Abstract
Objective
There is mounting evidence suggesting that arginine vasopressin via its V1a receptor interaction is involved in the regulation of the brain water channel, aquaporin-4 (AQP4). The role of AQP4 in brain edema resolution has been thoroughly investigated in knock-out animal studies, which showed that its depletion increases brain water content in models of vasogenic edema. As a result, we tested the hypothesis that the activation of V1a receptor by it selective agonist will decrease brain edema in a mouse intracerebral hemorrhage (ICH) model.
Materials and Methods
ICH was induced by injection of bacterial collagenase into the right basal ganglia in CD1 male mice (weight 30–35 g). The animals were divided into the following groups: sham, ICH + vehicle, and ICH + AVP V1a receptor agonist. Brain edema and neurological outcomes were evaluated at 24 and 72 h post-ICH.
Results
We found that collagenase injection increased brain edema and resulted in subsequent neurobehavioral deficits at both time points. Treatment with our agonist had no effect on the ICH outcomes at both time points.
Conclusions
Our results suggest that the activation of the V1a receptor has no beneficial effect on secondary brain injury following ICH in mice.
Keywords: Arginine vasopressin, Brain edema, ICH, Neuroprotection
Introduction
Development of perihematomal brain edema is one of the most life-threatening events following an intracerebral hemorrhage (ICH). Two types of edema can present after ICH – namely vasogenic and cytotoxic edema. Vasogenic edema occurs predominately as a result of BBB disruption, whereas cytotoxic edema refers to the intracellular accumulation of water that is released following death. Today, despite promising basic science research aimed at reducing and/or eliminating cerebral edema, bench work has not been translated into clinical improvements. This is partly due to the lack of clear understanding behind the pathophysiology of edema evolution.
Arginine vasopressin (AVP) is a cyclic non-peptide that regulates water and electrolyte homeostasis in the body [1]. Niermann et al. demonstrated that AVP via its G protein-coupled receptor V1a can modulate the most abundant bidirectional water channel in the brain, called aquaporin-4 (AQP4) [2]. Aquaporins play an important role in the maintenance of tissue water homeostasis. Specifically AQP-4, the most abundant water channel in the brain, has been implicated in a number of brain injury-induced cerebral edema cases [3–5]. AQP-4 is an integral membrane protein; it is located in the blood-brain interface and regulates the flow of water in and out of the membrane [6]
In this study, we investigated the role of AVP V1a receptor activation and its effects on cerebral edema and neurobehavioral functioning after intracerebral hemorrhage (ICH). We hypothesized that treatment with a selective V1a agonist will ameliorate the brain water increase and improve neurobehavioral deficits in mice after ICH injury.
Materials and Methods
This study was in accordance with the guidelines of the National Institute of Health for the treatment of animals and was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Male CD1 mice (weight 35–45 g, Charles River, MA) were housed in a 12-h light/dark cycle at controlled temperature and humidity with free access to food and water. Mice were divided into the following groups: sham (n = 5), ICH (n = 5), and ICH treated with agonist (NC1900 10 ng/kg0; n = 5),
Operative Procedure
The collagenase-induced ICH model was adapted as previously described in mice [7]. Briefly, mice were anesthetized intrapertioneally with a ketamine (100 mg/kg)/xylazine (10 mg/kg) cocktail, and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL). An electronic thermostat-controlled warming blanket was used to maintain the core temperature at 37°C. The calvarium was exposed by a midline scalp incision from the nose to the superior nuchal line, and the skin was retracted laterally. With a variable speed drill (Fine Scientific Tools, Foster City, CA), a 1.0-mm burr hole was made 0.9 mm posterior to the bregma and 1.45 mm right-lateral to the midline. A 26-G needle on a Hamilton syringe was inserted with stereotaxic guidance 4.0 mm into the right deep cortex/basal ganglia at a rate of 1 mm/min. Collagenase (0.075 units in 0.5 μL saline; Sigma, St Louis, MO) was then infused into the brain at a rate of 0.25 μL/min over 2 min using an automatic infusion pump (Stoelting, Wood Dale, IL). The needle was left in place for an additional 10 min after injection to prevent the possible leakage of collagenase solution. After removal of the needle, the incision was sutured closed, and mice were allowed to recover. Sham operation was performed with needle insertion only.
Treatment Method
The agonist (NC1900) was kindly provided by Nippon Chemiphar Co., Ltd., Japan. NC1900 (10 ng/kg) was dissolved in saline and administrated as a single dose subcutaneously 1 h after ICH.
Brain Water Content
Brain water content was measured as previously described [7]. Briefly, rats were sacrificed at 24 and 72 h post ICH, and brains were immediately removed and divided into five parts: ipsilateral frontal, contralateral frontal, ipsilateral parietal, contralateral parietal, and cerebellum. The cerebellum was used as an internal control for brain water content. Tissue samples were then weighed on an electronic analytical balance (APX-60, Denver Instrument; Arvada, CO) to the nearest 0.1 mg to obtain the wet weight (WW). The tissue was then dried at 105°C for 48 h to determine the dry weight (DW). The percent brain water content was calculated as [(WW − DW)/WW] × 100.
Assessment of Neurobehavioral Deficits
Neurobehavioral deficits were assessed by a blind observer at 24 and 72 h post ICH using the Modified Garcia Score [15]. The Modified Garcia Score is a 21-point sensorimotor assessment system consisting of seven tests with scores of 0–3 for each test (maximum score = 21). These seven tests included: (1) spontaneous activity, (2) side stroking, (3) vibris touch, (4) limb symmetry, (5) climbing, (6) lateral turning, and (7) forelimb walking.
Additionally, beam balance and wire hang testing were performed. Both the beam (590 cm in length by 51 cm in width) and wire (550 cm in length by 51 mm in width) were constructed and held in place by two platforms on each side. Mice were put on the center of the beam or wire and allowed to reach the platform. Mice were observed for both their time and behavior until they reached one platform and scored according to six grades. The test was repeated three times, and an average score was taken [minimum score 0; maximum score (healthy mouse)] [9].
Western Blotting of Aquaporin-4
Animals were perfused with 0.1 M PBS at 72 h post-ICH. The peri-hematomal region was then isolated and snap-frozen in liquid nitrogen for analysis. Individual protein samples (50 μg each) were subjected to electrophoresis and then transferred to a nitro-cellulose membrane for 80 min at 70 V (Bio-Rad). Blotting membranes were incubated for 2 h with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 and then incubated overnight at 4°C with primary antibody (anti-aquaporin 4) (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were incubated for 1 h with secondary antibodies (1:1,000; Santa Cruz Biotechnology) and processed with an enhanced chemiluminescent reagent kit (Amersham Bioscience, Arlington Heights, IL) on X-ray film (Kodak, Rochester, NY).
Statistical Analysis
Quantitative data were expressed as the mean ± SEM. One-way ANOVA and Tukey test were used to determine significance in differences between the means. Neurological scores were evaluated using the Dunn method. A p-value < 0.05 was considered statistically significant.
Results
AVP V1a receptor activation has no effect on cerebral edema after ICH injury
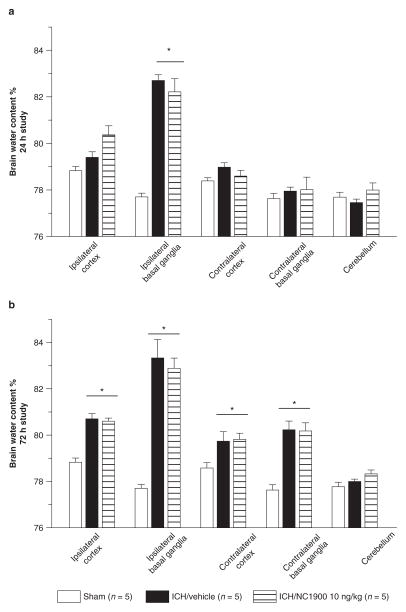
Vehicle groups demonstrated a consistently elevated level of cerebral edema in the ipsilateral hemisphere compared to the sham animals (p < 0.05). Activation of the AVP V1a receptor using NC1900 was not able to reduce the cerebral edema at 24 and 72 h post injury (Fig. 1).
Fig. 1.
All animals after collagenase injection have a significant increase of brain water content. NC1940 has no effect on brain edema at 24 h (a) and 72 h (b) as well. *Significant difference vs. sham (p < 0.05); (a) 24 h: sham = 5; (vehicle) = 5; (ICH + 10 ng/kg NC1900); (b) 72 h: sham = 5; (vehicle) = 5; (ICH + 10 ng/kg NC1900)
AVP V1a receptor activation does not improve neurobehavioral deficits
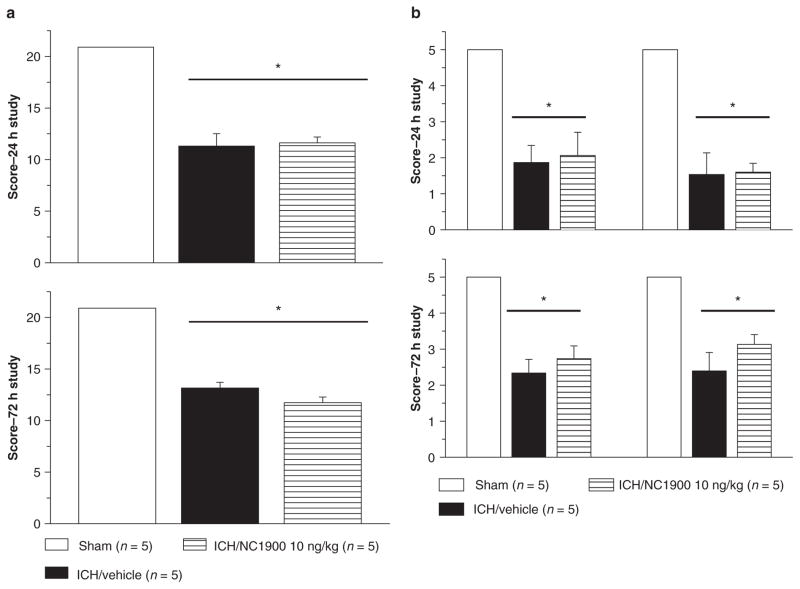
Neurobehavioral deficits were present in all animals following collagenase injection. No effects were observed with NC1900 treatment. The neurobehavioral deficits were evaluated by the Modified Garcia test (Fig. 2a(1) and (2)), and the beam balance and wire hanging test (Fig. 2b(1) and (2))
Fig. 2.
Neurobehavioral deficits were present in all animals following collagenase injection. NC1900 had no effect on ICH-induced neurobehavioral deficit at 24 (a) and at 72 h (b). *Significant difference vs. sham (p < 0.05)
AVP V1a receptor activation and AQP4 expression
Western blot measurement of AQP4 protein expression showed no difference between the vehicle and NC1900 (Fig. 3).
Fig. 3.
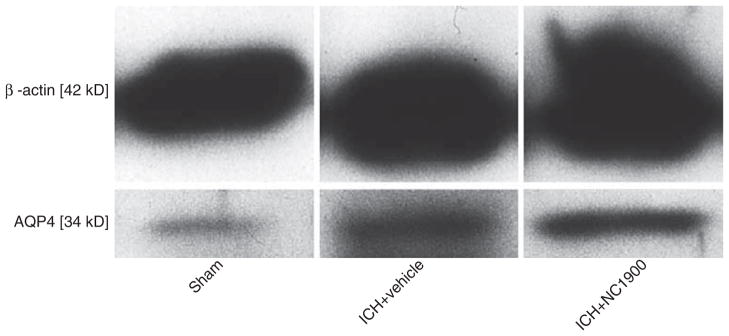
NC1900 does not change the production of AQP4 at 72 h
Discussion
The aim of this study was to determine the effects of the AVP V1a receptor agonist NC1900 on AQP4 production, and resulting effects on brain edema and neurobehavioral deficits. We demonstrated that activation of the AVP V1a receptor with NC1900 had no effect on AQP4 production, the ICH-induced increase in brain water content and neurobehavioral deficits.
AVP’s actions are modulated through one of its three receptors. In the brain, the predominant receptor is the V1 receptor, which exists in two subtypes V1a and V1b. It has been demonstrated that activation of V1a but not other types of receptors can activate AQP4 [2, 14]. AQP4 provides bidirectional water flow between the blood and the brain, and has been implicated as a key component in water homeostasis in normal brain and the cause of brain edema during injury [8]. Experiments with knock-out animals demonstrated that AQP4 is essential in reducing the duration and onset of vasogenic edema and that its depletion can cause a significant increase in brain water content in models of vasogenic edema [5]. Hirt et al. demonstrated that early aquaporin induction has a protective effect, and his team was able to reduce the edema formation after a middle cerebral artery occlusion model [10]. Similarly, Badaut et al. demonstrated that induction of AQP4 can preserve the tissue and ameliorate edema formation following injury [11]. In order to test whether the pharmacological activation of V1a receptor will have an effect on brain water content after ICH, we investigated the effect of V1a receptor agonist NC1900.
NC1900 is an AVP analog with a strong affinity for the V1a receptor in rodents compared to other known AVP agonist types [12, 16]. In this study, we tested the effects of this drug on various delayed time points in which vasogenic edema has been suggested to be most present. Since AQP4 upregulation peaks 72 h after ICH [13], we tested the effect of NC1900 on AQP4 production at this time point.
Conclusion
We conclude that AVP V1a receptor activation has no effect on AQP 4 production and ICH-induced brain damage in mice.
Acknowledgments
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Anatol Manaenko, Department of Physiology and Pharmacology, Loma Linda University, Loma Linda, CA, USA.
Tim Lekic, Department of Physiology and Pharmacology, Loma Linda University, Loma Linda, CA, USA.
Jiping Tang, Department of Physiology and Pharmacology, Loma Linda University, Loma Linda, CA, USA.
John H. Zhang, Email: johnzhang3910@yahoo.com, Department of Physiology and Pharmacology, Loma Linda University, Loma Linda, CA, USA and Department of Anesthesiology, Loma Linda Medical Center, Loma Linda, CA, USA and Department of Neurosurgery, Loma Linda University Medical Center, 11234 Anderson Street, Room 2562B, Loma Linda, CA, 92354, USA
References
- 1.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 2.Niermann H, Amiry-Moghaddam M, Holthoff K, Witte O, Ottersen O. A novel role of vasopressin in the brain: modulation of activity-dependent water flux in the neocortex. J Neurosci. 2001;21(9):3045–3051. doi: 10.1523/JNEUROSCI.21-09-03045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu YT, Zhang H, Xue YX. Dexamethasone treatment modulates aquaporin-4 expression after intracerebral hemorrhage in rats. Neurosci Lett. 2007;413:126–131. doi: 10.1016/j.neulet.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 4.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 6.Yukutake Y, Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2010;168(4):885–891. doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 8.Benarroch E. Aquaporin-4, homeostasis, and neurologic disease. Neurology. 2007;69:2266–2268. doi: 10.1212/01.wnl.0000286385.59836.e2. [DOI] [PubMed] [Google Scholar]
- 9.Manaenko A, Lekic T, Sozen T, Tsuchiyama R, Zhang JU, Tang J. Effect of gap junction inhibition on intracerebral hemorrhage-induced brain injury in mice. Neurol Res. 2009;31:173–178. doi: 10.1179/174313209X393591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirt L, Ternon B, Price M, Mastour N, Brunet JF, Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab. 2009;29(2):423–433. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- 11.Badaut J, Ashwal S, Tone B, Regli L, Tian HR, Obenaus A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr Res. 2007;62:248–254. doi: 10.1203/PDR.0b013e3180db291b. [DOI] [PubMed] [Google Scholar]
- 12.Mishima K, Tsukikawa H, Miura I, Inada K, Abe K, Matsumoto Y, Egashira H, Iwasaki K, Fujiwara M. Ameliorative effect of NC1900, a new AVP4–9 analog, through vasopressin V1A receptor on scopolamine-induced impairments of spatial memory in the eight-arm radial maze. Neuropharmacology. 2003;44:541–552. doi: 10.1016/s0028-3908(02)00408-2. [DOI] [PubMed] [Google Scholar]
- 13.Qing WG, Dong YQ, Ping TQ, Lai LG, Fang LD, Min HW, Xia L, Heng PY. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. J Neurosurg. 2009;110:462–468. doi: 10.3171/2008.4.JNS17512. [DOI] [PubMed] [Google Scholar]
- 14.Brinton RE, Gee KW, Wamsley JK, Davis TP, Yamamura HI. Regional distribution of putative vasopressin receptors in rat brain and pituitary by quantitative autoradiography. Proc Natl Acad Sci USA. 1984;81:7248–7252. doi: 10.1073/pnas.81.22.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka T, Sumiyoshi T, Tanaka K, Tsunoda M, Uehara T, Itoh H, Kurachi M. NC-1900, an arginine-vasopressin analogue, ameliorates social behavior deficits and hyperlocomotion in MK-801-treated rats: therapeutic implications for schizophrenia. Brain Res. 2005;1053:131–136. doi: 10.1016/j.brainres.2005.06.035. [DOI] [PubMed] [Google Scholar]