Abstract
EPR and 1H ENDOR spectroscopies have been used to analyze intermediate states formed during the hydroxylation of (1R)-camphor [H2-camphor] and (1R)-5,5-dideuterocamphor [D2-camphor] as induced by cryoreduction (77 K)/annealing of the ternary ferrous cytochrome P450cam-O2-substrate complex. Hydroxylation of H2-camphor produced a primary product state in which 5-exo-hydroxycamphor is coordinated with Fe(III). ENDOR spectra contained signals derived from two protons [Fe(III)-bound C5-OHexo and C5-Hendo] from camphor. When D2-camphor was hydroxylated under the same condition in H2O or D2O buffer, both ENDOR Hexo and Hendo signals are absent. For D2-camphor in H2O buffer, H/D exchange causes the C5-OHexo signal to reappear during relaxation upon annealing to 230 K; for H2-camphor in D2O, the C5-OHexo signal decreases through H/D exchange. These observations clearly show that Cpd I is the reactive species in the hydroxylation of camphor in P450cam.
The cytochromes P450 form a superfamily of cysteine thiolate-ligated heme enzymes that catalyze reductive activation of dioxygen for the insertion or addition of a single oxygen atom into a wide variety of substrates. These reactions include hydroxylations, epoxidations, sulfoxidations, and many more.1 P450 enzymes are critical to many biological processes, including steroid hormone biosynthesis, drug metabolism, and the detoxification of xenobiotics, and are found in most classes of organisms.1–5
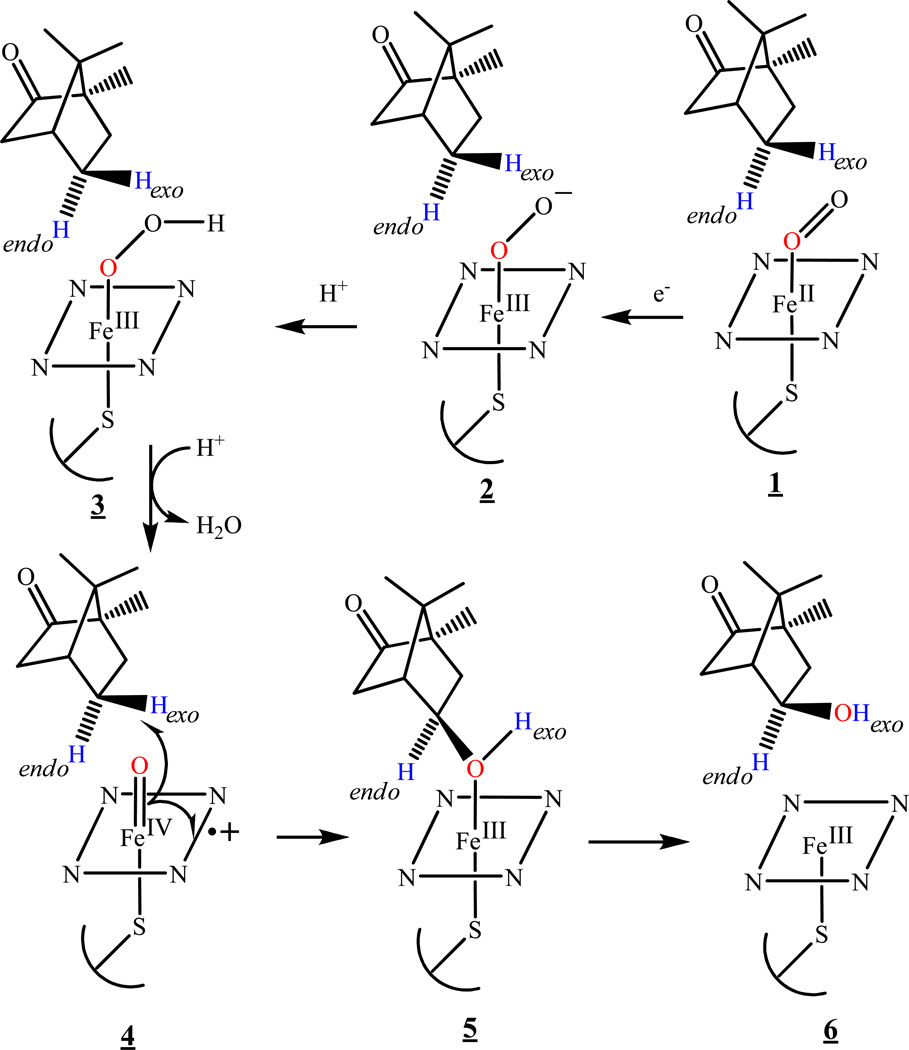
The reaction cycle of cytochrome P450s and the nature of the catalytically active state have been the object of intense study for over three decades.6–13 The classic cytochrome P450 catalytic cycle is initiated by binding substrate to the ferric state followed by one-electron reduction to the ferrous state by NAD(P)H-dependent reductases. Binding of molecular oxygen gives the ferrous-dioxygen (1) complex, which has been observed and characterized (Scheme 1). The addition of a second electron to the oxyferrous complex leads to a peroxoferric (2), which is protonated to generate a hydroperoxoferric (3) intermediate. Protonation of the distal oxygen in the hydroperoxo intermediate then leads to heterolytic O-O bond cleavage with loss of water and generation of iron(IV)oxo porphyrin π-cation radical (Fe(IV)=O P+·) center, known as Compound I (Cpd I) (4). In this classical scheme, Cpd I is the catalytically active species in substrate hydroxylation,2–5 but it has also been proposed that the peroxo or hydroperoxo intermediates can function as the hydroxylating species in some enzymes and/or with specific classes of substrates.2–5,14
Scheme 1.
Cytochrome P450 intermediates in the presence of D2-camphor. 1, Oxyferrous; 2, Peroxoferric; 3, Hydroperoxoferric, 4, Compound I.
The short lifetime of intermediates 2–4 makes it difficult to characterize them and their reactions with substrate by conventional kinetic and spectroscopic techniques under physiological conditions. Thus, until recently, the oxyferrous complex was the last observable intermediate in the reaction cycle of heme-containing monooxygenases; Cpd I now has been detected by direct oxidation of substrate-free ferric P450cam and Cyp 119 with m-chloroperoxobenzoic acid.15–17 These limitations have been largely overcome by application of radiolytic cryoreduction at 77 K, which both generates and traps one-electron reduced ternary ferrous heme monooxygenase-O2-substrate complexes that are catalytically competent during annealing to higher temperatures.14,18–25 Cryogenic stabilization of intermediates generated in this way has made possible the direct spectroscopic and structural characterization of peroxoferric and hydroperoxoferric intermediates, as well as measurement of the kinetics of their interconversion and the formation of product.14,26 In contrast, no spectroscopically detectable amounts of Cpd I are observed during these conversions in the presence of substrate because of the rapid reaction with bound substrate, although Cpd I again has been observed during annealing of cryoreduced oxyferrous dehaloperoxidase in the absence of bound substrate.23 Nonetheless, the cryoreduction approach in combination with EPR/ENDOR spectroscopy permits identification of the reactive species involved in conversion of bound substrate to product.14
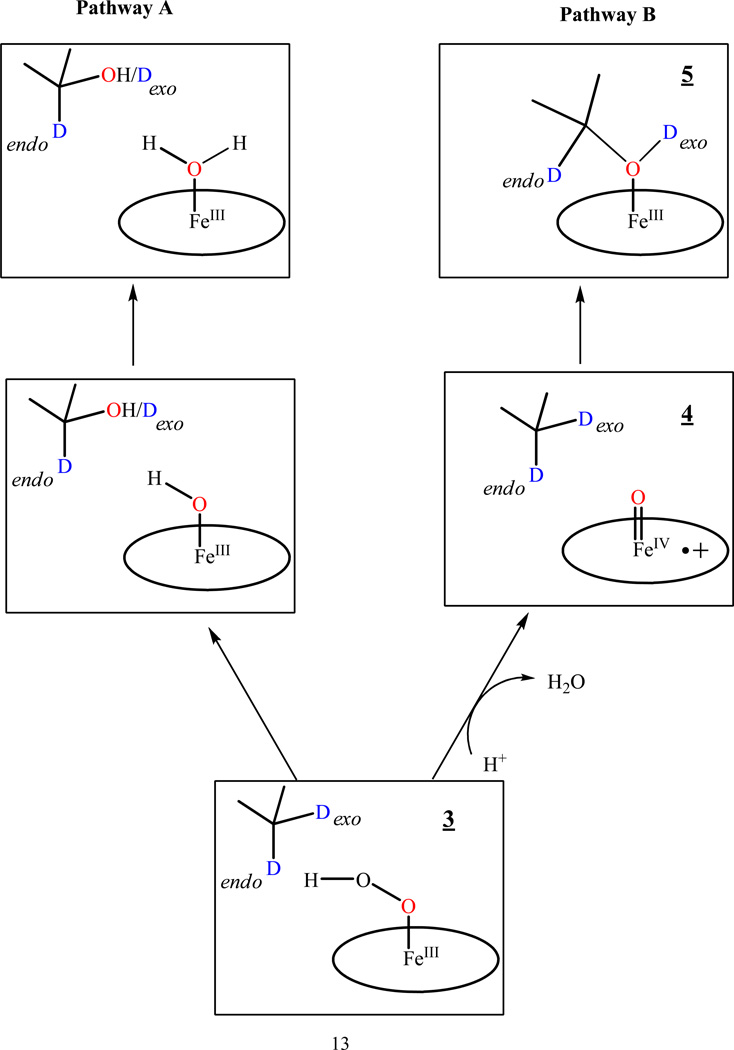
Identification of the active oxidizing species in cytochrome P450 reactions through cryoreduction techniques has been based on EPR/ENDOR on the solvent isotope of the isotopic composition of the primary product state trapped during annealing of the cryoreduced ternary complex of oxyferrous heme monooxygenase with bound substrate.14 If the product is formed by insertion of the ferryl oxygen of Cpd I into a C-H bond of substrate, the primary product state trapped by cryoreduction/annealing at low temperatures contains the product alcohol bound to the heme iron(III) (Scheme 2, Pathway B). When reaction occurs with deuterated substrate in H2O, the product hydroxyl group contains the D abstracted from the reaction; when reaction occurs in D2O with protonated substrate the product hydroxyl contains the H abstracted from substrate; in the relaxed product state that forms during annealing to higher temperatures, as visualized in the previously reported crystal structure of cytochrome P450cam with its product, 5-exo-hydroxycamphor bound by its OH group to the low-spin Fe(III) heme,27 the hydroxyl H/D of the primary product can exchange with solvent. The source of the exchangeable hydroxyl H or D in the bound product can be determined by 1H ENDOR spectroscopy.
Scheme 2.
Alternative reaction pathways during hydroxylation of D2-camphor by hydroperoxoferric P450 (Pathway A) intermediate and Cpd I (Pathway B).
If, instead, a peroxo/hydroperoxoferric heme intermediate is the reactive species (Scheme 2, Pathway A), the primary product trapped at low temperature contains a hexacoordinated aqua ferriheme, while the hydroxylated substrate is sequestered remote from the heme in the distal pocket of the active site.14 The same is true for the "somersault" mechanism suggested by Bach.13 In this case, the bound water incorporates H/D from solvent, not substrate, and again this can be revealed by 1H ENDOR, while the remote hydroxylated substrate in this state would at most show a 1,2 H ENDOR signal with small hyperfine coupling.
Previously, a cryoreduction/ENDOR study that employed such a variation in solvent isotope provided evidence that Cpd I is the active state in the hydroxylation of bound camphor by the cryoreduced oxyferrous mutant D251N P450cam.19 We herein cleanly demonstrate the accuracy of this conclusion through a complementary study that uses cryoreduction/ENDOR techniques to determine the variation of the isotopic composition of the primary product of hydroxylation with the isotopic composition of the substrate. Comparison of the primary product formed with (1R)-camphor [H2-camphor] and (1R)-5,5-dideuterocamphor [D2-camphor] by ENDOR spectroscopy directly reveals the agency of Cpd I as the reactive intermediate.
EXPERIMENTAL PROCEDURE
Chemicals
Solid Na2S2O4, chromium trioxide, d4-hydrazine, (R)-camphor and all other chemicals and reagents were purchased from standard commercial sources (Aldrich) and used as received. O2 gas was obtained from Matheson Co.
Materials
The synthesis of (1R)-5,5-dideuterocamphor (D2-camphor) was carried out in two steps. First step: To 1R (+)-camphor (10 g) in refluxing glacial acetic acid (150 mL), chromium trioxide (20.7g) was cautiously added in 3 portions over 30 mins.28 Refluxing was continued for 1 h. The solution was then cooled and diluted with water (150 mL). The organic layer was extracted with ether (3 × 100 mL). The combined extracts were washed with saturated sodium bicarbonate solution (6 × 50 mL) and brine (100 mL), dried over anhydrous MgSO4, filtered and the solvent removed under vacuum. Flash chromatography using silica and hexane-ethyl acetate (80:20) gave the product, 5-oxo-1R (+)-camphor (5-ketocamphor), as white solid. GCMS data: 6.1 min; m/z: 166 (M), 151, 123, 109, 95, 83, 69, 55; 1H NMR (300 MHz, CDCl3) δ 0.93 (s, 3H), 1.03 (d, J = 3 Hz, 6H), 2.01 (d, 1H, J = 19 Hz 6-exo), 2.13 (d, J = 17.5 Hz 1H, 3-endo), 2.31 (d, J = 18.5 Hz 1H, 6-endo), 2.55 (m, 2H, 3-exo, 4-bridge); 13C NMR δ 214.3, 212.5, 58.1, 57.6, 46.1, 42.7, 36.6, 19.5, 18.3, 9.0.(29) (Supporting Information Figure S1)
Second step: 5-oxo-1R (+)-camphor (0.05 g), d4-hydrazine (0.0432 g) and potassium t-butoxide (0.134 g) were taken in DMSO-d6 (10 mL). The mixture was refluxed for 18 h under anhydrous conditions (bath temperature maintained at 175 °C). D2O (10 mL) and DCl (30%, 1 mL) were added to the mixture and stirred for 30 minutes at room temperature. The product was examined by TLC and purified by flash chromatography using silica and hexane-ethyl acetate (90:10) as solvent. GCMS data: 9.37 min; m/z: 154 (M), 109, 97, 83, 69, 55. (Supporting Information Figure S2)
The E. coli expression system was used to express and purify P450cam protein as previously described. 24
Sample Preparation
EPR /ENDOR samples of oxyferrous P450cam complexes with H2-camphor and D2-camphor were prepared as described previously.24 The oxyferrous P450 samples were reduced at 77 K by γ-irradiation with a 60Co source to a dose of 3 Mrad and the annealing protocol for the irradiated samples also has been described.24
Spectroscopic Techniques
EPR/ENDOR measurements of cryoreduced samples were conducted as previously described.25,30
RESULTS AND DISCUSSION
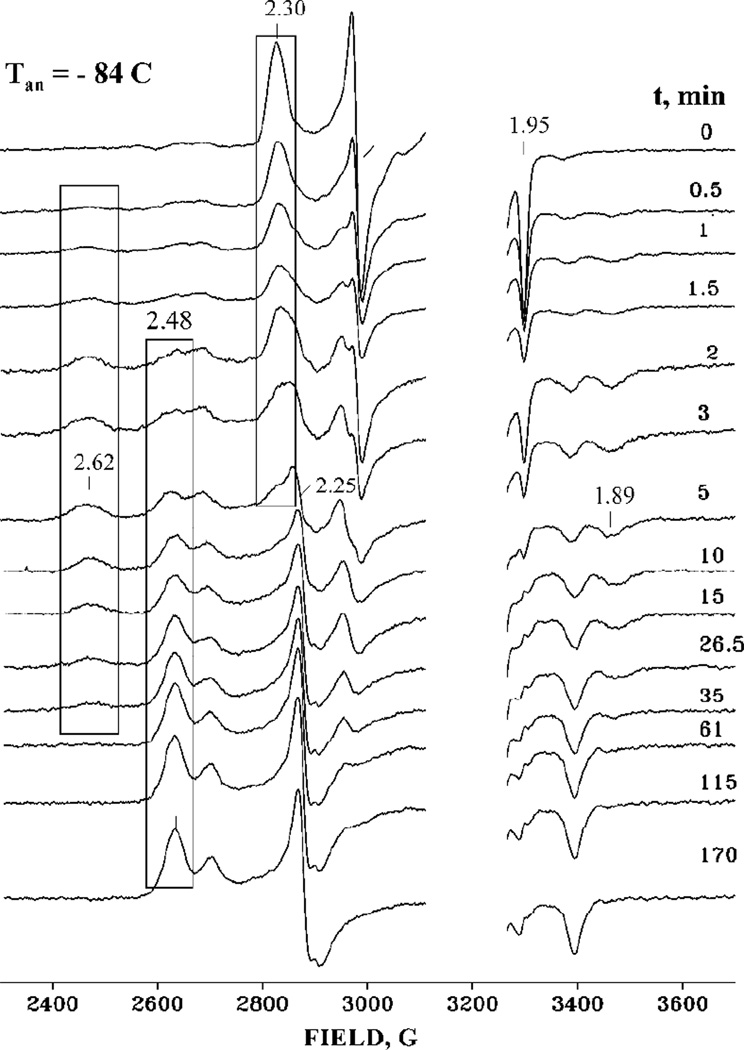
Studies of the catalytic intermediates in the P450cam catalytic cycle begin with the substrate-bound oxyferrous state of the protein. Figure 1 shows EPR spectra of the cryoreduced ternary oxyferrous P450-camphor complex taken during its progressive annealing at 190 K. The 77 K cryoreduced ternary complex shows a rhombic EPR signal with g = [2.30, 2.18, 1.9], characteristic of the S = ½ hydroperoxoferric heme species18,19,24 As seen previously, during annealing at 190 K, the hydroperoxoferric intermediate converts into the primary product, with g = [2.62, 2.25, 1.89], which then relaxes to the equilibrium state (Figure 1).19 This relaxed complex shows two low-spin EPR signals with g = [2.48, 2.25, 1.90] and [2.41, 2.23 1.96], assigned respectively to ferric P450-5-hydroxy camphor complex and aquo ferric P450cam. Decay of the hydroperoxoferric heme intermediate during annealing at 190 K was earlier shown to be two-fold slower in D2O solvent,18 establishing that the rate-limiting step of the decay of hydroperoxo intermediate involves activation of the hydroperoxo moiety by transfer of a proton to it, as presumed for Cpd I formation.
Figure 1.
X-band EPR spectra of cryoreduced oxyferrous P450cam-camphor complex taken during progressive annealing at 190 K at indicated times. Blue boxes isolate the loss of the signal at g = 2.30 from the hyrdoxoferric state, the appearance and loss of the g = 2.62 signal from the primary product state, and the appearance of the g = 2.48 signal from the relaxed product state. Instrument conditions: T = 29 K, MW frequency 9.369, MW power 13 db, modulation amplitude Am = 10 G.
According to Scheme 2 (Pathway B), hydroxylation of H2-camphor by Cpd I should produce a primary product with ENDOR signals from two protons derived from camphor, one from the C5-OHexo group coordinated to Fe(III), the other from the remaining C5-Hendo, whereas if hydroxylation occurs by the hydroperoxyferric heme, in the primary product the heme iron would be coordinated by water and the C5-Hendo proton of hydroxycamphor will be too far away to give an appreciable hyperfine interaction.
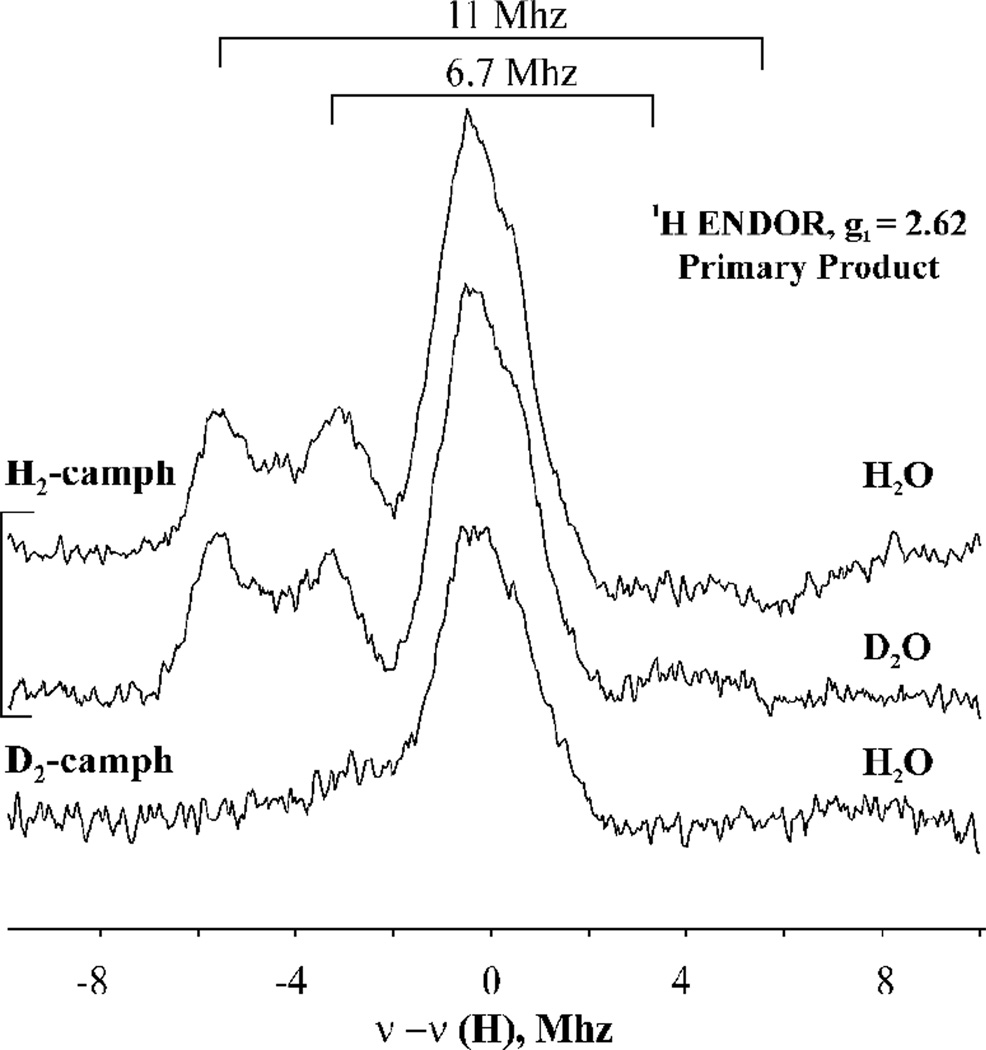
Our previous studies of the primary product complex formed in H2O buffer with C5-H2-camphor, showed two well-resolved, strongly-coupled 1H ENDOR signals. This observation is repeated in this work. Figure 2 presents 1H ENDOR spectra taken at the canonical g1 = 2.62 field position for the primary product intermediate. It shows signals from two protons, with hyperfine coupling, A(H1) = 11 MHz and A(H2) = 6.7 MHz, as previously reported. Figure 2 also shows the complementary ENDOR spectrum obtained when the primary product is instead formed with D2-camphor in H2O buffer: both the H1 and H2 signals disappear, unambiguously confirming that the two 1H signals seen the H2-camphor substrate arise from the C5 protons of substrate, as they both are absent when product is formed by hydroxylation of D2-camphor.
Figure 2.
1H CW 35 GHz ENDOR spectra taken at g1=2.62 field for the primary product trapped upon annealing at 190 K cryoreduced oxyferrous P450cam-camphor complex in H2O and D2O and cryoreduced oxyferrous P450cam-D2-camphor in H2O. Instrument: T = 2 K, 35.1 GHz, Field modulation 100 kHz, modulation amplitude 2 G, scan speed 1 MHz/s, 100 kHz broadening of RF excitation.
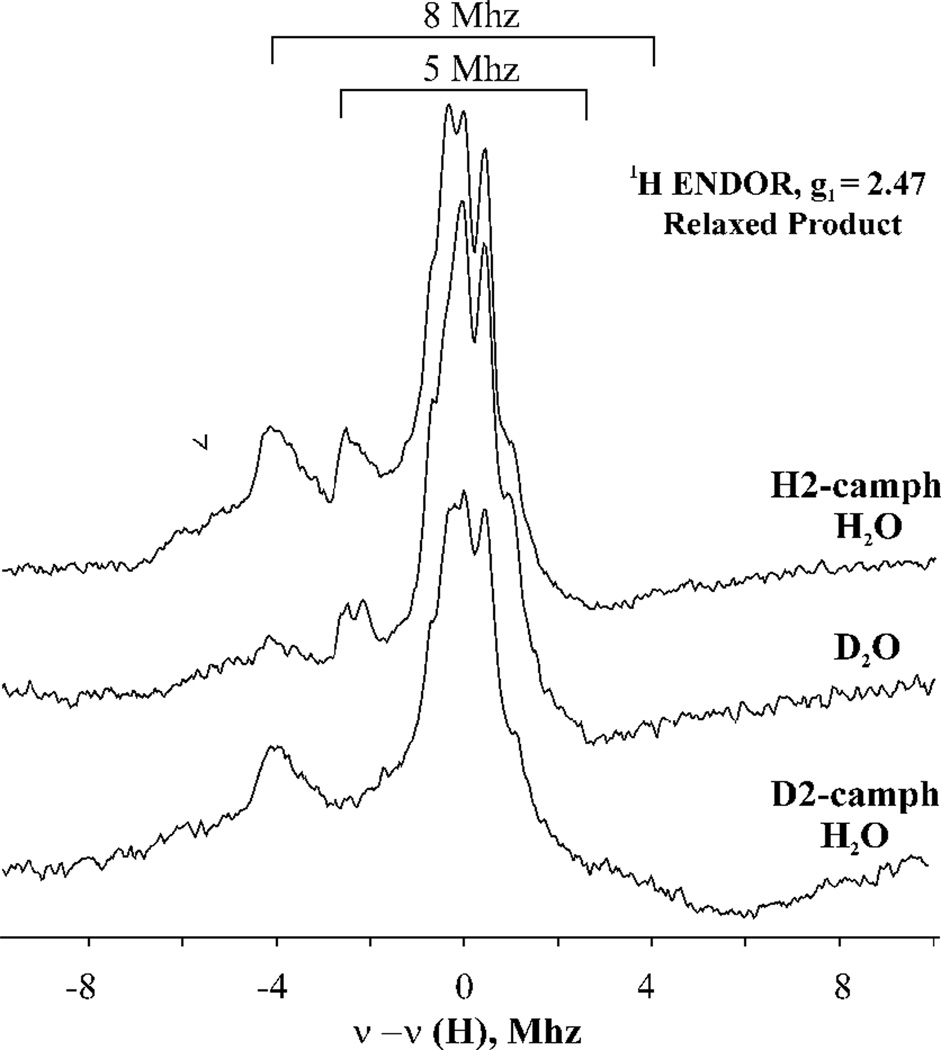
Relaxation of the primary product formed in H2O buffer with C5-H2-camphor gives the equilibrium product complex, which also shows two well-resolved, strongly-coupled 1H ENDOR signals, but with slightly different couplings, Amax (H1) = 9.2 MHz and Amax (H1) = 5.7MHz at g1 = 2.48, as seen in Figure 3. Relaxation experiments previously carried out with D2O buffer and repeated in Figure 3 show that the H1 signal is lost in the relaxed product complex but H2 is not. This indicates that H2 = Hendo(C5) of hydroxycamphor, and upon relaxation, H1 exchanges with solvent and so Hexo, is the proton of the hydroxycamphor hydroxyl coordinated to heme iron (III).19 The differences in hyperfine couplings of the two protons and g-tensor components of the EPR signals of the primary and relaxed (g1 = 2.48) product complexes reflect different geometries of hydroxycamphor coordinated to Fe(III).19
Figure 3.
1H CW 35 GHz ENDOR spectra taken at g1 = 2.47 field for the relaxed product complex formed upon annealing at 230 K cryoreduced oxyferrous P450 cam-camphor complex in H2O and D2O and cryoreduced oxyferrous P450cam-D2-camphor in H2O. Instrument: same as in Figure 2.
Correspondingly, when D2-camphor is the substrate, the primary product state formed in H2O buffer during hydroxylation shows neither Hexo or Hendo signals, but when it is annealed at 230 K the Hexo ENDOR signal reappears as the Fe-bound hydroxyl of 5,5’-OD, D-camphor in the primary product exchanges with H of solvent to form 5,5’-OH,D-camphor (Figure 3). As required, the Hendo signal from the nonexchangeable Hexo(C5) does not reappear (Figure 3)
In summary, complementary cryoreduction/ENDOR approaches in which the isotopic composition of the primary product of camphor hydroxylation by P450cam is correlated with either the solvent or substrate isotopic compositions demonstrate that Cpd I is the reactive hydroxylating species according to Scheme 2; Pathway B. Insertion of ferryl oxygen of Cpd I into the camphor C(5)-Hexo bond of bound camphor leads to formation of 5-OH-camphor with its hydroxyl coordinated to Fe(III), and with the hydroxyl proton derived from substrate. At the reaction temperature of 190 K, the hydroxyl proton does not exchange with solvent, but during annealing of the primary complex at 230 K, the conformation of the product complex relaxes and the OH/D group of the 5-hydroxycamphor product is exchanged with solvent. The ENDOR results are those predicted for Cpd I as the active hydroxylating intermediate (Scheme 2, Pathway B) and rule out the peroxo/hydroperoxo state (Pathway A) for that role.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Leticia Loredo for NMR work and Prof. H. Halpern, Pritzker School of Medicine, University of Chicago, for access to the 60Co Gamma cell irradiation.
Funding
This research was supported by National Institute of Health grants GM 26730 (J.H.D.) and HL13531 (B.M.H.).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Additional supporting spectral data pertaining to the synthesis of (1R)-5,5-dideuterocamphor are included. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem. Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz de Montellano PR, De Voss JJ. Oxidizing species in the mechanism of cytochrome P450. Natural Product Reports. 2002;19:477–493. doi: 10.1039/b101297p. [DOI] [PubMed] [Google Scholar]
- 3.Hlavica P. Models and mechanisms of O-O bond activation by cytochrome P450. A critical assessment of the potential role of multiple active intermediates in oxidative catalysis. Eur. J. Biochem. 2004;271:4335–4360. doi: 10.1111/j.1432-1033.2004.04380.x. [DOI] [PubMed] [Google Scholar]
- 4.Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 5.Perera R, Jin S, Sono M, Dawson JH. Cytochrome P450-catalyzed hydroxylations and epoxidations, in. Metal Ions in Life Sciences. 2007;3:319–359. [Google Scholar]
- 6.Roberts ES, Vaz ADN, Coon MJ. Catalysis by cytochrome-P-450 of an oxidative reaction in xenobiotic aldehyde metabolism - deformylation with olefin formation. Proc. Natl. Acad. SciU.SA. 1991;88:8963–8966. doi: 10.1073/pnas.88.20.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaz ADN, Roberts ES, Coon MJ. Olefin formation in the oxidative deformylation of aldehydes by cytochrome-P-450 - mechanistic implications for catalysis by oxygen-derived peroxide. J. Am. Chem. Soc. 1991;113:5886–5887. [Google Scholar]
- 8.Jin S, Makris TM, Bryson TA, Sligar SG, Dawson JH. Epoxidation of olefins by hydroperoxo-ferric cytochrome P450. J. Am. Chem. Soc. 2003;125:3406–3407. doi: 10.1021/ja029272n. [DOI] [PubMed] [Google Scholar]
- 9.Coon MJ, Vaz AD, McGinnity DF, Peng HM. Multiple activated oxygen species in P450 catalysis: contributions to specificity in drug metabolism. Drug Metab. Dispos. 1998;26:1190–1193. [PubMed] [Google Scholar]
- 10.Vaz AD, McGinnity DF, Coon MJ. Epoxidation of olefins by cytochrome P450: evidence from site-specific mutagenesis for hydroperoxo-iron as an electrophilic oxidant. Proc. Natl. Acad. SciU.SA. 1998;95:3555–3560. doi: 10.1073/pnas.95.7.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toy PH, Newcomb M, Coon MJ, Vaz ADN. Two distinct electrophilic oxidants effect hydroxylation in cytochrome P-450-catalyzed reactions. J. Am. Chem. Soc. 1998;120:9718–9719. [Google Scholar]
- 12.Lai W, Shaik S. Can ferric-superoxide act as a potential oxidant in P450(cam)? QM/MM investigation of hydroxylation, epoxidation, and sulfoxidation. J. Am. Chem. Soc. 2011;133:5444–5452. doi: 10.1021/ja111376n. [DOI] [PubMed] [Google Scholar]
- 13.Bach RD. The rate-limiting step in P450 hydroxylation of hydrocarbons a direct comparison of the "somersault" versus the "consensus" mechanism involving compound I. J. Phys. Chem. A. 2010;114:9319–9332. doi: 10.1021/jp1045518. [DOI] [PubMed] [Google Scholar]
- 14.Davydov R, Hoffman BM. Active intermediates in heme monooxygenase reactions as revealed by cryoreduction/annealing, EPR/ENDOR studies. Arch. Biochem. Biophys. 2011;507:36–43. doi: 10.1016/j.abb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 16.Spolitak T, Dawson JH, Ballou DP. Rapid kinetics investigations of peracid oxidation of ferric cytochrome P450cam: nature and possible function of compound ES. J. Inorg. Biochem. 2006;100:2034–2044. doi: 10.1016/j.jinorgbio.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Spolitak T, Funhoff EG, Ballou DP. Spectroscopic studies of the oxidation of ferric CYP153A6 by peracids: insights into P450 higher oxidation states. Arch. Biochem. Biophys. 2010;493:184–191. doi: 10.1016/j.abb.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Yang TC, Perera R, Jin S, Bryson TA, Sono M, Davydov R, Dawson JH, Hoffman BM. Cryoreduction EPR and 13C, 19F ENDOR study of substrate-bound substates and solvent kinetic isotope effects in the catalytic cycle of cytochrome P450cam and its T252A mutant. Dalton Trans. 2005;21:3464–3469. doi: 10.1039/b506764m. [DOI] [PubMed] [Google Scholar]
- 19.Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. Hydroxylation of camphor by reduced oxy-cytochrome P450cam: mechanistic implications of EPR and ENDOR studies of catalytic intermediates in native and mutant enzymes. J. Am. Chem. Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 20.Davydov R, Kofman V, Fujii H, Yoshida T, Ikeda-Saito M, Hoffman BM. Catalytic mechanism of heme oxygenase through EPR and ENDOR of cryoreduced oxy-heme oxygenase and its Asp 140 mutants. J. Am. Chem. Soc. 2002;124:1798–1808. doi: 10.1021/ja0122391. [DOI] [PubMed] [Google Scholar]
- 21.Davydov R, Razeghifard R, Im SC, Waskell L, Hoffman BM. Characterization of the microsomal cytochrome P450 2B4 O2 activation intermediates by cryoreduction and electron paramagnetic resonance. Biochemistry. 2008;47:9661–9666. doi: 10.1021/bi800926x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davydov R, Sudhamsu J, Lees NS, Crane BR, Hoffman BM. EPR and ENDOR characterization of the reactive intermediates in the generation of NO by cryoreduced oxy-nitric oxide synthase from Geobacillus stearothermophilus. J. Am. Chem. Soc. 2009;131:14493–14507. doi: 10.1021/ja906133h. [DOI] [PubMed] [Google Scholar]
- 23.Davydov R, Osborne RL, Shanmugam M, Du J, Dawson JH, Hoffman BM. Probing the oxyferrous and catalytically active ferryl states of Amphitrite ornata dehaloperoxidase by cryoreduction and EPR/ENDOR spectroscopy. Detection of compound I. J. Am. Chem. Soc. 2010;132:14995–15004. doi: 10.1021/ja1059747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davydov R, Perera R, Jin S, Yang TC, Bryson TA, Sono M, Dawson JH, Hoffman BM. Substrate modulation of the properties and reactivity of the oxy-ferrous and hydroperoxo-ferric intermediates of cytochrome P450cam as shown by cryoreduction-EPR/ENDOR spectroscopy. J. Am. Chem. Soc. 2005;127:1403–1413. doi: 10.1021/ja045351i. [DOI] [PubMed] [Google Scholar]
- 25.Davydov R, Ledbetter-Rogers A, Martasek P, Larukhin M, Sono M, Dawson JH, Masters BS, Hoffman BM. EPR and ENDOR characterization of intermediates in the cryoreduced oxy-nitric oxide synthase heme domain with bound L-arginine or N(G)-hydroxyarginine. Biochemistry. 2002;41:10375–10381. doi: 10.1021/bi0260637. [DOI] [PubMed] [Google Scholar]
- 26.Denisov IG, Mak PJ, Makris TM, Sligar SG, Kincaid JR. Resonance Raman characterization of the peroxo and hydroperoxo intermediates in cytochrome P450. J. Phys. Chem. A. 2008;112:13172–13179. doi: 10.1021/jp8017875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li HY, Narasimhulu S, Havran LM, Winkler JD, Poulos TL. Crystal-structure of cytochrome P450(cam) complexed with its catalytic product, 5-exo-hydroxycamphor. J. Am. Chem. Soc. 1995;117:6297–6299. [Google Scholar]
- 28.Bredt J, Lieser PET, Germar H. Formation of diketocamphane (ketocamphor) and of diketofenchane (ketofenchone) in the oxidation of camphor or fenchone by chromic acid. Journal fuer Praktische Chemie (Leipzig) 1923;106:336–347. [Google Scholar]
- 29.Bays DE, Cannon GW, Cookson RC. Absorption spectra of ketones part VIII. circular dichroism of some trimethylbicyclo[2,2,1]heptan-2-ones. J. Chem. Soc. B. 1966:885–892. [Google Scholar]
- 30.Davydov R, Kappl R, Huttermann J, Peterson JA. EPR-spectroscopy of reduced oxyferrous-P450cam. FEBS Lett. 1991;295:113–115. doi: 10.1016/0014-5793(91)81398-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.