.1006/exnr.2000.7564 S0014-4886(00)97564-2 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, Nagao S, Xi G, Keep RF, Itano T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39:463–469. doi: 10.1161/STROKEAHA.107.486654. doi:STROKEAHA.107.486654 [pii] 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- 11.Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27:627–637. doi: 10.1089/neu.2009.1163. doi:10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. doi:10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. doi:10.1161/01.STR.0000103140.52838.4501STR.0000103140.52838.45 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. doi:S1474-4422(05)70283-0 [pii] 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 15.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. doi:S0006-8993(05)00104-6 [pii] 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neuro surg. 1996;84:91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- 18.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 19.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Keep RF, Hua Y, Nagao S, Hoff JT, Xi G. Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:194–198. doi: 10.1007/3-211-30714-1_42. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. doi:10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 22.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. doi:JPI407 [pii] 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 23.Cervantes M, Morali G, Letechipia-Vallejo G. Melatonin and ischemia-reperfusion injury of the brain. J Pineal Res. 2008;45:1–7. doi: 10.1111/j.1600-079X.2007.00551.x. doi:JPI551 [pii] 10.1111/j.1600-079X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 24.Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235–246. doi: 10.1111/j.1600-079X.2008.00580.x. doi:JPI580 [pii] 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 25.Tesoriere L, D’Arpa D, Conti S, Giaccone V, Pintaudi AM, Livrea MA. Melatonin protects human red blood cells from oxidative hemolysis: new insights into the radical-scavenging activity. J Pineal Res. 1999;27:95–105. doi: 10.1111/j.1600-079x.1999.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 26.Tesoriere L, Allegra M, D’Arpa D, Butera D, Livrea MA. Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J Pineal Res. 2001;31:114–119. doi: 10.1034/j.1600-079x.2001.310204.x. doi:jpi310204 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Hayter CL, Bishop GM, Robinson SR. Pharmacological but not physiological concentrations of melatonin reduce iron-induced neuronal death in rat cerebral cortex. Neurosci Lett. 2004;362:182–184. doi: 10.1016/j.neulet.2004.02.024. doi:10.1016/j.neulet.2004.02.024 S0304394004002083 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Lin AM, Ho LT. Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic Biol Med. 2000;28:904–911. doi: 10.1016/s0891-5849(00)00169-6. doi:S0891-5849(00)00169-6 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. doi:S0149-7634(04)00073-9 [pii] 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. doi:10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. doi:S0006-8993(09)00520-4 [pii] 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. doi:S0006-8993(09)00957-3 [pii] 10.1016/j.brainres. 2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- 34.Morgan PJ, Barrett P, Howell HE, Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 35.Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. doi:10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- 36.Wan Q, Man HY, Liu F, Braunton J, Niznik HB, Pang SF, Brown GM, Wang YT. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–403. doi: 10.1038/8062. doi:10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 37.Hogan MV, El-Sherif Y, Wieraszko A. The modulation of neuronal activity by melatonin: in vitro studies on mouse hippocampal slices. J Pineal Res. 2001;30:87–96. doi: 10.1034/j.1600-079x.2001.300204.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. doi:EJN4408 [pii] 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorfine T, Zisapel N. Melatonin and the human hippocampus, a time dependent interplay. J Pineal Res. 2007;43:80–86. doi: 10.1111/j.1600-079X.2007.00446.x. doi:JPI446 [pii] 10.1111/j.1600-079X.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 40.Bob P, Fedor-Freybergh P. Melatonin, consciousness, and traumatic stress. J Pineal Res. 2008;44:341–347. doi: 10.1111/j.1600-079X.2007.00540.x. doi:JPI540 [pii] 10.1111/j.1600-079X.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 41.Talaei SA, Sheibani V, Salami M. Light deprivation improves melatonin related suppression of hippocampal plasticity. Hippocampus. 2009 doi: 10.1002/hipo.20650. doi:10.1002/hipo.20650. [DOI] [PubMed] [Google Scholar]
- 42.Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. doi:10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- 43.Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005;1046:187–194. doi: 10.1016/j.brainres.2005.04.011. doi:S0006-8993(05)00549-4 [pii] 10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, Imbesi M, Manev H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. doi:S0304-3940(05)01098-0 [pii] 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 45.Macleod MR, O’Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. doi:JPI172 [pii] 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]