Summary
The unfolded protein response (UPR) is a signaling pathway required to maintain endoplasmic reticulum (ER) homeostasis and hepatic lipid metabolism. Here, we identify an essential role for the inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α)-X-box binding protein 1 (XBP1) arm of the UPR in regulation of hepatic very low-density lipoprotein (VLDL) assembly and secretion. Hepatocyte-specific deletion of Ire1α reduces lipid partitioning into the ER lumen and impairs the assembly of triglyceride (TG)-rich VLDL, but does not affect TG synthesis, de novo lipogenesis, or the synthesis or secretion of apolipoprotein B (apoB). The defect in VLDL assembly is, at least in part, due to decreased microsomal triglyceride-transfer protein (MTP) activity resulting from reduced protein disulfide isomerase (PDI) expression. Collectively, our findings reveal a key role for the IRE1α-XBP1s-PDI axis in linking ER homeostasis with regulation of VLDL production and hepatic lipid homeostasis that may provide a therapeutic target for disorders of lipid metabolism.
Introduction
Hepatic very low-density lipoprotein (VLDL) secretion plays an essential role in regulating intrahepatic and plasma lipid homeostasis (Bamba and Rader, 2007). Elevated production of hepatic VLDL is a common cause of dyslipidemia and is tightly associated with an increased risk of cardiovascular disease, especially for individuals with obesity and type 2 diabetes (Adiels et al., 2008). The mechanism underlying dysregulation of hepatic VLDL production is not completely understood and new factors playing critical roles in this process are still emerging (Calandra et al., 2011; Chen et al., 2010)
The ER is the major site for lipid synthesis and VLDL assembly. ER homeostasis is maintained by an adaptive mechanism termed the unfolded protein response (UPR) through inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α), protein kinase R (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6α). Disturbed ER homeostasis can stimulate lipogenesis (Kammoun et al., 2009) and inhibit hepatic VLDL secretion (Ota et al., 2008; Qiu et al., 2006), resulting in hepatosteatosis. Although each arm of the UPR is required to alleviate hepatosteatosis under pharmacologically-induced, extreme levels of ER stress (Rutkowski et al., 2008), the specific contribution of each individual UPR pathway to either hepatic VLDL production and/or plasma lipoprotein metabolism under physiological conditions is an important understudied problem. Recently, it was shown that CREB-H, regulated through ER stress-induced intramembrane proteolysis (Zhang et al., 2006), similar to ATF6, acts to decrease plasma triglycerides (Zhang et al., 2011a) and that defective CREB-H alleles in humans associate with extreme hypertriglyceridemia, supporting the physiological significance of CREB-H in lipid homeostasis (Lee et al., 2011).
Hepatic VLDL assembly is a two–stage process (Sundaram and Yao, 2010). In the initial stage, the apolipoprotein B (apoB) is synthesized in the rough ER (rER) by co-translational lipidation upon translocation into the rER lumen. In the second stage, bulk neutral lipids, especially triglycerides (TG), are added to the VLDL precursors in the lumen of the smooth ER (sER) and/or the Golgi apparatus to form lipid-rich VLDL (Rusinol et al., 1993). ApoB is an obligate structural component of VLDL, whereas the microsomal triglyceride–transfer protein complex (MTP) is a co-factor that is absolutely required at both stages of VLDL biogenesis (Hussain et al., 2003). Protein disulfide isomerase (PDI) is a subunit of MTP necessary for normal MTP activity (Wetterau et al., 1991). Numerous studies demonstrate that increased expression of MTP elevates MTP activity and VLDL secretion but a similar role for PDI has not been demonstrated (Pan et al., 2010).
TGs stored in cytosolic lipid droplets (CLD) are the major source of lipid substrates for VLDL assembly (Yang et al., 1995). It is believed that cytosolic TG undergoes lypolysis (in the cytosol) and reesterification (in ER membranes) for delivery to the ER lumen for VLDL assembly (Lankester et al., 1998). The mechanism(s) that controls partitioning of newly synthesized TG between the cytosol and the ER lumen is poorly understood. Due to its lipid transfer activity, MTP is thought to play a critical role in facilitating the accretion of lumenal TG (Raabe et al., 1999). Although studies demonstrate ER stress interferes with hepatic lipid homeostasis, it is unknown whether the UPR pathways affect the lipid partitioning process and/or VLDL assembly.
IRE1α plays an essential role in maintaining ER homeostasis through initiating unconventional splicing of XBP1 mRNA to remove a 26 base intron, to create a translational frame-shift in XBP1 mRNA to produce a potent transcription factor (XBP1s) that regulates expression of genes encoding functions in ER protein folding and trafficking and ER-associated degradation to preserve ER homeostasis (Lee et al., 2002). Recently, XBP1s was shown to induce key hepatic lipogenic genes (Lee et al., 2008). It was reported that Xbp1-deletion in the liver reduces hepatic lipogenesis and leads to hypolipidemia in mice (Lee et al., 2008).
To determine the role of the IRE1α-XBP1 axis in regulation of hepatic metabolism, we generated hepatocye-specific Ire1α-deleted mice (Zhang et al., 2011b). These mice did not show basal UPR activation or any noticeable developmental or morphological abnormalities, but developed severe hepatosteatosis upon pharmacologically-induced ER stress (Zhang et al., 2011b). The present study was aimed at elucidating the role of the IRE1α-axis in hepatic lipid metabolism under physiological conditions of metabolic stress. We demonstrate that Ire1α-deletion in hepatocytes does not affect hepatic lipogenesis but impairs assembly of VLDL in the ER, leading to decreased hepatic export of TG-rich VLDL and increased hepatic TG accumulation. Impaired VLDL assembly occurs as a result of a reduced accumulation of TG in the ER lumen, due at least in part to deceased MTP activity resulting from decreased expression of PDI. This study demonstrates that the IRE1α-XBP1s-PDI axis plays a fundamental physiologic role to link ER homeostasis with MTP activity and VLDL assembly.
Results
Hepatocyte-specific Ire1α-deletion accentuates nutritional stress-induced hepatosteatosis without affecting de novo lipogenesis
Lethality of germ-line deleted Ire1α–null mice prompted us to generate a conditional allele to study the physiological functions of IRE1α in the murine liver. The hepatocyte (liver)-specific Ire1α-null (herein called L-Ire1α-KO) mice and their littermate heterozygous (herein called L-Ire1α-Het) or wild-type (L-Ire1α-WT) mice were generated by crossing homozygous Ire1α-floxed mice with the compound heterozygous Ire1α-null mice containing Cre recombinase under the control of albumin promoter (Zhang et al., 2011b). The resultant L-Ire1α-KO mice permit efficient CRE-mediated deletion of the single Ire1α-floxed allele in the liver of Ire1α-null heterozygous mice (Zhang et al., 2011b). We have not observed any difference in liver function and plasma lipid parameters between L-Ire1α-Het and L-Ire1α-WT mice. We assessed the physiologic role of IRE1α in regulation of hepatic lipid homeostasis by comparing L-Ire1α-KO with littermate L-Ire1α-Het and/or L-Ire1α-WT mice. We first investigated the effect of hepatocyte-specific Ire1α-deletion on hepatosteatosis induced by either fasting or over-nutrition.
L-Ire1α-KO mice displayed only mild hepatosteatosis when fed normal chow, however upon a 16 hr-fast they exhibit a 2-fold increase in hepatic TG content compared to L-Ire1α-Het mice (Figure 1A and Figure 1B). Cholesterol ester levels were also elevated in L-Ire1α-KO mice under fasted conditions (Figure 1A and Figure 1B). An increased delivery of adipose tissue-derived free fatty acids (FFA) to the liver is a key mechanism of fasting-induced hepatostatosis in mice. However, deletion of Ire1α in hepatocytes did not alter plasma FFA levels under either fed or fasting conditions (Figure S1A), indicating that hepatic uptake of FFA from plasma is unlikely to contribute to the difference in hepatic lipid accumulation observed between L-Ire1α-Het and L-Ire1α-KO mice.
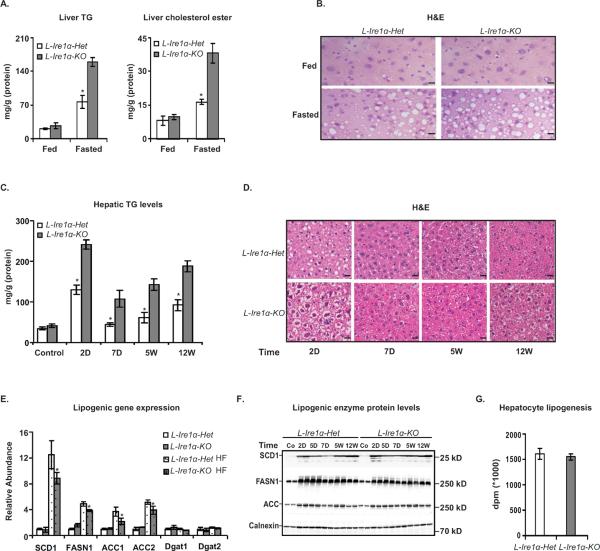
Figure 1. Hepatocyte-specific Ire1α-deletion accentuates nutritional stress-induced hepatosteatosis without affecting de novo lipogenesis.
(A) and (B) A 16-hr fast increases lipid accumulation in L-Ire1α-KO livers. (A) Liver TG and cholesterol esters were measured from L-Ire1α-Het and L-Ire1α-KO mice, in fed and 16-hr fasted condition n=4. *P< 0.01 vs L-Ire1α-KO. (B) Micrographs of liver sections from mice that were fed or fasted for 16 hrs were stained with Hematoxylin and Eosin (H&E). Scale bars, 40 μm. (C) and (D) A high-fructose diet (HFD) increases TG accumulation in L-Ire1α-KO mice. (C) Liver TG was measured in mice fed with HFD for the indicated times (n ≥ 4). *P< 0.05 vs L-Ire1α-Het. (D) Mice were fed with HFD for indicated time periods and liver sections prepared and stained with H&E. Scale bars, 30μm. (E) and (F) Livers from mice fed a HFD were analyzed for mRNA and proteins levels of lipogenic genes. (E) The abundance of lipogenic gene mRNA was normalized to actin mRNA. Values are relative to mRNA levels of L-Ire1α-Het mice fed with chow. (F) Liver lysates were prepared from mice fed with chow (Co) or HFD (HF) and analyzed by immunoblotting. Calnexin was used as a loading control. (G) Fatty acid synthesis was measured in hepatocytes by labeling with [14C]-acetic acid for 4 hrs (n=6).
We next fed mice with a high fructose diet (HFD) to study the effects of over-nutrition on hepatic lipid homeostasis. This diet stimulates de novo lipogenesis and causes lipid accumulation in the liver (Fukuda et al., 1983). Most of the metabolic parameters were comparable between L-Ire1α-KO and L-Ire1α-Het mice after a 12-week HFD-feeding period (Table S1). Insulin signaling was also similar between these mice (Figure S1B, C and D). However, the L-Ire1α-KO livers were substantially more steatotic than livers from L-Ire1α-Het mice at every time point examined during feeding of HFD (Figure 1C and 1D). These findings suggest that L-Ire1α-KO hepatocytes may have a defect in disposing of FFA derived from de novo lipogenesis.
Since it was reported that Xbp1-deletion impaired hepatic lipogenesis (Lee et al., 2008), we were surprised to find that L-Ire1α-KO mice display an augmented steatotic phenotype. To address this issue, we first analyzed gene expression of key lipogenic transcription factors, including liver X receptor (LXR), SREBP-1c and the carbohydrate response element binding protein (ChREBP). There were no differences in the expression levels of these genes between the two groups of mice (Figure S1E). In addition, although mRNA levels of lipogenic genes, including Scd1, Acc and Fasn, were moderately attenuated in L-Ire1α-KO mice (Figure 1E), their protein levels were not altered (Figure 1F). Moreover, metabolic labeling experiments using hepatocytes confirmed that Ire1α-deletion did not alter de novo lipogenesis (Figure 1G). Thus, the previously reported effect of Xbp1-deletion on lipogenesis was not observed in L-Ire1α-KO mice.
Mitochondrial β-oxidation of fatty acids and VLDL secretion are the primary catabolic pathways by which the liver maintains fatty acid homeostasis. However, we found no difference in the expression of key genes in the fatty acid oxidation pathway including the peroxisome proliferator-activated receptor (Pparα) and acyl-CoA oxidase (Acox) and carnitine palmitoyltransferase-1α (Cpt1) between L-Ire1α-KO and L-Ire1-Het mice (Figure S2A). Furthermore, the rate of [3H]-palmitic acid oxidation was not altered due to Ire1α-deletion in hepatocytes (Figure S2B). In addition, there were no significant differences in plasma ketone body levels between L-Ire1α-KO and L-Ire1-Het mice (Figure S2B). Taken together, these results indicate that the decreased capacity of the L-Ire1α-KO livers to handle the increased amounts of hepatic fatty acids during long-term fasting or feeding of HFD was not due to impaired fatty acid oxidation, pointing to a possible defect in hepatic TG export in these mice.
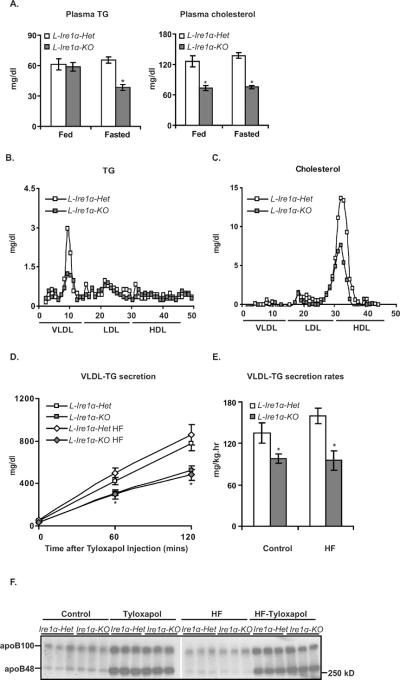
Figure 2. Hepatocyte-specific Ire1α-deleted mice display hypolipidemia and reduced hepatic VLDL secretion.
(A) Plasma TG and cholesterol levels are reduced in L-Ire1α-KO mice. Levels of plasma TG and cholesterol were measured in fed and 16-hr fasted mice, n>40. *P< 0.01 vs LIre1α-Het. (B) and (C) Plasma VLDL-TG and HDL-cholesterol levels are reduced in L-Ire1α-KO mice. (D) and (E) VLDL-TG secretion is reduced in L-Ire1α-KO mice. Mice (n≥5) fed with chow or HFD for 2d were fasted 4-hr followed by i.v. injection with tyloxapol. *P< 0.05 vs L-Ire1α-Het. (F) Plasma levels of apoB from the “0” time point to 120 min in Figure 2D were measured.
Hepatocyte-specific Ire1α-deleted mice display hypolipidemia and reduced hepatic VLDL-TG secretion
To investigate the potential effect of Ire1α-deletion on hepatic VLDL production, we first measured plasma lipid levels under fed and fasted conditions. Plasma TG levels were reduced approximately 40% in long-term fasted L-Ire1α-KO mice than L-Ire1α-Het mice (Figure. 2A). The decreased plasma TG levels were also observed in short-term fasted L-Ire1α-KO mice (Figure S2C). Plasma cholesterol levels were substantially decreased in both fed and long-term fasted L-Ire1α-KO compared to L-Ire1α-Het mice. Fast protein liquid chromatography (FPLC) analysis demonstrated that these changes in plasma lipids were mainly due to decreases in VLDL-TG and HDL-cholesterol levels in L-Ire1α-KO mice (Figure 2B and 2C). The decrease in VLDL-TG level suggested defective secretion of hepatic VLDL in these mice.
We measured hepatic VLDL secretion by injecting mice with tyloxapol to inhibit VLDL catabolism. Accumulation of plasma TG was significantly less in L-Ire1α-KO mice than L-Ire1α-Het mice after tyloxapol injection (Figure 2D), leading to a 40% decrease in the rates of hepatic VLDL-TG secretion (Figure 2E). In contrast to the decreased hepatic VLDL-TG secretion, there was no phenotypic difference in the plasma levels of apoB before or after tyloxapol injection (Figure 2F and Figure S2D and S2E), suggesting that the apoB-containing lipoprotein particles secreted from the livers of L-Ire1α-KO have reduced TG content.
Ire1α-deletion impairs secretion of TG-rich VLDL particles
We next labeled hepatocytes with [3H]-glycerol to examine the effect of Ire1α-deletion on TG synthesis and TG secretion. Under basal conditions where intracellular TG is mainly derived from de novo lipogenesis or a more physiological-relevant condition with oleic acid (OA)-supplementation, there was no difference in TG synthesis between hepatocytes isolated from the two groups of mice (Fig 3A). These findings reinforce the notion that Ire1α-deletion does not alter lipogenesis. However, the rate of TG secretion was significantly reduced in L-Ire1α-KO hepatocytes (Fig 3B) under both conditions.
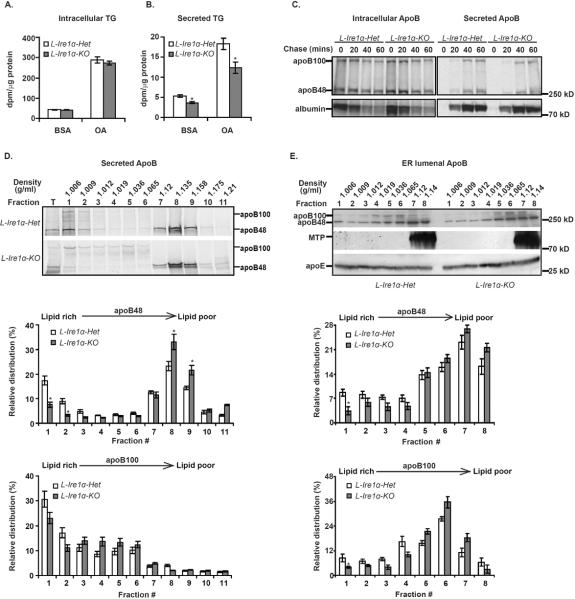
Figure 3. Ire1α-deletion impairs secretion of TG-rich VLDL particles.
(A) and (B) TG secretion is reduced without a change in TG synthesis in L-Ire1α-KO hepatocytes. Hepatocytes were labeled with [3H]-glycerol in the presence of BSA or OA for 3-hr and intracellular (A) and secreted (B) was measured (n=6). *P< 0.05 vs L-Ire1α-Het hepatocytes. (C) ApoB synthesis and secretion are not altered in L-Ire1α-KO hepatocytes. Primary hepatocytes were incubated 48 hrs in vitro and then pulse-labeled with [35S]-Met/Cys for 20 min. Intracellular apoB and secreted apoB were analyzed by immunoprecipitation and SDS-PAGE. (D) Secretion of lipid-rich apoB particles is reduced in L-Ire1α-KO hepatocytes. Secreted apoB-containing lipoproteins from hepatocytes were analyzed by DGUC and immunoblotting (n=3). The abundance of apoB in each fraction was quantified by ImageJ and relative amounts were calculated from the percentage of intensity in each fraction relative to the total intensity across the gel. *P< 0.05 vs L-Ire1α-Het hepatocytes. (E) VLDL assembly is defective in L-Ire1α-KO ER lumen. Lumenal apoB was analyzed by DGUC (n=3). Fractions were either immunoprecipitated with anti-apoB antibody or concentrated and analyzed by immunoblotting for MTP and apoE. The analysis was quantified as described in (D). *P< 0.05 vs L-Ire1α-Het.
We also performed pulse-chase analysis to compare rates apoB synthesis and secretion between L-Ire1α-Het and L-Ire1α-KO hepatocytes in the presence of OA. Murine hepatocytes predominantly produce apoB48 over apoB100 but prolonged culture in vitro increases the ratio of apoB100 to apoB48, most likely due to the decreased apoB RNA editing activity that accompanies dedifferentiation. To examine the synthesis and secretion of apoB48, as well as apoB100, we conducted pulse-chase analysis in primary hepatocytes, after an overnight-(Figure S3B) or a 2-day culture period (Figure 3C). Neither the synthesis nor secretion of apoB48 or apoB100 was altered in L-Ire1α-KO hepatocytes (Figure 3C and S3A and S3B). Under these experimental conditions, we noticed very little degradation of either apoB isoform from either L-Ire1α-Het or L-Ire1α-KO hepatocytes (Figure S3A). Accordingly, these observations suggest that hepatocytes from both genotypes secrete similar amounts of apoB-containing lipoprotein particles. In addition, the secretion of albumin was also comparable between the genotypes. These results together demonstrate that the secretory pathway is generally intact in L-Ire1α-KO hepatocytes.
To determine the effect of Ire1α-deletion on the density of secreted VLDL particles, we performed density gradient ultracentrifugation (DGUC) to fractionate apoB-containing lipoproteins secreted into the media from hepatocytes after a 3-hr continuous labeling with [35S]-Met/Cys and analyzed the relative density-distribution of the labeled apoB. The amount of apoB48 in lipid-rich VLDL fractions decreased approximately 50% and the amount of apoB48 in lipid-poor particles correspondingly increased in L-Ire1α-KO hepatocytes (Figure 3D). Although Ire1α-deletion did not affect apoB100 lipidation to the same extent as apoB48 lipidation, more apoB100 did reside in lipid-rich particles from L-Ire1α-Het hepatocytes than from L-Ire1α-KO hepatocytes (Figure 3D). Taken together, the results suggest that Ire1α-deletion specifically impairs the ability of hepatocytes to assemble or/and secrete lipid-rich VLDL particles.
Ire1α-deletion impairs assembly of lipid-rich VLDL particles in the sER
The ER is the major site for VLDL assembly in hepatocytes. To directly determine whether the defective VLDL secretion in Ire1α-deleted hepatocytes reflects a reduced ability to assemble lipid-rich VLDL particles in the ER, we conducted DGUC experiments on ER lumenal extracts to separate the lipoprotein complexes based on their buoyant densities that reflect lipid content. Approximately 10% of both apoB48 and apoB100 were present in the lipid-rich fraction from the ER lumen of L-Ire1α-Het mice (Figure 3E), and the abundance of each isoform significantly decreased to ~5% in the ER lumen from L-Ire1α-KO mice (Fraction 1 in Figure 3E). Correspondingly, the levels of apoB in lipid-poor fractions increased in the L-Ire1α-KO ER lumen (Figure 3E). Although the distribution of both apoB48 and apoB100 shifted toward the lipid-poor fractions in the L-Ire1α-KO ER lumen, the distribution of apoE shifted only slightly toward the lipid-poor fractions and MTP was concentrated in lipid-poor fractions. These observations collectively indicate that decreased secretion of TG-rich VLDL particles results from impaired assembly of these lipoprotein particles in the ER lumen of L-Ire1α-KO mice.
We next analyzed expression of genes encoding currently known protein factors affecting VLDL assembly, including Mtp, apoE, apoC3, ADRP, CideB, PGC-1α, PGC-1β. There were no changes in expression of these genes (Figure S2D and S3C). There was also no change in the expression of other genes encoding proteins modulating hepatic TG storage such as PPARγ, CideA and CideC (Figure S3C), suggesting that IRE1α regulates VLDL assembly through pathways that do not involve regulation of these genes.
XBP1s complementation restores TG secretion in Ire1α-deleted hepatocytes
IRE1α directly initiates unconventional splicing of XBP1 mRNA. To assess the specific contribution of XBP1s to the defective VLDL secretion in L-Ire1α-KO hepatocytes, we overexpressed XBP1s, wild-type IRE1α, kinase-dead IRE1α (K599A), and RNase-dead IRE1α (K907A) individually using adenovirus to test whether expression of these proteins can restore TG secretion in L-Ire1α-KO hepatocytes. Overexpression of wild-type IRE1α, but not kinase-dead IRE1α or RNase-dead IRE1α, increased expression of XBP1s, as expected (Figure 4A). Forced expression of XBP1s yielded significantly greater levels of XBP1s. The amount of TG secreted by L-Ire1α-KO hepatocytes infected with adenovirus expressing either wild-type IRE1α or XBP1s was comparable to that secreted by L-Ire1α-Het hepatocytes, which was at least thirty percent higher than that secreted from L-Ire1α-KO hepatocytes infected with adenovirus expressing GFP, kinase-dead IRE1α, or RNase-dead IRE1α (Figure 4B). Overexpression of these proteins did not affect TG synthesis (Figure 4B) or lipogenic gene expression (Figure S4A). These observations demonstrate that IRE1α-mediated splicing of XBP1 mRNA corrects the defective TG secretion in L-Ire1α-KO hepatocytes. Thus, the defective TG secretion in L-Ire1α-KO hepatocytes was primarily due to a lack of XBP1s.
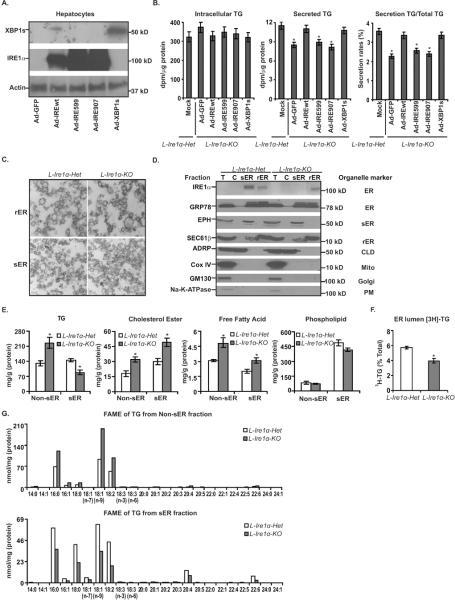
Figure 4. Ire1α-deletion reduces TG content in the sER of the liver.
(A) and (B) Complementation of IRE1α or spliced XBP1 (XBP1s) restores TG secretion in L-Ire1α-KO hepatocytes. (A) Protein levels of XBP1s and IRE1α were measured by immunoblotting of hepatocytes infected with adenoviruses expressing GFP (Ad-GFP), XBP1s (Ad-XBP1s) and wild-type IRE1α (Ad-IREwt), kinase-dead IRE1α (Ad-IRE599) or RNase-dead IRE1α (Ad-IRE907) (n=6). (B) Rates of TG synthesis and secretion were measured in L-Ire1α-Het hepatocytes and L-Ire1α-KO hepatocytes infected with the above adenoviruses (n=6). *P< 0.05 vs XBP1s infected hepatocytes. (C) Electron micrographs of isolated rER and sER from livers are shown. (D) Immunoblotting analysis of protein markers was performed on isolated cellular organelles. (E) TG accumulation is reduced in L-Ire1α-KO sER. Levels of TGs, cholesterol esters, fatty acids and phospholipids were measured in sER and non-sER fractions (n=4). *P< 0.05 vs L-Ire1α-Het. (F) ER-localized TG is reduced in L-Ire1α-KO hepatocytes. Hepatocytes were incubated with [3H]-glycerol-DMEM media for 3-hr with 0.4mM OA. [3H]-labeled sER was isolated from 5×106 hepatocytes and radioactivity incorporated into TG was measured by TLC of non-sER and sER fractions and presented relative to total protein content (n=4). *P< 0.05 vs L-Ire1α-Het. (G) FAME samples derived from sER-TG and non-sER-TG were analyzed by GC. FAME samples were isolated from 4 livers of each genotype.
Ire1α-deletion reduces TG content in the sER of the liver
Given that VLDL assembly is highly dependent on availability of TG in the ER, the defective assembly of VLDL particles in L-Ire1α-KO ER may reflect decreased lipid availability in the sER. To test this possibility, we isolated sER from the L-Ire1α-Het and L-Ire1α-KO livers following an established method (Gilchrist et al., 2006) and quantified lipid contents in purified sER fraction and the pooled fraction containing rER, Golgi and cytosol (designated as non-sER fractions). EM analysis showed that the fractions were relatively homogeneous and without any discernible morphological differences in the isolated sER or rER between L-Ire1α-KO and L-Ire1α-Het livers (Figure 4C). The purity of the sER was also quantified by western blotting of proteins from different cellular organelles. The results revealed that the contaminants from Golgi, plasma membrane, CLD and mitochondria were largely removed (Figure 4D). In addition, the levels of Sec61β, a rER specific marker, (Gilchrist et al., 2006) and epoxide hydrolase, a sER marker, (Galteau et al., 1985) were not significantly different between genotypes, suggesting the relative mass of rER and sER was similar between L-Ire1α-KO and L-Ire1α-Het livers.
Analysis of lipid content demonstrated that TG levels were significantly decreased in sER fractions but increased in non-sER fractions from L-Ire1α-KO livers (Figure 4E). Since total sER-TG only accounted for ~7% of TG in non-sER fractions, the total intracellular TG was mainly from non-sER-TG, which was greater in L-Ire1α-KO livers, reflecting the steatotic phenotype of L-Ire1α-KO mice. Interestingly, elevated levels of cholesterol ester (CE) were observed in both sER fractions and non-sER fractions from L-Ire1α-KO livers (Figure 4E). TG and CE are major lipid constituents in CLD; therefore, the inverse relationship between TG and CE levels in sER fractions from L-Ire1α-KO livers confirmed the minimal contamination of CLD in the isolated sER fractions. Moreover, FFA levels were higher in both fractions from L-Ire1α-KO livers compared to L-Ire1α-Het livers, which may result from elevated lipolysis of the overaccumulated TG. Phospholipid levels in the fractions were comparable between the two groups of livers (Figure 4E).
To confirm there was also a decrease in accumulation of newly synthesized TG in L-Ire1α-KO ER, we employed [H3]-glycerol labeling to measure TG partitioning between the cytosol and the ER in L-Ire1α-Het and L-Ire1α-KO hepatocytes. Indeed, [H3]-labeled TG in the ER from L-Ire1α-Het hepatocytes was ~40% greater than that from the L-Ire1α-KO hepatocytes (Figure 4F). Taken together, the decreased TG content associated with defective TG-rich VLDL assembly in the L-Ire1α-KO ER (Figure 3D) indicates that the defect in TG-rich VLDL assembly occurs in the ER due to Ire1α-deletion. However, we cannot completely exclude the possibility that Ire1α-deletion additionally affects the process of VLDL maturation in the Golgi.
Lipid composition in the sER and non-sER fractions was further analyzed by gas chromatography (GC). Fatty acid methyl esters (FAMEs) derived from TG increased uniformly in the non-sER fraction and decreased in the sER fraction from L-Ire1α-KO livers (Figure 4G, S4B and S4C). Similarly, FAMEs of CE in both sER and non-sER fractions were elevated in L-Ire1α-KO livers and FAMEs of phospholipids in both sER and non-sER fractions were comparable between L-Ire1α-KO and L-Ire1α-Het livers (Figure S4B and S4C). We did not observe any changes in the distribution of FFA species, suggesting that IRE1α-XBP1s does not alter fatty acid composition to reduce TG accumulation in the sER. In addition, the contents of TG, CE and phospholipids calculated according to the concentration of FAMEs in the GC analysis again revealed a reduction of TG and an increase of CE in L-Ire1α-KO sER (Figure S4B). Taken together, the observations from both enzymatic assays and GC analysis consistently indicate that intervention in the IRE1α-XBP1s pathway specifically reduces TG accumulation in the ER, which, in turn, impairs the assembly of TG-rich VLDL particles.
Hepatocyte-specific Ire1α-deletion decreases PDI levels and MTP activity in the liver
To identify the defective protein component(s) responsible for the diminished TG accumulation in L-Ire1α-KO ER, we performed microarray mRNA expression profiling (Zhang et al., 2011b) and proteomic analysis. Comparative 2-D proteomic analysis on sER isolated from L-Ire1α-Het and L-Ire1α-KO livers demonstrated they were very similar (Figure 5A). Nevertheless, several protein species that had increased intensity in L-Ire1α-Het sER compared to L-Ire1α-KO sER were isolated for mass spectrum analysis. Interestingly, one of these species was identified as protein disulfide isomerase PDI (also known as PDIa1 and prolyl 4-hydroxylase subunit beta). PDI was also identified in the microarray studies as an IRE1α-dependent target gene (Zhang et al., 2011b). Given that PDI is a structural component of MTP (Bradbury et al., 1999), this observation raised the possibility that reduced levels of PDI in L-Ire1α-KO hepatocytes decrease MTP activity, affecting TG accumulation in the ER.
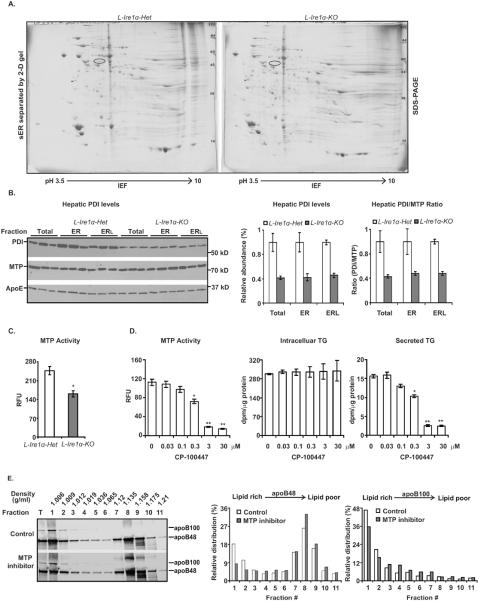
Figure 5. Hepatocyte-specific Ire1α-deletion decreases PDI levels and MTP activity in the liver.
(A) Proteomic analysis of sER fractions was performed using IEF followed by SDS-PAGE. One spot identified by the circle was subjected to mass spectrum analysis. (B) PDI levels are reduced in L-Ire1α-KO livers. Immunoblot analysis of PDI and MTP in total liver lysates (Total), ER (ER) and ER lumen (ERL) is shown. The intensities of PDI and MTP in the blot were quantified by ImageJ and normalized to the intensity of apoE. The relative abundances are shown relative to PDI levels in each fraction from L-Ire1α-Het samples. The ratios of PDI and MTP in L-Ire1α-KO livers are presented relative to their amount in respective fractions from L-Ire1α-Het samples. (C) MTP activity is reduced in L-Ire1α-KO livers. MTP activity was measured in liver lysates (n=5). *P< 0.01 vs L-Ire1α-Het. (D) Treatment of hepatocytes with 0.3 μM CP-100447 reduces both MTP activity and TG secretion by ~40%. MTP activity, intracellular TG and secreted TG were measured in wild-type hepatocytes treated with MTP inhibitor, CP-100447. (E) Hepatocytes treated with 0.3 μM CP-100447 reduce secretion of apoB in lipid-rich particles. DGUC was repeated twice and the average of the relative intensities were plotted as described in Figure 3D.
To test this idea, we used immunoblot analysis to quantify PDI contents in whole liver homogenates and ER fractions and measured liver MTP activity. PDI levels in L-Ire1α-KO livers were decreased to one-half of those in L-Ire1α-Het livers (Figure 5B). Importantly, MTP activity was decreased ~40% in L-Ire1α-KO livers compared to L-Ire1α-Het livers (Figure 5C), although hepatic MTP protein levels were comparable between L-Ire1α-KO and L-Ire1α-Het mice (Figure 5B). Taken together, these findings revealed a reduced PDI content associated with decreased MTP activity in L-Ire1α-KO hepatocytes.
To support our finding that the defective TG-rich VLDL secretion could be due to decreased MTP activity in L-Ire1α-KO hepatocytes. We analyzed TG secretion under different concentrations of MTP inhibition by CP-100447, to reduce MTP activity to a similar extent (40%) as that obtained upon Ire1α-deletion (Figure 5D). We found that 0.3 μM CP-100447 decreased MTP activity by ~40% and significantly reduced TG secretion but not TG synthesis (Figure 5D). In addition, DGUC experiments demonstrated that 0.3 μM CP-100447 impaired secretion of TG-rich VLDL particles to a similar level as observed upon Ire1α-deletion in hepatocytes (Figure 5E and Figure 3D). Thus, these observations strengthen our conclusion that decreased MTP activity at least in part contributes to defective TG-rich VLDL assembly and secretion in L-Ire1α-KO hepatocytes.
Overexpression of PDI but not MTP promotes TG secretion in Ire1α-deleted hepatocytes
To determine whether the IRE1α-XBP1s axis regulates hepatic PDI gene expression under physiological conditions, we measured PDI mRNA levels under short fasting (Figure 6A) and fed conditions (Figure S5A). PDI mRNA levels, together with other XBP1-dependent UPR genes including Erdj4, Edem2 and Wfs1, were reduced approximately 2-fold in L-Ire1α-KO livers compared to L-Ire1α-Het livers, whereas the expression of Bip and Grp94 and other abundant hepatic PDI family members, Erp57 and Erp72 (Grubb et al., 2012), which are not regulated by XBP1s, was unchanged (Figure 6A and Figure S5A). Enforced expression of XBP1s in vitro using XBP1s adenovirus markedly increased PDI mRNA levels but not lipogenic genes in both L-Ire1α-KO and L-Ire1α-Het hepatocytes (Figure 6B). These observations indicate that XBP1s acts as a primary transcription factor that regulates PDI levels in hepatocytes.
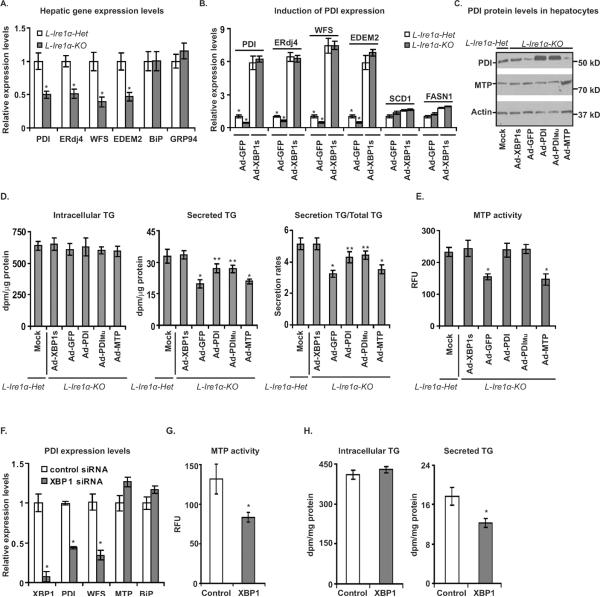
Figure 6. Overexpression of PDI, but not MTP, promotes TG secretion in Ire1α-deleted hepatocytes.
(A) PDI expression is reduced in L-Ire1α-KO livers. Relative gene expression was normalized to actin mRNA levels and values are compared to mRNA levels from LIre1α-Het mice. *P< 0.05 vs L-Ire1α-Het. (B) and (C) Adenovirus-mediated overexpression of XBP1s increases PDI expression. (B) Values are relative to mRNA levels of L-Ire1α-KO hepatocytes infected with adenovirus expressing GFP. (C) PDI and MTP protein levels were measured at 48-hr after infection of hepatocytes with adenovirus expressing GFP (Ad-GFP), XBP1s (Ad-XBP1s), MTP (Ad-MTP), PDI (Ad-PDI) or mutant PDI (Ad-PDIMu). Actin was used as a loading control. (D) and (E) Overexpression of PDI restores MTP activity and promotes hepatic TG secretion in LIre1α-Het hepatocytes. (D) TG synthesis and secretion rates were measured at 48-hr after of infection with the indicated adenoviruses (n=6). *P< 0.05 vs Ad-PDI or mutant PDI Ad-PDIMu; **P< 0.05 vs Ad-XBP1s. (E) MTP activity was measured in each sample of (D). *P< 0.05 vs Ad-PDI or Ad-PDIMu. (F), (G) and (H) XBP1 knockdown decreases PDI levels, MTP activity and TG secretion in hepatocytes. (F) XBP1 and PDI levels were measured in hepatocytes transfected with control siRNA or XBP1 siRNA. (G) MTP activity was measured in hepatocytes transfected with above siRNAs (n=6). **P<0.05 vs control. (H) TG synthesis and secretion were measured in hepatocytes transfected with above siRNAs (n=6). **P<0.05 vs control.
To further test whether decreased PDI is responsible for the reduced MTP activity and defective VLDL assembly in L-Ire1α-KO mice, we determined whether overexpression of wild-type or mutant PDI or alternatively overexpression of MTP using adenovirus can correct defective VLDL secretion in L-Ire1α-KO hepatocytes. The mutant PDI contains four cysteine-to-serine mutations in both of the thioredoxin-like active-sites that destroy PDI activity but retains chaperone activity (Quan et al., 1995). Overexpression of either wild-type or mutant PDI, but not MTP, restored MTP activity and significantly improved TG secretion in L-Ire1α-KO hepatocytes without affecting TG synthesis (Figures 6C to 6E). These findings suggest that the chaperone activity of PDI, but not the catalytic activity, is required for MTP activity to rescue TG secretion in L-Ire1α-KO hepatocytes. However, while fully restoring MTP activity, PDI overexpression only partially restored TG secretion, suggesting another XBP1s-dependent factor(s) limits TG-rich VLDL assembly in addition to PDI in L-Ire1α-KO hepatocytes.
Unlike PDI overexpression, XBP1s overexpression fully restored not only the PDI level and MTP activity, but also TG secretion in L-Ire1α-KO hepatocytes (Figures 6C to 6E), demonstrating the importance of XBP1s in regulating VLDL assembly. In confirmation of the immediate impact of XBP1s-PDI on TG secretion, knockdown of XBP1 in primary hepatocytes also decreased PDI expression, MTP activity, and TG secretion, but not TG synthesis (Figure 6F, 6G and 6H). Thus, the IRE1α-XBP1s-PDI axis is an important regulatory pathway to maintain TG-rich VLDL production that controls hepatic and plasma lipid content.
Discussion
Accumulating evidence suggests that the UPR plays an essential role in hepatic lipid metabolism (Flamment et al., 2010). VLDL secretion is critical in maintaining intrahepatic and plasma lipid homeostasis. Although ER stress was linked to dysregulation of hepatic VLDL production (Ota et al., 2008; Qiu et al., 2006), little is known about the mechanism by which the UPR signaling pathways impact VLDL assembly and secretion. Here, we reveal a specific requirement for the IRE1α-XBP1s axis in VLDL assembly. Using L-Ire1α-KO mice, we first demonstrated that these mice developed hepatosteatosis and hypolipidemia primarily due to a defective TG-rich VLDL secretion. Through metabolic labeling in primary hepatocytes, we further revealed reduced sER-TG accretion is a key underlying cellular mechanism for defective TG-rich VLDL assembly in L-Ire1α-KO hepatocytes. Our studies identified no defects in lipogenesis or apoB synthesis and secretion in L-Ire1α-KO hepatocytes. In contrast, we show that IRE1α is required for adequate lipid partitioning into the sER for TG-rich VLDL assembly. Finally, proteomic analysis and genetic complementation approaches demonstrated that this defect in TG-rich VLDL assembly is caused in a large part by reduced MTP activity due to decreased expression of PDI. Together, our study provides mechanistic insight into the requirement for the IRE1α-XPB1s-PDI axis in promoting TG-rich VLDL secretion and maintaining lipid homeostasis.
VLDL assembly is highly dependent on efficient trafficking of fatty acid stored in CLD into ER lumen (Yang et al., 1995). Decreased TG accumulation in the sER, coupled with increased accumulation of CLD-TG indicates defective lipid partitioning between CLD and the ER in L-Ire1α-KO hepatocytes. Previous studies established a pivotal role for MTP in facilitating accretion of TG in the VLDL secretory pathway (Raabe et al., 1999). Interestingly, Ire1α-deletion caused proportionate decreases in PDI expression and MTP activity (~40%). Overexpression of IRE1α, XBP1s, or PDI all completely restored PDI levels, MTP activity and significantly increased TG secretion in Ire1α-deleted hepatocytes. Thus, transcriptional regulation of PDI is a key mechanism whereby IRE1α-XPB1s regulates VLDL assembly. However, overexpresson of PDI only partially complemented the TG secretion defect in L-Ire1α-KO hepatocytes, suggesting that IRE1α may increase TG-rich VLDL assembly through additional XBP1s-dependent but MTP-PDI-independent mechanism(s).
Despite the reduced secretion of TG-rich VLDL particles, rates of apoB secretion were not altered and degradation of apoB100 was not increased in L-Ire1α-KO hepatocytes under the OA-supplemented experimental conditions employed in this study. These observations suggest that the residual MTP activity (~60% of full activity) in L-Ire1α-KO hepatocytes is sufficient to support co-translational lipidation of apoB100 and prevent its degradation, a key component of the first step of VLDL assembly which is crucial for apoB secretion. In contrast, decreased MTP activity mainly affected the second step of VLDL assembly in L-Ire1α-KO hepatocytes, which involves bulk TG addition to primordial VLDL particles. These findings are consistent with the observation that Mtp-deletion did not affect apoB48 secretion, but the apoB48 was secreted only as lipid-poor particles (Rustaeus et al., 1998). The secretion of apoB100 was more susceptible to alterations in MTP activity than that of apoB48 both in vitro (Kulinski et al., 2002) and in vivo (Chen et al., 2008). Since apoB100 represents only a small proportion of the total secreted apoB molecules, the precise effect of Ire1α-deletion on apoB100 secretion alone will require future studies using genetically engineered mice that produce exclusively apoB100 from the apoB gene (Hirano et al., 1996).
IRE1α initiates production of XBP1s that is a potent transcription factor that regulates expression of a wide range of ER homeostatic genes. Disruption of XBP1 in secretory cells profoundly reduces secretory functions of those cells (Hetz, 2012). Deletion of Xbp1 in the liver was reported to cause a defect in hepatic lipogenesis (Lee et al., 2008). However, lipogenic activity was not altered in L-Ire1α-KO hepatocytes. There are several possibilities that might account for the discrepant phenotypes between Ire1α-and Xbp1-deleted mice. First, albumin-promoter-driven CRE deletion of Ire1α may not be as complete as Mx1-promoter-driven CRE recombinase (Mx1-CRE). Residual XBP1s may sustain some functions of XBP1s in hepatocytes. Second, Mx1-CRE-mediated deletion of Xbp1 occurs in multiple cell types in the liver, including Kupffer cells and stellate cells (Kuhn et al., 1995). Inactivation of Xbp1 in Kupffer cells may contribute to the impaired lipogenesis observed in Xbp1-deleted hepatocytes because Kupffer cells can affect lipogenesis in hepatocytes and even contribute to diet-induced hepatosteatosis (Huang et al., 2010). In fact, we did observe decreased expression of hepatic lipogenic genes in Mx1-CRE Ire1α-deleted mice (unpublished data, SW and RJK). Third, loss of XBP1 causes hyperactivation of IRE1α (Lee et al, 2008) that can activate downstream signaling pathways or mediate mRNA degradation to further complicate the phenotype observed in Mx1-CRE Xbp1 mice (Zhang and Kaufman, 2008). Regardless, both our XBP1s-complementation and XBP1-knockdown experiments in primary hepatocytes support the notion that XBP1 is the major determinant in the IRE1α pathway that regulates TG-rich VLDL-assembly.
Previous studies have documented a requirement for the IRE1α-XBP1 pathway to expand the ER during differentiation of cells that secrete large amounts of proteins (Hetz, 2012). Recent studies have also implicated IRE1α in mRNA degradation, such as insulin mRNA in a pancreatic β-cell line (Lipson et al., 2006), and IRE1β in degradation of MTP mRNA in intestinal epithelial cells to limit chylomicron production (Iqbal et al., 2008), suggesting a physiological role for IRE1β in lipid metabolism. Here, we demonstrate a unique physiological requirement for IRE1α in metabolic regulation of lipid homeostasis by regulating expression of PDI, an MTP chaperone, in response to metabolic stress. Thus, it is of great interest to understand the role of the IRE1α-XBP1-PDI pathway in development of dyslipidemia and hepatosteatosis under pathophysiological conditions such as obesity and diabetes.
In summary, we identified a specific role for IRE1α in regulating TG-rich VLDL assembly and plasma lipid levels. The regulatory mechanism requires XBP1s-PDI that increases MTP activity for efficient TG partitioning into the ER lumen to provide TG substrates for VLDL assembly. Thus, manipulation of the IRE1α-XBP1 pathway may provide a unique approach to treat hyperlipidemia.
Experimental Procedures
Mice
L-Ire1α-KO mice were generated and bred as described (Zhang et al., 2011b). All the transgenic mice in this study were backcrossed to C57/Bl6 background at least 6 generations. For long fasting experiments, food was removed from mice overnight (16 hr) before sacrifice. A high fructose diet (TD. 89247, Harlan Teklad; Madison, WI) was given for increasing periods of time and mice were sacrificed after a 4-hr fast.
Plasma parameters and hepatic MTP activity
Plasma levels of triglyceride, ketone body, free cholesterol, cholesterol ester and fatty acid were determined using EnzyChromTM Triglyceride and ketone body Assay Kit (Bioassay, Hayward, CA), Free Cholesterol E, Cholesterol E assay kit, and NEFA C commercial kit (Wako Chemicals GmbH, USA). For lipoprotein analysis, plasma was fractioned by FPLC using a Superose-6 column. The rates of VLDL-TG secretion were measured after mice were intravenously injected with tyloxapol (500mg/kg body weight) after 6-hr fasting. The Roar MTP activity assay kit was used for MTP activity according to manufacturer's instruction.
RNA isolation and quantitative PCR analysis
Total RNAs were isolated using Trizol reagent (Life Technologies, Inc. Carlsbad, California). cDNA was synthesized using iScript™ Reverse Transcription Supermix (Bio-Rad, Hercules, CA). All primers from IDT (Coralville, IA) are listed in Table S2.
Protein isolation and Western blotting
Proteins were extracted using RIPA buffer, resolved by SDS-PAGE gels (Bio-Rad, Hercules, CA) and electroblotted onto PVDF membranes, blocked with nonfat milk. Membranes were incubated with different primary antibodies (Table S3) followed by incubation with HRP secondary antibody or infrared-labeled antibody. Blots were analyzed by ImageJ (NIH) or quantified by Odyssey Imager (LI-Cor Biosciences, Lincoln, Nebraska)
Pulse-chase analysis of apoB, VLDL-TG secretion and fatty acid oxidation in primary hepatocytes
Hepatocytes were isolated as described (Chen et al., 2010). Cells were starved 20 mins with Met and Cys-free media followed by 20 min chase with [35S]-Met/Cys. After chase, every 20 min up to 60 min, samples were collected and harvested for IP with apoB antibody. Labeled apoB immuno-complexes were resolved by SDS-PAGE and exposed to a phosphoimager screen. Quantification was performed using ImageJ. TG synthesis and secretion was measured in hepatocytes incubated with [3H]-glycerol (1μCi/ml) for 3-hr. Labeled TG was isolated from both cells and media, separated by TLC and analyzed by scintillation counting. Fatty acid oxidation was measured as described (Purushotham et al., 2009).
Density gradient analysis of lipoprotein from primary hepatocytes and ER lumen
Hepatocytes were exposed to 140μCi/ml [35S] with 0.4mM OA for 4-hr. The labeled media or ER luminal contents released by 0.1 M Na2CO3 was adjusted to 1.25g/ml using KBr and layered below the salt solutions with density of 1.063g/ml, 1.019g/ml and 1.006g/ml followed by an ultracentrifugation. Eleven fractions were isolated and analyzed as described for the pulse-chase experiments.
Isolation of rER and sER from livers
The rER and sER was isolated as described (Gilchrist et al., 2006). Livers were homogenized in 0.25M sucrose followed by centrifugation at 6,000rpm. The supernatant was centrifuged at 25,000rpm for 7 min followed by another ultracentrifugation at 55,000 rpm. The pellets were resuspended in 1.85M sucrose and adjusted to 1.37M sucrose, which were laid to a step gradient containing 1.0M, 0.8M and 0.6 M sucrose. The pellet rER fraction and the loading zone sER fraction were collected. 2D-gel was performed by Kendrick Labs (Madison, WI) and mass spectroscopy was performed by the Protein Core Facility at the Columbia University College of Physicians & Surgeons.
Hepatic lipidomic analysis
FAMEs were analyzed by gas chromatography by the lipidomics core at the Nutrition Obesity Research Center at the University of Michigan.
Statistical analysis
Data in the figures are presented with means ± SEM applying the unpaired two-tailed t test. P < 0.05 was considered as a significant difference.
Supplementary Material
Highlights
Ire1α-deletion impairs hepatic VLDL assembly, but not lipogenesis or apoB secretion
IRE1α-XBP1s regulates TG partitioning into the smooth ER for VLDL assembly
Inactivation of IRE1α in hepatocytes reduces PDI expression and MTP activity
PDI restores MTP function and promotes VLDL secretion in Ire1α-deleted hepatocytes
Acknowledgments
This work was supported by NIH grants P01HL057346, R37DK042394, R01DK088227, R24DK093074 and R01HL052173 to RJK, grants HL-38180, DK-56260 and DK-52574 to NOD, DK-78187 to BNF and an AHA postdoctoral fellowship to SW. We thank Dr. Chang, Benny Hung-Junn for ADRP antibody, Dr. Yong Liu for wild-type IRE1α, kinase-dead IRE1α and RNase-dead IRE1α adenovirus, Dr. Umut Ozcan for XBP1(s) adenovirus, Dr. Stuart Lipton for PDI and PDImu adenovirus, Dr. John Bergeron for generating EM micrographs, Dr. Henry Ginsberg and Dr. Richard Lehner for insightful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions SW and ZC conceived and designed the experiments with RJK. SW, ZC, VL and JH performed the experiments. SW and ZC wrote the paper with RJK. BF and ND provided technical support.
References
- Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–2190. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- Bradbury P, Mann CJ, Kochl S, Anderson TA, Chester SA, Hancock JM, Ritchie PJ, Amey J, Harrison GB, Levitt DG, et al. A common binding site on the microsomal triglyceride transfer protein for apolipoprotein B and protein disulfide isomerase. J Biol Chem. 1999;274:3159–3164. doi: 10.1074/jbc.274.5.3159. [DOI] [PubMed] [Google Scholar]
- Calandra S, Tarugi P, Speedy HE, Dean AF, Bertolini S, Shoulders CC. Mechanisms and genetic determinants regulating sterol absorption, circulating LDL levels, and sterol elimination: implications for classification and disease risk. J Lipid Res. 2011;52:1885–1926. doi: 10.1194/jlr.R017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Newberry EP, Norris JY, Xie Y, Luo J, Kennedy SM, Davidson NO. ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. J Lipid Res. 2008;49:2013–2022. doi: 10.1194/jlr.M800240-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Norris JY, Finck BN. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) stimulates VLDL assembly through activation of cell death-inducing DFFA-like effector B (CideB) J Biol Chem. 2010;285:25996–26004. doi: 10.1074/jbc.M110.141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamment M, Kammoun HL, Hainault I, Ferre P, Foufelle F. Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr Opin Lipidol. 2010;21:239–246. doi: 10.1097/MOL.0b013e3283395e5c. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Iritani N, Tanaka T. Effects of high-fructose diet on lipogenic enzymes and their substrate and effector levels in diabetic rats. J Nutr Sci Vitaminol (Tokyo) 1983;29:691–699. doi: 10.3177/jnsv.29.691. [DOI] [PubMed] [Google Scholar]
- Galteau MM, Antoine B, Reggio H. Epoxide hydrolase is a marker for the smooth endoplasmic reticulum in rat liver. EMBO J. 1985;4:2793–2800. doi: 10.1002/j.1460-2075.1985.tb04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Grubb S, Guo L, Fisher EA, Brodsky JL. Protein disulfide isomerases contribute differentially to the endoplasmic reticulum-associated degradation of apolipoprotein B and other substrates. Mol Biol Cell. 2012;23:520–532. doi: 10.1091/mbc.E11-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hirano K, Young SG, Farese RV, Jr., Ng J, Sande E, Warburton C, Powell-Braxton LM, Davidson NO. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J Biol Chem. 1996;271:9887–9890. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
- Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 2002;277:31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- Lankester DL, Brown AM, Zammit VA. Use of cytosolic triacylglycerol hydrolysis products and of exogenous fatty acid for the synthesis of triacylglycerol secreted by cultured rat hepatocytes. J Lipid Res. 1998;39:1889–1895. [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med. 2011;17:812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–186. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Avramoglu RK, Rutledge AC, Tsai J, Adeli K. Mechanisms of glucosamine-induced suppression of the hepatic assembly and secretion of apolipoprotein B-100-containing lipoproteins. J Lipid Res. 2006;47:1749–1761. doi: 10.1194/jlr.M500363-JLR200. [DOI] [PubMed] [Google Scholar]
- Quan H, Fan G, Wang CC. Independence of the chaperone activity of protein disulfide isomerase from its thioredoxin-like active site. J Biol Chem. 1995;270:17078–17080. doi: 10.1074/jbc.270.29.17078. [DOI] [PubMed] [Google Scholar]
- Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinol A, Verkade H, Vance JE. Assembly of rat hepatic very low density lipoproteins in the endoplasmic reticulum. J Biol Chem. 1993;268:3555–3562. [PubMed] [Google Scholar]
- Rustaeus S, Stillemark P, Lindberg K, Gordon D, Olofsson SO. The microsomal triglyceride transfer protein catalyzes the post-translational assembly of apolipoprotein B-100 very low density lipoprotein in McA-RH7777 cells. J Biol Chem. 1998;273:5196–5203. doi: 10.1074/jbc.273.9.5196. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau JR, Combs KA, McLean LR, Spinner SN, Aggerbeck LP. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991;30:9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Yang LY, Kuksis A, Myher JJ, Steiner G. Origin of triacylglycerol moiety of plasma very low density lipoproteins in the rat: structural studies. J Lipid Res. 1995;36:125–136. [PubMed] [Google Scholar]
- Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, Zhang K. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology. 2011a doi: 10.1002/hep.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, et al. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 2011b;30:1357–1375. doi: 10.1038/emboj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.