Abstract
Objective
Sinapic acid (SA, Sinapine), small naturally occurring hydroxycinnamic acid, has a GABA(A) receptor agonistic property and free radical scavenging activity. We examined potential neuroprotective effects of sinapic acid (SA) using global cerebral ischemia animal model.
Methods
MTT assay was performed to determine cytotoxic effects of SA. To examine the neuroprotective effects of SA, SA was administrated for 14 d before 4-vessel occlusion. Also, to determine whether SA prevents cognitive impairment, Morris water maze was performed.
Results
In this study, the efficacy of SA for the prevention of neuronal damage and for the reduction of memory impairment was investigated.
Conclusion
The results indicate that SA confers significant neuroprotection especially for ischemic hippocampal neurons.
Keywords: Sinapic acid, Neuroprotection effect, Memory, Hippocampus
INTRODUCTION
Neuroprotection for ischemic stroke refers to strategies that antagonize the injurious biochemical and molecular events that eventuate in irreversible ischemic injury. Global cerebral ischemia resulting from cardiac arrest, stroke and hypoxia is a problem of increasing clinical significance. Rigorously conducted experimental studies in animal models of brain ischemia provide incontrovertible proof-of-principle that high-grade protection of the ischemic brain is an achievable goal.1)
Dysfunction of mitochondria induced by ischemia is considered to be key event triggering neuronal cell death after brain ischemia.2) Several cyclooxygenase (COX) inhibitors have proved to be neuroprotective in stroke models. By performing 4-VO model of global cerebral ischemia, neuronal damage of selective vulnerable cells, most notably the CA1 cells of the hippocampus is generated. Global cerebral ischemia produces delayed neuronal death in hippocampal CA1 region.3) The hippocampus bas been shown to be essentially involved in learning and memory processes.4) Nimodipine, a dihydropyridine derivative, is a well-known neural protective drug that has been applied to some ischemic vascular diseases and may have a beneficial effect on cerebral ischemia after subarachnoid hemorrhage.5)
SA is found abundantly in Polygala tenuifolia WILLDENOW (PT), which was reported that PT improved scopolamine-induced memory impairment in mice.6) SA might be not only a main active structure in the pharmacological effects of tenuifoliside B and 3, 6'-disinapolysucrose but also a candidate for cerebral protective and cognition-improving medicine.7,8) In the present, in order to ascertain the hypothesis, we examined whether SA possesses cerebral protective cognition-improving effects in global cerebral ischemia.
METHODS
Cell Culture
The SH-SY5Y human neuroblastoma cells were cultured in Dulbecco's modified Eagle medium/Ham's F-12 nutrient mixture (1:1) containing 10% fetal bovine serum. The culture medium was changed every 2 days. The cells were kept in a 95% air/5% CO2 humidified incubator at 37℃.
MTT Assay for Cell Viabilities
Cell viability was determined by the MTT assay. Cells were seeded in triplicate at a concentration of 1×105 cells per well on a 96-well plate. In a dose-dependent test, SH-SY5Y cells were treated with SA at concentrations of 0, 1, 10, and 100µg/ml for 24 h. After MTT (Sigma, St Louis, MO, USA) was added to each group, the cells were incubated for 4 h. Then, they were further incubated for 1 h in the solution in which MTT was dissolved. The viability was measured with a microtiter plate reader (Bio-Tek, Winooski, VT, USA) at a test wavelength of 595 nm with a reference wavelength of 690 nm. The optical density (O.D.) was calculated as the difference between the reference wavelength and the test wavelength. Percent viability was calculated as (O.D. of drug-treated sample/O.D. of untreated sample)×100.
Animals
Male Wistar rats (6 weeks old) weighing 180-190 g were purchased from SLC Japan (Hamamatsu, Shizuoka, Japan). The rats were housed in clear polycarbonatecage (22.5×33.8×14.0 cm) in groups of 5 or 6 per cage under a controlled 12-h light/12-h dark cycle (lights on from 7:00 AM to 7:00 PM), with room temperature at 23±1℃ and humidity at 55±5%. The rat was given free access to water and food pellets. Animal treatment and maintenance were carried out in accordance with the Principle of Laboratory Animal Care (NIH publication No. 85-23, revised 1985) the Animal Care and Use Guide lines issued by Kyung Hee University.
Acute Toxicity Studies
The acute toxicity of SA was investigated in mice and rats of both sexes (6 male/6 female in each dosing group) receiving single administrations by intravenous, intraperitoneal, and oral routes. The appearance and behavior of the animals were observed for 6 h after dosing and then daily for 2 weeks. Deaths were recorded daily, and post mortem examinations were performed on all dead animals, as well as on the survivors at the end of the observation period.
Cerebral Ischemia
Adult male wistar rats 6 weeks of age (weight of 180-200 g at the time of surgery) were used in the study. The animals were initially anesthetized with 3.5% isofluorane and then maintained during operation on 1.5% isofluorane in N2O:O2 (70:30) mixture on the first day and the vertebral arteries were electrocauterized in the alar foramina at the level of the first cervical vertebrae. Bilateral common carotid arteries were exposed and carefully separated from the carotid sheath, cervical sympathetic and vagus nerves through a ventral cervical incision. The rats were placed on a heating pad during recovery from anesthetized to maintain the body temperature at 37±0.5℃ after surgery.
The next day, both common carotid arteries were occluded for 10 min. while the animals awake. It results in damage limited to the hippocampal area. Rats that become unresponsive and loss the righting reflex within 2 min occlusion but show no seizure during and after ischemia are used further experiments. Reperfusion was achieved by releasing the clips at the end of 10 min ischemic period. Animals were that developed post-operative complications such as excessive weight loss (>20% of preoperative body weight) and showed evidence of unilateral hippocampal damage were excluded from the study. The rats which received the same operation without carotid arteries ligation served as the sham-operated control. The rats were allowed to survive for 7 days (8 controls, 8 sham rats and 8 ischemia rats) or for 14 days (8 controls, 8 sham rats and 8 ischemia rats). The rats were placed on a heating pad during recovery from anesthetized to maintain the body temperature at 37±0.5℃ after surgery.
Drugs
SA and nimodipine were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other materials were of the highest grade commercially available. SA (10 mg/kg) was suspended in a 10% aqueous solution of Tween 80. SA was administered intraperitoneally (i.p.) to rats 0 and 90 min after induction of ischemia. Ischemia-only animals were injected i.p. with 180µl distilled water at the same time points. Beginning the day after ischemia induction, some animals were administered SA solution p.o. daily during seven days for behavior test.
Morris Water Maze
In the standard use of water maze the rat is placed into the pool at one of several randomly ordered start locations near the wall and swims to a submerged platform in a fixed position (simple task). Escape latency, swim speed and swim distance are the main parameters of these escape trials which provide information about the ability of learning and about the motor performance.9) The commercially available video tracking programe, the EthoVision®, from Noldus Information Technologies, can analyze rat behavior in an arena. The maze was a stainless pool (186 cm diameter 50 cm in height) filled with clear water (25±2℃) from which they could escape onto a hidden platform. The platform (10 cm diameter 49 cm in height) was hidden 1cm below the water level. In the probe trial, the platform is removed and the rat is permitted to swim freely about the pool for a given time.
The pool was situated in a room measuring 10 cm with different markers on three of the walls as cues. So the water was made opaque by the addition of milk powder. The maze was divided conceptually into four quadrants (1-4) and three concentric annuli. A counter area, twice the size of the platform in diameter was used as a measure of search accuracy.
Place learning
Each rat underwent two trials per day for five consecutive days. For each trial, the rat was placed in the water facing the pool wall at one of eight around the tank. Latency to finding the hidden platform (escape latency) was recorded. Each rat was allowed a maximum of 180 second to find a hidden platform and remain on it for 10 second. If a rat did not find platform after 180 second, rat was gently put on it by the investigator. Once the rat located the platform (or was put on it) it was permitted to remain there for 10 second. At the end of the five trials the rat was dried with paper towels.
Probe trial
On the sixth day of learning test, the platform was withdrawn and the time the rat swam in each of the four quadrants of the tank was record for 180 second. Learning was defined as at spending a time significantly longer than 75 second in the quadrant where the platform was located (training quardrant).
Statistical analysis
Results are expressed as the mean±SD. Statistical analysis was evaluated by Student's t-test, one-way ANOVA followed by Dunnett's t-test. Results were considered statistically significant when p-values were *p<0.05, †p<0.005 and ‡p<0.001.
RESULTS
Cytotoxicity of SA in SH-SY5Y Cells
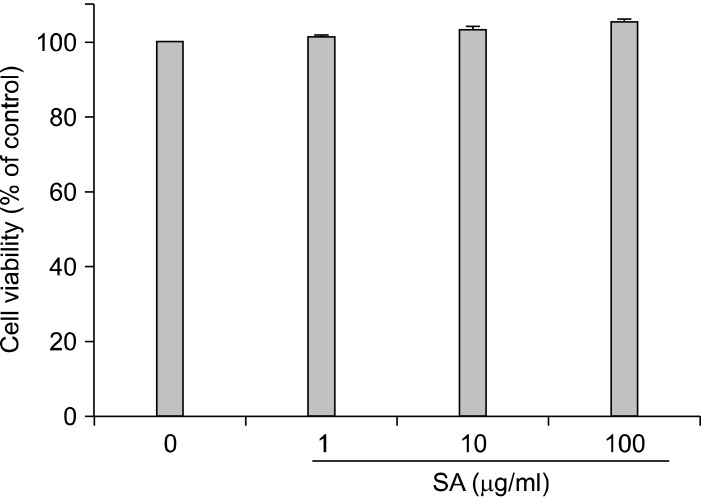
To determine cytotoxic effects of SA on SH-SY5Y cells, cells were incubated with various concentrations of SA (0, 1, 10, and 100µg/ml) for 24 h. In 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, the viabilities of SH-SY5Y cells exposed to SA for 24 h were 100%, 101.1%, 103.2%, and 105.2%, respectively (Fig. 1). The treatment of SA did not induce cytotoxic effects on SH-SY5Y cells. These results show that SA can be used safely.
Fig. 1.
Cytotoxicity by SA was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. SH-SY5Y cells. These results show that SA can be used safely.
To examine the neuroprotective effect of SA (1, 3, and 10 mg/kg) were injected i.p. into rats 0 and 90 min after the induction of cerebral ischemia. For the ischemia group, 0.89% physiological saline was injected at a volume of 180µl. When reperfusion is conducted after cerebral ischemia caused by 4-VO, pyramidal neurons in the hippocampus CA1 subfield are the most susceptible to the ischemia and start undergoing cell death 72 h after reperfusion.10) In the present study, rats were sacrificed 7 days after reperfusion, the time point by which all signs of neuronal cell damage have become manifest. Dorsal hippocampal tissue sections were stained with cresyl violet to visualize CA1 neurons in the ischemic group, the sham-operated group, the SA treated group, and the nimodipine treated group (positive control group).
Neuroprotective Effect of SA on Global Cerebral Ischemia in vivo
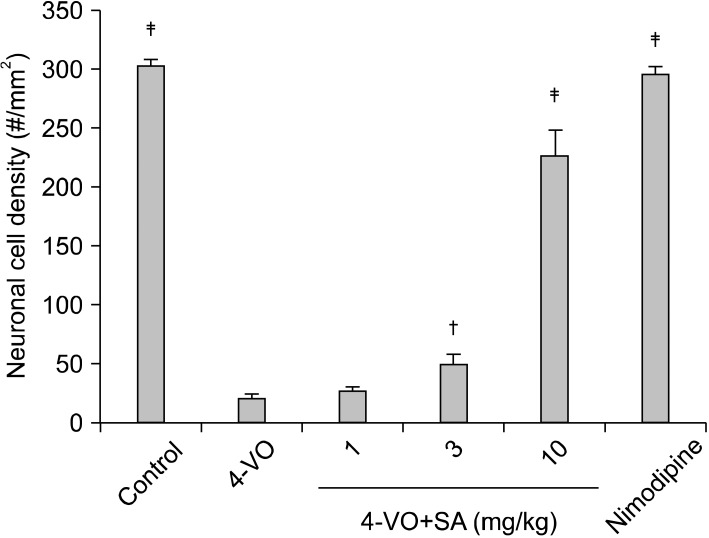
To examine the neuroprotective effect of SA, a dose of 10 mg/kg was injected i.p. into rats 0 and 90 min after the induction of cerebral ischemia. For the ischemia group, 0.89% physiological saline was injected at a volume of 180µl. When reperfusion is conducted after cerebral ischemia caused by 4-VO, pyramidal neurons in the hippocampus CA1 subfield are the most susceptible to the ischemia and start undergoing cell death 72 h after reperfusion. In the present study, rats were sacrificed 7 days after reperfusion, the time point by which all signs of neuronal cell damage have become manifest. There was no significant difference in body temperature between ischemic and SA treated groups at any time point recorded indicating that neuroprotective effects of SA were not due to a decrease in body temperature. Normal CA1 pyramidal neurons from three hemispherical sections each having a size of 1×1 mm2, were counted and averaged. In the ischemic group the viable cell density was 20.6±3.9 cells/mm2, which is far lower than that in the sham group, 303.7±4.8 cells/mm2. In the group injected with SA, viable cells were measured to be 26.9±3.1, 49.4±9.2, and 226.4±22.6 cells/mm2 at 1, 3, and 10 mg/kg. Thus SA rescued 72.7% of the ischemic neurons at 10 mg/kg injected (Fig. 2).
Fig. 2.
Neuroprotective effects of SA (1, 3, and 10 mg/kg). Either saline of SA was injected in i.p. into the animals following 10 min ischemia. Seven days later, neuronal cell density in CA1 neurons. Statistically significant differences from saline-treated group (†p<0.005, ‡p<0.001). Control, Normal animals (n=8); 4-VO, saline-treated animals following ischemia (n=8). SA, SA treated animals following ischemia (n=8 for 1, 3, and 10 mg/kg, respectively). The male wistar rats were 6 weeks.
Cognitive-Enhancing Activity of SA Following Ischemia/Reperfusion
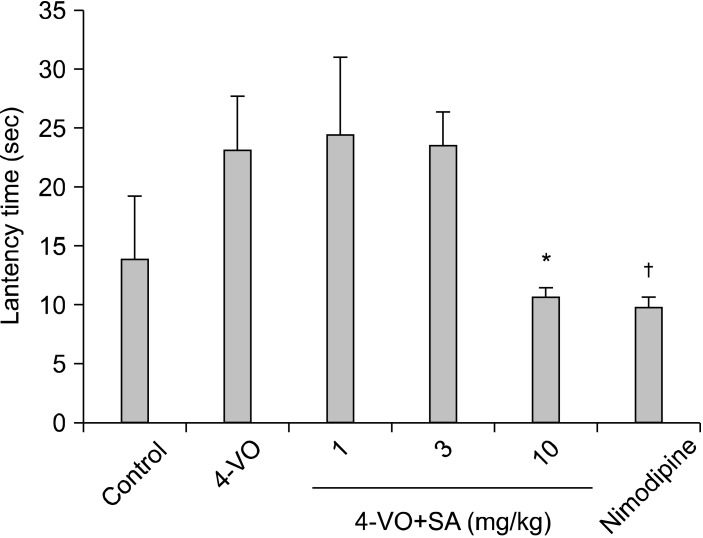
The water maze reveals an impairment in spatial learning and memory which can be easily quantified. To compare spatial learning of rat, we tested them on the hidden platform and testing 1 week after induced ischemia. Seven days following ischemia induced by 4-VO. During the escape trials all rats were able to find the hidden platform. With increasing number of trials the escape latency decreased in both groups. Latency times for the 4-VO rats were consistently longer than times for either the sham-operated or SA treated animals, as was the time to reach the platform region during the probe trial on day 6. Ischemic animals receiving SA took slightly longer to swim to the platform than sham operated animals, but performed just as well in the probe trial (Fig. 3). In the probe trials, rats after ischemia take longer than sham-operated rats. Thus prolongation of latency was markedly shortened by SA at a dose of 10 mg/kg.
Fig. 3.
Effects of SA on Morris water maze performance on the probe trial deficits induced by 10 min cerebral ischemia in rats (n=8). Oral administration of SA (10 mg/kg) for 14 days. Mean daily latencies of escaping from the start point onto the hidden platform. Each rat was subjected to two trials per day for 5 consecutive days. Data represent means±SD: Control, 13.83±5.49 sec; 4VO, 23.17±4.54 sec; Nimodipine, 9.83±0.79 sec; SA, 24.5±6.57, 23.5±2.93, 10.67±0.76 sec at 1, 3, 10 mg/kg (*p<0.05, †p<0.0051).
DISCUSSION
In the present study, the efficacy of SA (1, 3, and 10 mg/kg) for the prevention of neuronal damage and for the reduction of memory impairment was investigated. We examined potential neuroprotective effects of SA using the 4-VO model in rats. The results indicate that SA confers significant neuroprotection especially for ischemic hippocampal neurons. Moreover, we observed that SA showed no cytotoxic effects on SH-SY5Y human neuroblastoma cells. This study is the first work that investigated neuroprotection under SA-treated conditions in global cerebral ischemia.
The hippocampus in brain is known to demonstrate selective vulnerability to hypoxic and ischemic damage. Hippocampal structure is directly involved in learning and memory processes. Cell deaths in the hippocampal CA1 region due to transient cerebral ischemia do not occur immediately after completion of ischemia. The hippocampal morphology remains normal until four days after ischemia and cell deaths begin four or five days after ischemia. Such cell deaths are referred to delayed neuronal deaths.10) The delayed neuronal death implicates important meaning that neurons are not destroyed instantaneously and directly at the end of the period of ischemia. Rather, the cells have a normal appearance early after ischemia because they are still alive. Furthermore, they are not irreversibly committed to be destroyed. Thus, the immediate post ischemic period represents the "therapeutic window" during which interventions could prevent the delayed neuronal death.11)
A latest review article showed a narrow survey of the most extensively evaluated neuroprotective agents and classes. Among the agent-classes considered are calcium channel blockers; glutamate antagonists; GABA agonists; antioxidants/radical scavengers; phospholipid precursor; nitric oxide signal-transduction down-regulator; leukocyte inhibitors; hemodilution; and a motley of other agents. Among promising ongoing efforts, therapeutic hypothermia, high-dose human albumin therapy, and hyperacute magnesium therapy were considered in detail. The potential of combination therapies was highlighted. SA is a phenylpropanoid derivative and possesses antioxidant activity.12) Until now, however, no report has been issued on the pharmacological activities of SA on the global cerebral ischemia. During previous screening studies for neuroprotective effect from natural sources, we found that SA has good neuroprotection, and thus, we researched its neuroprotective effect using behavioral methods. Karakida ascertain that SA possesses cerebral protective and cognition-improving effects in various animal models of hypoxia and amnesia.13) SA might play different reginonal roles. We performed water maze task. To investigate the possibility that SA allows neurons to remain functionally active in cognitive impairment in mice.
In conclusion, SA is characterized a good neuroprotective agent and cognition-enhancing effect in improving the cognitive impairment caused by global cerebral ischemia. Future work will focus on potential treatment for neurodegenerative disorders like Alzheimer's disease and Parkins disease.
References
- 1.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Racay P, Tatarková Z, Drgová A, Kaplan P, Dobrota D. Ischemia-reperfusion induces inhibition of mitochondrial protein synthesis and cytochrome c oxidase activity in rat hippocampus. Physiol Res. 2009;58:127–138. doi: 10.33549/physiolres.931383. [DOI] [PubMed] [Google Scholar]
- 3.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 5.Drejer J, Benveniste H, Diemer NH, Schousboe A. Cellular origin of ischemia-induced glutamate release from brain tissue in vivo and in vitro. J Neurochem. 1985;45:145–151. doi: 10.1111/j.1471-4159.1985.tb05486.x. [DOI] [PubMed] [Google Scholar]
- 6.Naito R, Tohda C. Characterization of anti-neurodegenerative effects of Polygala tenuifolia in Abeta(25-35)-treated cortical neurons. Biol Pharm Bull. 2006;29:1892–1896. doi: 10.1248/bpb.29.1892. [DOI] [PubMed] [Google Scholar]
- 7.Karakida F, Ikeya Y, Tsunakawa M, Yamaguchi T, Ikarashi Y, Takeda S, et al. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol Pharm Bull. 2007;30:514–519. doi: 10.1248/bpb.30.514. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Yang J, Yu S, Chen N, Xue W, Hu J, et al. Triterpenoid saponins with neuroprotective effects from the roots of Polygala tenuifolia. Planta Med. 2008;74:133–141. doi: 10.1055/s-2008-1034296. [DOI] [PubMed] [Google Scholar]
- 9.Corbett D, Evans SJ, Nurse SM. Impaired acquisition of the Morris water maze following global ischemic damage in the gerbil. Neuroreport. 1992;3:204–206. doi: 10.1097/00001756-199202000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- 11.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 12.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 13.Karakida F, Ikeya Y, Tsunakawa M, Yamaguchi T, Ikarashi Y, Takeda S, et al. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol Pharm Bull. 2007;30:514–519. doi: 10.1248/bpb.30.514. [DOI] [PubMed] [Google Scholar]