Abstract
Prior small studies have shown multiple benefits of frequent nocturnal hemodialysis compared to conventional three times per week treatments. To study this further, we randomized 87 patients to three times per week conventional hemodialysis or to nocturnal hemodialysis six times per week, all with single-use high-flux dialyzers. The 45 patients in the frequent nocturnal arm had a 1.82-fold higher mean weekly stdKt/Vurea, a 1.74-fold higher average number of treatments per week, and a 2.45-fold higher average weekly treatment time than the 42 patients in the conventional arm. We did not find a significant effect of nocturnal hemodialysis for either of the two coprimary outcomes (death or left ventricular mass (measured by MRI) with a hazard ratio of 0.68, or of death or RAND Physical Health Composite with a hazard ratio of 0.91). Possible explanations for the left ventricular mass result include limited sample size and patient characteristics. Secondary outcomes included cognitive performance, self-reported depression, laboratory markers of nutrition, mineral metabolism and anemia, blood pressure and rates of hospitalization, and vascular access interventions. Patients in the nocturnal arm had improved control of hyperphosphatemia and hypertension, but no significant benefit among the other main secondary outcomes. There was a trend for increased vascular access events in the nocturnal arm. Thus, we were unable to demonstrate a definitive benefit of more frequent nocturnal hemodialysis for either coprimary outcome.
Keywords: hemodialysis, left ventricular mass, nocturnal hemodialysis, RAND physical health composite (PHC) SF-36, randomized clinical trial, vascular access
Patients on maintenance hemodialysis in the United States have suffered from annual mortality rates of ~18–20% for two decades.1 The National Cooperative Dialysis Study demonstrated that an insufficient dialysis dose was associated with a higher hospitalization rate.2 However, the HEMO study indicated that an increased dose of dialysis provided during the three times per week hemodialysis did not significantly improve survival, cardiovascular, or infection-related hospitalizations3 or improve health-related quality of life, functional status, or nutritional status.4,5
Investigators have hypothesized that an increased frequency of hemodialysis could lead to improved patient outcomes, as increased frequency results in both an increased clearance of solutes and a reduced interdialytic change in volume. More frequent dialysis can be performed through either a short daily schedule6 or a longer overnight or nocturnal schedule. Observational studies7–14 of nocturnal hemodialysis performed 5 to 6 nights per week and one randomized trial15 published after we started have suggested that frequent nocturnal hemodialysis was associated with decreased left ventricular (LV) mass,8,15 improved control of hypertension,8–11,15 a marked increase in phosphorus clearance, often leading to the discontinuation of phosphate binders,10–12,14,15 and, in some studies, with improved nutritional status,14 health-related quality of life,11 and sleep apnea.13 The Frequent Hemodialysis Network (FHN) Nocturnal Trial was designed to rigorously compare frequent nocturnal home hemodialysis six times per week with conventional three times per week hemodialysis using a randomized controlled clinical trial design.
RESULTS
Baseline characteristics
A total of 118 patients were enrolled into the trial, and 87 patients were randomized: 42 in the conventional arm and 45 in the frequent nocturnal arm (Figure 1). The baseline characteristics in Table 1 demonstrate that demographic, socioeconomic, and medical characteristics were similar between the two randomized groups.
Figure 1. FHN Nocturnal Trial patient flow.
FHN, Frequent Hemodialysis Network; GFR, glomerular filtration rate; LV, left ventricular; MRI, magnetic resonance imaging; PHC, physical health composite.
Table 1.
Baseline characteristicsa
| Factor | All patients (n=87) | Conventional hemodialysis (n=42) | Frequent nocturnal hemodialysis (n=45) | P-value |
|---|---|---|---|---|
| Age (years) | 52.8±13.6 | 54.0±12.9 | 51.7±14.4 | 0.48 |
| Female (%) | 34.5 | 33.3 | 35.6 | 0.83 |
| Race | 0.67 | |||
| Black (%) | 26.4 | 26.2 | 26.7 | |
| White (%) | 55.2 | 50.0 | 60.0 | |
| Native American, Aboriginal Canadian, Alaskan Native, First Nation (%) | 3.4 | 4.8 | 2.2 | |
| Asian (%) | 13.8 | 16.7 | 11.1 | |
| Native Hawaiian or other Pacific Islander (%) | 1.1 | 2.4 | 0 | |
| Body mass index (kg/m2) | 29.0±8.0 | 28.3±7.8 | 29.7±8.3 | 0.67 |
| Weight after HD (kg) | 85.5±25.4 | 83.3±23.8 | 87.6±27.0 | 0.43 |
| Anthropometric volume (l) | 42.2±9.8 | 41.6±9.5 | 42.7±10.0 | 0.73 |
| Cause of ESRD | 0.47 | |||
| Diabetic nephropathy (%) | 34.5 | 35.7 | 33.3 | |
| Glomerulonephritis (%) | 35.6 | 40.5 | 31.1 | |
| Hypertensive nephrosclerosis (%) | 8.0 | 7.1 | 8.9 | |
| Polycystic kidney disease (%) | 21.8 | 16.7 | 26.7 | |
| ESRD vintage | ||||
| <1 year (%) | 51.7 | 59.5 | 44.4 | 0.38 |
| 1 to <2 years (%) | 14.9 | 11.9 | 17.8 | |
| 2 to 5 years (%) | 14.9 | 11.9 | 17.8 | |
| >5 years (%) | 18.4 | 16.7 | 20.0 | |
| Urine volume (ml) | 0.47 | |||
| <100 ml/day | 27.6 | 26.2 | 28.9 | |
| 100–499 ml/day | 20.7 | 16.7 | 24.4 | |
| 500–999 ml/day | 34.5 | 38.1 | 31.1 | |
| >1000 ml/day | 17.2 | 19.1 | 15.6 | |
| Comorbid medical conditions | ||||
| Hypertension (%) | 89.7 | 90.5 | 88.9 | 0.81 |
| Myocardial infarction (%) | 10.3 | 9.5 | 11.1 | 0.81 |
| Heart failure (%) | 13.8 | 16.7 | 11.1 | 0.45 |
| Atrial fibrillation (%) | 6.9 | 0 | 13.3 | 0.014 |
| Peripheral vascular disease (%) | 17.2 | 16.7 | 17.8 | 0.89 |
| Abdominal aortic aneurysm repair or bypass grafting (%) | 8.0 | 11.9 | 4.4 | 0.20 |
| Stroke (CVA) (%) | 2.3 | 2.4 | 2.2 | 0.96 |
| Dementia (%) | 0 | 0 | 0 | — |
| Tumor without metastases (%) | 1.1 | 0 | 2.2 | 0.33 |
| Diabetes and diabetic complications (%) | 42.5 | 42.9 | 42.2 | 0.95 |
| Hemiplegia (%) | 0 | 0 | 0 | — |
| Chronic pulmonary disease (%) | 4.6 | 4.8 | 4.4 | 0.94 |
| Moderate-to-severe liver disease (%) | 1.1 | 2.4 | 0 | 0.30 |
| Residual kidney function (urea clearance in ml/min) | 0.95 | |||
| Anuric (%) | 27.6 | 26.2 | 28.9 | |
| >0–1 (%) | 18.4 | 21.4 | 15.6 | |
| >1–3 (%) | 34.5 | 33.3 | 35.6 | |
| >3 (%) | 19.5 | 19.0 | 20.0 | |
| Predialysis diastolic blood pressure (mm Hg) | 81.3±12.1 | 83.1±13.5 | 79.6±10.6 | 0.28 |
| Serum creatinine (mg/dl)b | 8.8±3.0 | 8.9±3.1 | 8.6±3.0 | 0.66 |
| Weekly standard Kt/Vurea | 2.34±0.34 | 2.34±0.34 | 2.38±0.35 | 0.58 |
| Equilibrated Kt/Vurea | 1.38±0.37 | 1.34±0.30 | 1.42±0.42 | 0.33 |
| Dialysis access | 0.48 | |||
| Fistula (%) | 47.1 | 40.5 | 53.3 | |
| Synthetic graft (%) | 8.0 | 9.5 | 6.7 | |
| Catheter (%) | 44.8 | 50.0 | 40.0 |
Abbreviations: CVA, cerebrovascular accident; ESRD, end-stage renal disease; HD, hemodialysis.
Shown are either mean±s.d. or percentage for each baseline factor.
To convert values for creatinine to μmol/l, multiply by 88.4.
Adherence to the dialysis prescription
Measurements obtained during monthly kinetic modeling sessions indicated that 86% of patients in the frequent nocturnal arm had a delivered mean standard (std)Kt/Vurea of ≥4.0, and that 100% of patients in the conventional arm had a delivered mean stdKt/Vurea of ≥2.0. Adherence was defined a priori as a patient attending at least 80% of dialysis treatments in a given month. As expected, patients in the frequent nocturnal arm had lower adherence to the prescribed dialysis prescription (72.7%) than patients in the conventional arm (97.6%, Table 2). Nevertheless, frequent nocturnal arm participants had a 1.82-fold higher mean weekly stdKt/Vurea, a 1.74-fold higher average number of treatments per week, a 2.45-fold higher average weekly treatment time than the conventional arm participants (Table 2 and Figure 2), as well as a 1.23-fold higher total weekly ultrafiltration and a 0.74-fold lower interdialytic normalized weight change. The dialysate concentrations of potassium and calcium, but not sodium, were higher in the frequent nocturnal arm compared with the conventional arm. Finally, the use of the buttonhole technique was significantly more common in the frequent nocturnal arm compared with the conventional arm.
Table 2.
Features of randomized interventiona
| Conventional hemodialysis (n=42) | Frequent nocturnal hemodialysis (n=45) | Ratio of means (frequent nocturnal vs conventional) | P-value | |
|---|---|---|---|---|
| Number of hemodialysis treatments per week | 2.91±0.21 | 5.06±0.80 | 1.74 | <0.001 |
| Percent of expected treatments attendedb | ||||
| >80% | 97.6 | 72.7 | — | <0.001 |
| <65–80% | 0 | 13.6 | — | |
| <65% | 2.4 | 13.6 | — | |
| Time per dialysis session (min) | 256±65 | 379±62 | 1.48 | <0.001 |
| Total dialysis time per week (h) | 12.6±3.9 | 30.8±9.1 | 2.45 | <0.001 |
| Blood flow rate (ml/min) | 350±49 | 262±61 | 0.75 | <0.001 |
| Dialysate flow rate (ml/min) | 554±126 | 354±106 | 0.64 | <0.001 |
| Dialyzer urea clearance (ml/min) | 236±26 | 181±30 | 0.77 | <0.001 |
| Ultrafiltration (weight change) | ||||
| Per session (l) | 2.52±1.01 | 1.95±0.66 | 0.77 | 0.003 |
| Per session (% of post weight) | 3.10±1.00 | 2.29±0.83 | 0.74 | <0.001 |
| Per week (l) | 7.41±3.02 | 9.13±3.26 | 1.23 | 0.01 |
| Kt/Vurea | ||||
| Total weekly standard | 2.91±0.86 | 5.03±1.23 | 1.73 | <0.001 |
| Dialysis weekly standard | 2.59±0.69 | 4.72±1.18 | 1.82 | <0.001 |
| Equilibrated (per session) | 1.48±0.5 | 1.87±0.8 | 1.26 | 0.009 |
| Blood urea nitrogen (mg/dl)c | ||||
| Before dialysis | 54.8±13.0 | 38.5±9.8 | 0.70 | <0.001 |
| After dialysis | 15.5±5.6 | 10.1±4.5 | 0.65 | <0.001 |
| Dialysate composition (initial concentration) | ||||
| Sodium (mEq/l) | 139±1 | 139±9 | 1.00 | 0.24 |
| Potassium (mEq/l) | 1.98±0.43 | 2.23±0.52 | 1.13 | <0.001 |
| Calcium (mEq/l) | 2.61±0.25 | 2.89±0.37 | 1.11 | <0.001 |
| Use of buttonhole technique for access of arteriovenous fistulad | 27.4% | 45.5% | — | <0.001 |
Shown are either mean±s.d. or percentage for each baseline factor.
In the conventional group, 65 and 80% adherence represent an average of 1.95 and 2.40 treatments/week, and 3.9 and 4.8 treatments/week in the frequent nocturnal group.
To convert values for blood urea nitrogen (BUN) to mmol/l, multiply by 0.357.
Data on the type of cannulation were prospectively collected from July 2006 until the conclusion of the study.
Figure 2. Separation in treatment parameters between groups.
Shown are distributions of the number of dialysis treatments per week (left), weekly treatment time in hours (center), and weekly standard Kt/Vurea (right) for the conventional (top) and frequent nocturnal (bottom) treatment groups. Each quantity was first averaged over the follow-up period separately for each patient. See Table 2 for means and s.d.s.
Coprimary outcomes
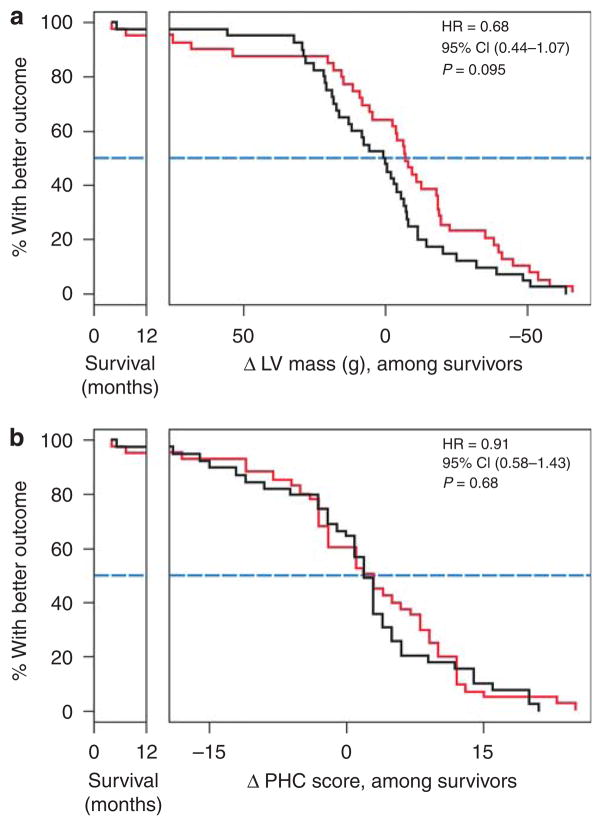
Three patients died (one from air embolism, one from an epidural hematoma in the frequent nocturnal arm, and one from ventricular fibrillation in the conventional arm) and five patients were transplanted (three in the frequent nocturnal arm; Figure 1). In the frequent nocturnal arm, three patients had no 12-month LV mass data and three patients had incomplete data for the baseline to 12-month physical health composite (PHC) score comparisons; the respective numbers in the conventional arm were 0 and 1, respectively. Frequent nocturnal hemodialysis did not yield a statistically significant improvement for either of the coprimary composite outcomes (Figures 3a, b, 4a and b).
Figure 3. Mortality/LV mass composite and mortality/PHC composite results.
Kaplan–Meier curves (conventional arm, black; frequent nocturnal arm, red) for the (a) death/LV mass composite and the (b) death/PHC composite. For any given value of the composite outcome indicated on the horizontal axis (time of death on the left, change in either LV mass or PHC among survivors on the right), the Kaplan–Meier curves indicate the proportion of patients in the respective treatment groups with an equal or more favorable outcome. The horizontal distance between the Kaplan–Meier curves at the 50% value on the vertical axis indicates the median composite outcome results.
CI, confidence interval; HR, hazard ratio; LV, left ventricular; PHC, physical health composite.
Figure 4. Histogram of change in left ventricular (LV) mass and physical health composite (PHC).
Shown are distributions of the change in LV mass (n = 76, a) and PHC (n = 77, b) in the nocturnal and conventional arms of the trial. Change in PHC summary includes one additional patient whose final PHC score occurred in the 8th month of follow-up whose PHC was censored in the primary PHC composite time-to-event analysis.
Main secondary outcomes
The mean difference in the change in LV mass between the frequent nocturnal and conventional arms was −8.8 g (95% confidence interval (CI) −21.8 to +4.2 g without covariate adjustment, and was −10.9 g (95% CI −23.7 to +1.8 g) after adjustment for the pre-specified covariates; Table 3 and Figures 4 and 5). After covariate adjustment, the estimated treatment effect for the change in LV mass factored by baseline body surface area was −5.2 g/m2 (95% CI −11.4 to +1.0 g/m2). The mean difference in the change in the PHC score between the frequent nocturnal and conventional arms was 1.2 points (95% CI −3.1 to 5.4 points) without covariate adjustment and was 0.6 points (95% CI −3.4 to 4.7 points) after covariate adjustment.
Table 3.
Secondary outcomes
| Outcome | Treatment | Na | Observed data (Mean±s.d.) (patients with non-missing baseline and follow-up values)
|
Main secondary analyses, controlling for baseline value and pre-specified covariates
|
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change from baseline to follow-up | Adjusted mean change from baseline±s.e. | Treatment comparison of change: nocturnal vs conventional (95% CI) | P-value | |||
| Left ventricular mass (g)b,c | Conventional | 39 | 132±41 | 133±42 | 0.6±24.9 | 1.7±4.5 | −10.9 (−23.7, 1.8) | 0.09 |
| Nocturnal | 37 | 141±48 | 132±55 | −8.2±31.7 | −9.2±4.6 | |||
| Physical health compositeb | Conventional | 38 | 38.4±8.5 | 40.6±9.2 | 2.1±9.6 | 2.1±1.5 | 0.6 (−3.4, 4.7) | 0.75 |
| Nocturnal | 39 | 37.0±9.3 | 40.3±12.3 | 3.3±9.0 | 2.7±1.4 | |||
| Beck depression inventoryb | Conventional | 38 | 11.7±9.3 | 11.1±10.2 | −0.6±9.6 | −0.4±1.3 | −1.5 (−4.9, 1.9) | 0.39 |
| Nocturnal | 39 | 11.8±7.9 | 9.7±8.6 | −2.1±5.2 | −1.9±1.2 | |||
| Predialysis albumin (g/dl)b,d | Conventional | 39 | 3.93±0.53 | 4.12±0.38 | 0.19±0.46 | 0.19±0.06 | −0.02 (−0.18, 0.15) | 0.85 |
| Nocturnal | 37 | 3.88±0.49 | 4.08±0.53 | 0.20±0.41 | 0.18±0.06 | |||
| Predialysis phosphorus (mg/dl)b,e | Conventional | 39 | 5.65±1.84 | 5.91±2.00 | 0.25±2.01 | 0.3±0.3 | −1.4 (−2.1, −0.7) | <0.001 |
| Nocturnal | 37 | 5.75±1.63 | 4.72±1.31 | −1.03±1.71 | −1.1±0.3 | |||
| Erythropoiesis-stimulating agents (EPO equivalent units)b,f | Conventional | 39 | 42,600±53,761 | 42,735±53,261 | 135±75,813 | −2±17% | 1.35 (0.87, 2.09) | 0.18 |
| Nocturnal | 37 | 43,939±68,173 | 56,678±58,436 | 12,739±63,244 | 33±24% | |||
| Weekly average predialysis systolic BP (mm Hg) | Conventional | 39 | 153±22 | 151±19 | −1.9±16.0 | −0.1±2.6 | −9.7 (−16.9, −2.5) | 0.009 |
| Nocturnal | 38 | 145±14 | 137±21 | −7.9±18.4 | −9.8±2.7 | |||
| Number of prescribed antihypertensive agents | Conventional | 39 | 1.74±1.27 | 2.00±1.43 | 0.26±1.43 | — | — | <0.001 |
| Nocturnal | 37 | 2.38±1.66 | 1.41±1.92 | −0.97±2.09 | — | |||
| N patients (%) | N patients (%) | — | — | Risk ratio, nocturnal vs conventional (95% CI) | P-value | |||
|
| ||||||||
| Trail Making B (failure to complete in 5 min)b | Conventional | 36 | 7 (19.4%) | 8 (22.2%) | — | — | 1.20 (0.52, 2.77) | 0.66 |
| Nocturnal | 34 | 8 (23.5%) | 8 (23.5%) | — | — | |||
| Non-access hospitalization and deatha | Conventional | 42 | 15 (38.1%) | — | 1.33 (0.67, 2.65) | 0.42 | ||
| Nocturnal | 45 | 18 (40.0%) | — | |||||
Abbreviation: EPO, erythropoietin.
Number of randomized patients for the non-access hospitalization/death outcome, and number of patients providing both baseline and follow-up measurements for the remaining outcomes.
Pre-specified main secondary outcomes.
When factored by body surface area per 1.73 m2, the mean±s.d. baseline LV mass index was 118±31 g per 1.73 m2 in the conventional group and 125±44 g per 1.73 m2 in the frequent nocturnal group.
To convert values for serum albumin to grams per liter, multiply by 10.
To convert values for phosphorus to millimoles per liter, multiply by 0.32.
The 12-month i.v. iron administration was significantly lower in the frequent nocturnal than the conventional group (exact stratified Wilcoxon P=0.007), with median and 90th percentile levels of 100 and 250 mg per month, respectively, in the frequent nocturnal group, and 200 and 425 mg per month, respectively, in the conventional group.
Figure 5. Main secondary results.
The calculation of the standardized effect sizes is described in ref. 16.
BP, blood pressure; CI, confidence interval; ESA, erythropoiesis-stimulating agent; HR, hazard ratio; LV, left ventricular.
Patients in both arms of the trial showed an improvement in the PHC score from baseline to follow-up, with an average increase of 2.4 points (95% CI 0.3 to 4.5 points, P = 0.02). Frequent nocturnal dialysis improved control of hyperphosphatemia and hypertension. There were no statistically significant differences between the study arms for the other main secondary outcomes (Table 3 and Figure 5). Although there was no difference in monthly erythropoietin dose between the two arms, the monthly dose of intravenous iron was lower in the frequent nocturnal arm compared with the conventional arm. The rate of the composite outcome of death or first non-access hospitalization did not differ significantly between the two groups (hazard ratio = 1.33, 95% CI 0.67–2.65).
Complications of therapy
There was a trend toward an increased rate of access complications in the frequent nocturnal arm, driven by a higher number of vascular access procedures (Table 4). A total of 34 vascular access events (17 failures and 17 access procedures) occurred in the frequent nocturnal arm and 21 events (13 failures and 8 access procedures) in the conventional arm (P = 0.10). The fraction of events affecting fistulas, grafts, and catheters were 50, 6, and 44% in the frequent nocturnal arm and 19, 24, and 57% in the conventional arm. A total of 51% of patients in the frequent nocturnal arm and 36% of patients in the conventional arm suffered a vascular access failure or underwent at least one vascular access procedure (time to first access event HR = 1.88, 95% CI 0.97–3.64, P = 0.06, Figure 6). During months 0–3, 3–6, 6–9, and 9–12, there were 14, 9, 5, and 6 interventions in the frequent nocturnal arm and 4, 7, 4, and 6 interventions in the conventional arm, respectively. There were fewer recorded hypotensive events in the frequent nocturnal arm, but no significant differences in the number of laboratory results indicating the presence of either hypokalemia or hypophosphatemia in the two trial arms.
Table 4.
Adverse events
| Outcome | Conventional (n=42)a | Frequent nocturnal (n=45)a | Hazard ratio (95% confidence interval) | P-value |
|---|---|---|---|---|
| Deaths | 1 | 2 | ||
| All hospitalizations | 30 (16) | 43 (19) | 1.42 (0.69, 2.90) | 0.34 |
| Non-access hospitalizations | 26 (15) | 35 (17) | 1.32 (0.60, 2.89) | 0.48 |
| Cardiovascular hospitalizations | 4 (3) | 6 (5) | 1.60 (0.49, 5.22) | — |
| Infection hospitalizations | 7 (5) | 14 (8) | 2.04 (0.80, 5.17) | — |
| Access hospitalizations | 4 (3) | 8 (5) | 2.15 (0.67, 6.89) | 0.20 |
| All vascular access interventions | 21 (15) | 34 (23) | 1.62 (0.91, 2.87) | 0.10 |
| Failures | 13 (10) | 17 (13) | 1.27 (0.60, 2.71) | 0.54 |
| Other procedures | 8 (6) | 17 (12) | 2.25 (0.87, 5.83) | 0.095 |
| Hypotensive episodes | ||||
| Number of hypotensive episodes | 136 (28) | 71 (25) | — | — |
| Percent of dialysis treatments with a hypotensive episode | 9.5 | 3.1 | <0.001 | |
| Hypokalemia | ||||
| Potassium <3.0 mEq/l | 0 | 8 (2) | — | 0.49 |
| Potassium <3.5 mEq/l | 16 (9) | 62 (13) | — | 0.47 |
| Hypophosphatemia (phosphorus <2.17 mg/dl) | ||||
| Without phosphorus added to the dialysate | 5 (3) | 11 (10) | — | 0.071 |
| With phosphorus added to the dialysate | 4 (2) | 6 (3) | — | 1.00 |
Indicated are total numbers of events and (numbers of patients with events) during the follow-up period of the study.
Figure 6. Time to first vascular access event.
Shown are Kaplan–Meier curves representing the conventional therapy (black) and the frequent nocturnal (red) groups for the time from randomization to each patient’s first access event, defined as an access failure or other access procedure. CI, confidence interval; HR, hazard ratio.
DISCUSSION
The frequent nocturnal hemodialysis intervention did not meet the prespecified criteria for statistical significance for either coprimary composite outcome of death/LV mass or death/PHC. Frequent nocturnal hemodialysis substantially improved the control of hyperphosphatemia and of systolic blood pressure, did not produce detectable improvements in any of the remaining prespecified main secondary outcomes relative to the three times per week group, and tended to increase vascular access events. These results differ from the FHN Daily Trial, as in that study there was a statistically significant benefit of more frequent hemodialysis for both coprimary outcomes. The other secondary outcome findings and vascular access findings of the FHN Daily Trial were similar to those found in this trial.16
There are several possible explanations for the nonsignificant effects of the intervention on the coprimary end points, some of which differ between the LV mass and PHC outcomes.
First, the estimated treatment effect on the mean change in LV mass (−10.9 g) should be interpreted in the context of its wide 95% CI (−23.7 to +1.8). The CI contains the value of 0, corresponding to no treatment effect. At the same time, changes in LV mass close to the center of the interval (for example, 10 g) have been associated with differences in mortality of up to 50% in observational studies of dialysis patients.17,18 Thus, when taken by themselves, the results of the FHN Nocturnal Trial neither prove nor disprove the hypothesis that frequent nocturnal dialysis leads to clinically important reductions in LV mass. On the basis of the dropout rates and variability in the change in LV mass observed in the study, one would have needed a sample size of 275 patients to obtain 80% power to detect a mean effect on LV mass of 10 g, and 125 patients to detect a mean change of 15 g.
Second, the FHN nocturnal LV mass results can be interpreted in the context of previous studies. The FHN nocturnal confidence interval contains the estimated effects of the six times per week interventions in the recently published FHN Daily Trial16 of −13.8 g (95% CI −21.8 to −5.8 g) and the shorter-term Culleton trial15 of −15.3 g (95% CI −29.6 to −1.0 g). Hence, the differences between the estimated effects on LV mass across the three randomized trials of frequent hemodialysis are consistent with chance variation. Given the positive effects on LV mass demonstrated in the two previous randomized trials of frequent dialysis, the inconclusive result of the FHN Nocturnal Trial could be interpreted as consistent with the hypothesis that nocturnal dialysis reduces LV mass to some extent. Although more sophisticated statistical analyses may provide additional hypothesis-generating information, the only method by which this question can be properly answered is to perform an adequately powered randomized clinical trial.
Finally, it is possible that differences in study population or design may have contributed to differences between our LV mass findings and those of the Culleton trial.15 The FHN Nocturnal Trial included a larger proportion of incident patients (~50%) than the Culleton trial. The median duration of dialysis in the Culleton trial was 5.2 years compared with 3.45 years in the FHN Nocturnal Trial. Although the Culleton trial did not report residual renal function, the difference in both the percentage of incident patients and the mean duration of dialysis suggests that urine volume and renal solute clearances are likely to have been substantially larger in the FHN trial, thus reducing the relative contribution of the dialysis regimens to total solute and fluid removal. Design differences between the trials include follow-up time (12 months in the FHN Nocturnal vs 6 months in the Culleton trial) and the method for measuring LV mass (which included papillary muscles in the Culleton trial but not in the FHN Nocturnal Trial).
Both the conventional and frequent hemodialysis treatments were performed primarily at home. The mean PHC score for the entire FHN Nocturnal cohort (both frequent nocturnal and conventional arms) increased by 2.4 points (95% CI 0.3 to 4.5 points, P = 0.02). However, the increase in the PHC score was similar between the two groups, with an estimated mean difference of only 0.6 points, corresponding to less than ~1/10th of the s.d. of PHC, with a 95% CI from −3.4 to 4.7 points (Table 3). Hence, although the overall increase in the mean PHC in both treatment groups is consistent with a positive effect of the change in venue from in-center hemodialysis at baseline to home hemodialysis during follow-up, our data provide no suggestion that the PHC score was improved by the frequent dialysis therapy itself. Although nocturnal dialysis has been reported to be associated with improved quality of life in some previous observational studies,11 our observation of an increase in the PHC in both treatment arms suggests that these differences may have resulted in part from differences in venue (home for frequent nocturnal hemodialysis vs in-center hemodialysis for conventional) rather than the therapy itself.
Adverse effects of more frequent nocturnal home hemodialysis included a trend for more frequent vascular access complications, which were due to an increase in vascular access procedures other than failures. This observation is most likely secondary to the more frequent use of the vascular access with more frequent hemodialysis. Hypotensive episodes were less common in the frequent nocturnal arm, which could reflect either the lower ultrafiltration rates used or differences in the reporting rates for hypotension between the two study arms.
The strengths of the FHN Nocturnal Trial include the relatively diverse patient population in terms of demographics and comorbidity, excellent separation between trial arms for both weekly dialysis dose, as measured by stdKt/Vurea, and the number of prescribed treatments per week, the use of blinded cardiac magnetic resonance imaging measurements for assessment of LV mass, and the wide variety of secondary outcomes examined, including adverse events. In contrast, previous observational studies have methodological limitations, including selection bias, nonrandomized trial study design, minimal data on access complications, and other potential safety and feasibility issues.19
The major limitations of the trial were the relatively small sample size and the lower adherence to the dialysis prescription in the frequent nocturnal arm, both of which reduced the power of the study. Recruitment was difficult as the initial protocol forced random assignment to in-center versus home hemodialysis; most patients interested in this study uniformly wanted to be dialyzed at home. Recruitment increased when patients were offered a choice between two different types of home hemodialysis, but was still difficult in part because of the proximity of other home hemodialysis programs offering the use of new home hemodialysis technologies and also because of barriers to acceptance of home hemodialysis therapies.20 In addition, only 87.3% of the patients who were randomized completed 12 months of follow-up and had measures of both coprimary outcomes. Moreover, ~25% of patients in the frequent nocturnal arm performed less than five hemodialysis treatments per week. In addition, patients in the FHN Nocturnal Trial were younger, had more residual renal function, and were less likely to be African American,21 thus limiting generalizability to the broader population of patients receiving maintenance hemodialysis therapy. Finally, the trial was not powered to determine the effect of the intervention on mortality or hospitalization rate. Owing to difficulty in recruitment, the power to detect modest differences in the coprimary composite outcomes and the main secondary outcomes was lower than anticipated.
In conclusion, frequent nocturnal home hemodialysis, compared with three times per week hemodialysis, did not result in significant benefits on the coprimary composite outcomes of death/LV mass or death/PHC. Moderate effects on LV mass may have gone undetected because of a small sample size; PHC improved in both groups, perhaps secondary to the effect of performing dialysis at home. Frequent nocturnal hemodialysis improved control of hyperphosphatemia and hypertension, but tended to increase vascular access events.
MATERIALS AND METHODS
Study setting
The FHN Nocturnal Trial was a multicenter, randomized, prospective trial of frequent home nocturnal hemodialysis sponsored by the National Institute of Health, National Institutes Diabetes, Digestive and Kidney Diseases (NIDDK), and the Center for Medicare and Medical Services (CMS). The design of the FHN Nocturnal Trial has been previously described.19,21 Patients were enrolled between March 2006 and May 2009 and the trial concluded in May 2010. The study was approved by the local institutional review board at each participating site. An independent data safety monitoring board provided oversight.
Study design
Study population
The inclusion and exclusion criteria are listed in Supplementary Table S1 online. Written informed consent was obtained from all participants.
Randomization
Patients were randomized in a 1:1 ratio to either three times per week hemodialysis for <5 h per session (conventional hemodialysis) or six times per week hemodialysis for ≥6 h per session (frequent nocturnal hemodialysis). Randomization was performed centrally using random permuted blocks, stratified by clinical center and by diabetic status.
Interventions and follow-up
The weekly stdKt/Vurea was defined as the ratio of urea generation rate to the average predialysis urea concentration, adjusted for the number of hemodialysis treatments per week.22 The delivered stdKt/Vurea was calculated by formal urea kinetic modeling as the ratio of the urea generation rate to the averaged predialysis blood urea nitrogen concentration with a correction for residual renal function.23 Equilibrated eKt/V was computed using a modified Tattersall correction to the single pool Kt/V.24 The residual renal function exclusion criterion was higher in the FHN Nocturnal Trial than in the FHN Daily Trial (>10 ml/min per 1.73 m2 as calculated as the average of the urea and creatinine clearances versus >3 ml/min per 1.73 m2 of urea clearance, respectively)21 as it was anticipated that the dose separation would be greater in the Nocturnal Trial.
Patients in the conventional arm remained on their usual three times per week hemodialysis prescription subject to a prescribed eKt/Vurea >1.1, a stdKt/Vurea of >2.0, and a treatment time ≥2.5 h/session. Patients randomized to the frequent nocturnal arm followed dialysis prescriptions subject to a stdKt/Vurea of ≥4.0 and a treatment time of ≥6 h, parameters designed to yield the maximum feasible dose using current dialysis technology.
Treatment parameters were monitored while subjects remained under the care of FHN centers. All treatment parameters were averaged over the first modeled dialysis within each follow-up month after 2 to 3 months. Adherence to the hemodialysis prescription was calculated as the ratio of the number of delivered hemodialysis treatments per month divided by the number of prescribed hemodialysis sessions per month.
All study participants were dialyzed using single-use high-flux dialyzers. A committee on standards of care, blinded to intervention, periodically reviewed and reported to clinical centers results of prespecified measures (phosphate, hemoglobin, bicarbonate, normalized protein nitrogen appearance, and blood pressure relative to achieved target postdialysis weight) that were outside of values recommended in published guidelines. Demographic, clinical, and laboratory data were obtained locally by site investigators and study coordinators. Additional data on missed dialysis sessions were obtained in both study arms on a prospective basis. Detailed information on the delivered dialysis prescription was obtained for all dialysis sessions that took place during 1 week of each follow-up month. Standardized assessments of comorbidity were obtained using a modification of the Charlson Index,25 supplemented by additional items from the Index of Co-existing Disease Score.26
Outcomes
The two coprimary end points were: (1) death or 12-month change in LV mass (death/LV mass), and (2) death or 12-month change in the SF-36 RAND PHC (death/PHC).27 We stipulated that demonstration of favorable effects on both coprimary outcomes would be interpreted as providing evidence of overall benefit. LV mass was measured by cardiac magnetic resonance imaging and evaluated in a blinded manner by a central reading center.28 The SF-36 RAND PHC was obtained via blinded telephone interviews conducted by a central quality of life core. Cost and recruitment constraints precluded the possibility of examining survival or hospitalization rates with adequate statistical power. LV mass, however, has been shown to be an independent predictor of survival,17 and studies in patients with end-stage renal disease18 have found that a decrease in LV mass over time is associated with lower rates of death and cardiovascular events. In addition, cross-sectional values of self-reported physical health in end-stage renal disease patients correlate with mortality and hospitalization rates.29
Nine conceptually distinct therapeutic outcome domains were chosen to reflect the potential impact on multiple aspects of end-stage renal disease. A single outcome measure was considered to be the main secondary outcome for seven of these domains. These included the change from baseline to 12 months in LV mass, PHC score, Beck Depression Inventory,30 serum albumin, Trail Making B,31,32 predialysis serum phosphorus level, and erythropoiesis-stimulating agent dose. Other key secondary outcomes included the rate of non-access hospitalization or death, and, for hypertension, predialysis systolic blood pressure and the number of prescribed antihypertensive agents.
Data regarding serious adverse events, including hospitalizations and deaths, were collected prospectively and adjudicated in a blinded manner by an outcomes committee. Vascular access events were defined as access failures, infections requiring a procedure, thrombectomies, angioplasties, catheter replacements, and fibrin stripping of catheters. Hypotensive episodes were defined as the need for a lower ultrafiltration rate, reduced blood flow, or saline administration to ameliorate hypotension. The investigators could not be blinded to the patient’s assigned intervention; however, investigators and patients remained blinded to outcome comparisons throughout the trial.
Vanguard design and protocol changes
Because the feasibility of conducting a randomized trial comparing nocturnal home hemodialysis with conventional in-center hemodialysis was not known, the trial was initially conducted using a Vanguard design.33 Recruitment, retention, and adherence were closely monitored during the initial year of the study to evaluate viability of the sample size targets and delivery of the intervention. Vanguard phase subjects were retained for final analyses.
During and after the Vanguard phase, several study design parameters were changed. The original sample size of 250 patients was estimated to provide 80% power to detect a 12-month change of 11.0 g in LV mass and a 4.2-point change in the SF-36 PHC. The Vanguard phase of the trial revealed that this goal was not feasible, and the target sample size was reduced to 150. The lower target sample size was justified in part by newly published data15 suggesting that frequent nocturnal hemodialysis might reduce average LV mass by ~15 g, which could be detectable with slightly fewer than 150 patients. However, the sample size was ultimately reduced to 90 patients because of continued difficulties with recruitment. This smaller sample size allowed for the detection of only large effects in the two coprimary outcomes, with 80% power to detect a 19.6-g reduction in LV mass and a 7.4-point improvement in PHC.
In addition, the initial protocol specified that conventional arm patients receive in-center hemodialysis three times a week. Follow-up was 14 months to allow up to 2 months of in-center training for the nocturnal home hemodialysis patients, resulting in at least 12 months of follow-up in the nocturnal arm. Because of the difficulties with recruitment, a revised protocol was adopted in which all of the last 72 participants were first trained in home hemodialysis. Those patients randomized to the conventional arm received hemodialysis at home rather than in-center hemodialysis. Follow-up was also shortened to 12 months for the last 72 patients randomized under the revised protocol; the 14-month follow-up was retained for the first 15 patients.
Statistical analyses
The two coprimary composite outcomes were analyzed using the Hochberg correction of the Bonferroni procedure,34 with a study-wise two-sided significance level of 0.05. Each of the two coprimary outcomes was analyzed using a rank-based nonparametric procedure. Patients who had died were ranked lowest, with the order of ranking determined by the duration of survival. Those who survived were ranked based on the change in the LV mass (or PHC) from baseline, with the ranking ordered from the most unfavorable change to the most favorable change. Patients were right censored at the time of transplantation or lost to follow-up; hence, patients who survived but did not provide 1-year LV mass or PHC measurements were credited with 1-year survival in the analysis. Ranks between the treatment arms were compared using the log rank test, and Cox regression was used to determine the associated HR and 95% CIs.
The analyses of the prespecified main secondary outcomes were performed on a comparison-wise basis without adjustment for multiple comparisons. Analyses of quantitative secondary outcomes were performed on the observed data without imputation of missing values by applying mixed-effects analyses using an unstructured covariance model to account for correlations in measurements over time,35 with covariate adjustment for age, diabetic status, baseline level of the glomerular filtration rate, and the baseline variable under analysis and the interactions of these factors with time. These models were used to compare mean changes from baseline to month 12 between the treatment groups while incorporating values at baseline, 4 months (all but LV mass), and 12 months. The 4- and 12-month values were averaged from three monthly assessments (months 3–5 and 10–12) for predialysis levels of albumin, phosphorus, hemoglobin, and average weekly systolic blood pressure. In addition, treatment group comparisons of unadjusted mean changes are provided for patients completing their 12-month assessments for the LV mass and the PHC.
Certain modifications of this strategy were necessary for the analyses of erythropoiesis-stimulating agents, the Trail Making B, and the number of antihypertensive medications. Darbepoetin dose levels were converted to approximate equivalent erythropoietin dose using the expression erythropoietin dose (units) = 250 × darbepoetin (mg).36 The erythropoietin (or equivalent transformed darbepoetin) dose was set to a minimum of 5000 units per 4 weeks for patients using <5000 units, and log transformed before application of the mixed-effects analysis described above. The treatment effect on erythropoiesis-stimulating agents was expressed as the ratio of the geometric mean changes between the frequent nocturnal and conventional groups. Standard errors of the adjusted means for erythropoiesis-stimulating agents were computed using the δ-method. Treatment group comparisons for the number of antihypertensive agents and the Trail Making B (with patients failing to complete the Trail Making B assigned the lowest rank) were obtained using exact Wilcoxon rank-sum tests stratified by quartiles of the corresponding baseline values.
Time to death, first non-access hospitalization/death, and first access intervention were analyzed with Cox regression, controlling for diabetes, age, and baseline glomerular filtration rate. HRs and P-values comparing treatment arm event rates for multiple hospitalizations and vascular access events per patient were calculated using the Andersen–Gill model. Comparison of other adverse events between the two treatment arms was made using Fisher’s exact test. All analyses were performed according to intent-to-treat principle and were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Supplementary Material
FHN Nocturnal Trial eligibility criteria.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the Centers for Medicare and Medicaid Services, and the NIH Research Foundation. The investigators and sponsors are grateful for the support of contributors to the NIH Foundation: Amgen, Baxter, and Dialysis Clinics, and support from Fresenius Medical Care. We are indebted to the patients who participated in the study, and to the United States Renal Data System for providing additional hospitalization data. This trial was registered at Clinical Trials.gov #NCT 00271999.
Appendix
List of institutions and investigators in the FHN Trial Group
Chair, Steering Committee: Kliger A; NIDDK: Eggers P, Briggs J, Hostetter T, Narva A, Star R; Centers for Medicare and Medical Services: Augustine B, Mohr P; Data Coordinating Center–Cleveland Clinic: Beck G (PI), Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, MacKrell J, Wiggins K, Sherer S, Weiss B; MRI Core–Ohio State University and Mt Sinai Medical Center: Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M; Tran T, West J; Central Quality of Life Core–University of Pittsburgh: Unruh M; Keene R, Schlarb J; Central Holter Core–Toronto General Hospital: Chan C; McGrath-Chong M; Biospecimen Repository–Fisher BioServices: Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C; Data Safety and Monitoring Board: Eknoyan G (Chair), Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E; Nocturnal Trial Clinical Sites—Wake Forest University School of Medicine Consortium: Rocco M (PI); Barnes-Jewish/Washington University: Miller B, Riley J, Schuessler R; Lynchburg Nephrology: Lockridge R, Pipkin M, Peterson C; Rubin Dialysis: Hoy C, Fensterer A, Steigerwald D; University of Iowa: Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E; University of Toronto: Chan C, McGrath-Chong M; University of British Columbia: Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D; University of Western Ontario: Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E; Wake Forest University School of Medicine: Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T; Humber River Regional Hospital: Pierratos A, Chan W, Regozo K, Kwok S.
Footnotes
The results from this clinical trial were presented in the abstract form at the American Society of Nephrology meeting in Denver, Colorado in November 2010.
DISCLOSURE
GMC is a member of the Scientific Advisory Board of DaVita Clinical Research and the Board of Directors of Satellite Healthcare. MC is a member of Baxter Healthcare Dialysis Advisory Board. TG is a consultant for Eli Lilly, Amgen, Cormedix, Keryx Biopharmaceuticals, and Nephrogenex. ASK is an investigator whose research originated in Amgen. NWL owns stock in Fresenius Medical Care North America. MVR is a consultant for Amgen and DaVita. MLU is a consultant for Baxter Healthcare, Merck, and Sigma Tau with investigator initiated research from Baxter, DCI, and Satellite Dialysis. All other authors declared no competing interests.
References
- 1.U.S. Renal Data System, USRDS 2008 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2008. http://www.usrds.org. 2009. [Google Scholar]
- 2.Lowrie EG, Laird NM, Parker TF, et al. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305:1176–1181. doi: 10.1056/NEJM198111123052003. [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G, Beck GJ, Cheung AK, et al. for the Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 4.Unruh M, Benz R, Greene T, et al. Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int. 2004;66:355–366. doi: 10.1111/j.1523-1755.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Rocco MV, Dwyer JT, Larive B, et al. for the HEMO Study Group. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2321–2334. doi: 10.1111/j.1523-1755.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 6.Suri RS, Nesrallah GE, Mainra R, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1:33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Chan CT, Jain V, Picton P, et al. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int. 2005;68:338–344. doi: 10.1111/j.1523-1755.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 10.Pierratos A. Nocturnal home hemodialysis: an update on a 5-year experience. Nephrol Dial Transplant. 1999;14:2835–2840. doi: 10.1093/ndt/14.12.2835. [DOI] [PubMed] [Google Scholar]
- 11.Lockridge RS, Jr, Spencer M, Craft V, et al. Nightly home hemodialysis: five and one-half years of experience in Lynchburg, Virginia. Hemodial Int. 2004;8:61–69. doi: 10.1111/j.1492-7535.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 12.Mucsi I, Hercz G, Uldall R, et al. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53:1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 14.Sikkes ME, Kooistra MP, Weijs PJ. Improved nutrition after conversion to nocturnal home hemodialysis. J Ren Nutr. 2009;19:494–499. doi: 10.1053/j.jrn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. J Am Med Assoc. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 16.Chertow GW, Levin NW, Beck GJ, et al. Effects of frequent in-center hemodialysis: the Frequent Hemodialysis Network Daily Trial. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoccali C, Benedetto FA, Mallamaci F, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–1498. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 18.London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 19.Suri RS, Garg AX, Chertow GM, et al. For the Frequent Hemodialysis Network (FHN) Trial Group. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 20.Pipkin M, Eggers PW, Larive B, et al. for the Frequent Hemodialysis Network Trial Group. Recruitment and training for home hemodialysis: experience and lessons from the nocturnal dialysis trial. Clin J Am Soc Nephrol. 2010;5:1614–1620. doi: 10.2215/CJN.02440310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocco MV, Larive B, Eggers PW, et al. for the Frequent Hemodialysis Network. Baseline characteristics of participants in the Frequent Hemodialysis Network Daily and Nocturnal Trials. Am J Kidney Dis. 2011;57:90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotch FA. Evolution of the single pool urea kinetic model. Semin Dial. 2001;14:252–256. doi: 10.1046/j.1525-139x.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 23.Daugirdas JT, Depner TA, Greene T, et al. Standard Kt/V(urea): a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77:637–644. doi: 10.1038/ki.2009.525. [DOI] [PubMed] [Google Scholar]
- 24.Daugirdas JT, Greene T, Depner TA, et al. Factors that affect postdialysis rebound in serum urea concentration, including the rate of dialysis: results from the HEMO Study. J Am Soc Nephrol. 2004;15:194–203. doi: 10.1097/01.asn.0000103871.20736.0c. [DOI] [PubMed] [Google Scholar]
- 25.Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–132. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 26.Miskulin DC, Athienites NV, Yan G, et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int. 2001;60:536–540. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 28.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 29.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 30.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 31.Kurella M, Chertow GM, Luan J, et al. Cognitive impairment in chronic kidney disease. J Am Soc Geriatrics. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 32.Umans JG, Pliskin NH. Attention and mental processing speed in hemodialysis patients. Am J Kidney Dis. 1998;32:749–751. doi: 10.1016/s0272-6386(98)70129-5. [DOI] [PubMed] [Google Scholar]
- 33.Tavel JA, Fosdick L for the ESPRIT Vanguard Group and the ESPRIT Executive Committee. Closeout of four phase II Vanguard trials and patient rollover into a large international phase III HIV clinical endpoint trial. Control Clin Trials. 2001;22:42–48. doi: 10.1016/s0197-2456(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg YA. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–803. [Google Scholar]
- 35.Verbeke G, Molenbergh G. Linear Mixed Models for Longitudinal Data (Springer Series in Statistics) Springer; New York, NY: 2000. p. 568. [Google Scholar]
- 36.Bock HA, Hirt-Minkowksi P, Brünisholz M, et al. Darbepoetin alpha in lower-than-equimolar doses maintains haemoglobin levels in stable haemodialysis patients converting from epoetin alpha/beta. Nephrol Dial Transplant. 2008;23:301–308. doi: 10.1093/ndt/gfm579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FHN Nocturnal Trial eligibility criteria.