SUMMARY
Calorie restriction (CR) slows aging and consistently reduces circulating sex hormones in laboratory animals. However, nothing is known regarding the long-term effects of CR with adequate nutrition on serum sex hormones concentration in lean healthy humans. In this study, we measured body composition, and serum total testosterone, total 17-β-estradiol, sex hormone binding globulin (SHBG), and dehydroepiandrosterone sulfate (DHEA-S) concentrations in 24 men (mean age 51.5±13 yrs), who had been practicing CR with adequate nutrition for an average of 7.4±4.5 years, in 24 age- and body fat-matched endurance runners (EX), and 24 age-matched sedentary controls eating Western diets (WD).
We found that both the CR and EX volunteers had significantly lower body fat than the WD volunteers (total body fat, 8.7±4.2%; 10.5±4.4%; 23.2±6.1%, respectively; P=0.0001). Serum total testosterone and the free androgen index were significantly lower, and SHBG was higher in the CR group than in the EX and WD groups (P≤0.001). Serum 17β-estradiol and the estradiol:SHBG ratio were both significantly lower in the CR and EX groups than in the WD group (P≤0.005). Serum DHEA-s concentrations were not different between the 3 groups. These findings demonstrate that, as in long-lived CR rodents, long-term severe CR reduces serum total and free testosterone and increases SHBG concentration in humans, independently of adiposity. More studies are needed to understand the role of this CR-mediated reduction in sex hormones in modulating the pathogenesis of age-associated chronic diseases such as cancer and the aging process itself.
Keywords: calorie restriction, endurance exercise, sex hormones, 17-β-estradiol, testosterone, sex hormone binding globulin, DHEA-s
INTRODUCTION
Long-term calorie restriction (CR) without malnutrition slows aging and extends maximal lifespan in many species, including yeast, worms, flies, spiders, fishes, and rodents (Weindruch & Walford, 1988). Although it has been suggested that the slowing of aging by CR is a general phenomenon that applies to all species, there is currently no evidence that CR extends maximal lifespan in non-human and human primates (Fontana & Klein, 2007). However, recent data from a 20-yr longitudinal study demonstrate that long-term moderate CR started in adult rhesus monkeys significantly reduced age-related morbidity and mortality, and appears to slow brain and skeletal muscle aging, suggesting that CR may also slow aging in long-lived species (Colman et al., 2009).
The mechanisms by which CR slows aging and protects against age-associated diseases are not known yet. Many interrelated and overlapping metabolic factors have been proposed to play a role, including a reduction in growth factors (e.g. insulin-like growth factor 1 (IGF-1)), anabolic hormones (e.g. insulin and testosterone), and circulating inflammatory cytokines (Fontana & Klein, 2007). Calorie restriction and reduced growth factor signalling both elevate resistance to oxidative stress, reduce cellular damage, and increase lifespan in experimental animals (Mair & Dillin, 2008; Russell & Kahn, 2007). Interestingly, in the majority of the animal models of longevity, lifespan extension is accompanied by reduced growth and fecundity and, paradoxically, by delayed age of reproduction cessation (Partridge et al., 2005; Bartke & Turyn, 2001; McShane & Wise, 1996; Merry et al., 1979). However, these findings may not have relevance to humans who begin CR as adults, long after the growth phase is completed.
A hormonal adaptation that occurs in CR rodents is a reduction in serum anabolic sex hormone concentrations, which control fertility, reproduction, metabolism and growth (Mooradian et al., 1987; Gruber et al., 2002). In 1971, Grewall and colleagues published the first report that in male rats 50% CR for 23 weeks reduced serum levels of testosterone without affecting epididymal or testicular sperm concentrations (Grewall et al., 1971). Since then many studies have shown that short- and long- term CR reduce serum luteinising hormone (LH) and testosterone concentrations in rodents (Howland, 1975; Merry & Holehan, 1981; Govic et al., 2008).
No information on the effects of long-term CR without malnutrition on circulating sex hormones in healthy lean humans has been published. The purpose of the present study was to compare the effects of long-term CR and endurance exercise training on sex hormone levels in equally lean healthy individuals to differentiate the effects due to leanness from those due to decreased calorie intake. Data from experimental studies have shown that rats that maintain a low body fat mass by performing regular exercise had only an extension of average lifespan, whereas both average and maximal lifespan is increased in sedentary rats that are calorie-restricted (Holloszy, 1997). The failure of life-long endurance exercise training to increase maximal lifespan and slow intrinsic aging provides evidence that slowing of aging is mediated by an effect of decreased food intake, not by leaness.
In this study, circulating levels of total testosterone, total 17-β-estradiol, sex hormone binding globulin (SHBG), and dehydroepiandrosterone sulfate (DHEA-S) were determined in men practising long-term severe CR, in age-, and total body fat-matched endurance runners, and in age-matched non-obese sedentary men consuming Western diets.
RESULTS
NUTRIENT INTAKE
Nutrient intakes differed significantly between the three groups. The CR men designed their diets in order to consume a balance of foods that supply more than 100 percent of the Recommended Daily Intake for all the essential nutrients, while minimizing energy content (1350–2415 kcal/d). They eat a wide variety of vegetables, fruits, nuts, low-fat dairy products, egg whites, wheat and soy proteins, fish and meat (~24% calories from protein, ~27% from fat, ~48% from complex carbohydrates and 1% from alcohol). All of CR group strictly avoid refined and processed foods containing trans-fatty acids and high glycemic foods (e.g. refined carbohydrates, desserts, snacks and soft drinks). The untrained comparison group ate typical U.S. diets (2145-3537 kcal/d; ~16% calories from protein, ~31% from fat, ~49% from carbohydrates and ~4% from alcohol). The EX group also ate typical American diets but with a higher energy intake to compensate the energy expenditure due to the high volume of daily training (2564-4345 kcal/d; ~16% calories from protein, ~33% from fat, ~48% from carbohydrates and 3% from alcohol).
BODY WEIGHT AND COMPOSITION
Mean values for body weight and BMI were significantly different between the three groups (Table 1). Total body weight and BMI were significantly lower in the CR group than in the EX and WD groups, while, total body fat and trunk fat were similar in the CR and EX groups, and lower than in the WD group (Table 1). Lean body mass was significantly lower in the CR group than in the EX and WD group (Table 1).
Table 1.
Characteristics of the study subjects
| CR group (n=24) | EX group (n=24) | WD group (n=24) | Among group P | |
|---|---|---|---|---|
| Age (yrs) | 51.5±13 | 53.9±11 | 52.3±9.7 | NS |
| Height (m) | 1.75±0.07 | 1.77±0.08 | 1.78±0.06 | NS |
| Weight (Kg) | 58.6±4.61,2 | 70.8±5.11 | 85.1±9.7 | 0.0001 |
| BMI (Kg/m2) | 19.1±1.41,2 | 22.6±1.91 | 26.7±2.5 | 0.0001 |
| Body fat (% body weight) | 9.7 ± 4.61 | 10.9 ± 4.51 | 23.2 ± 6.2 | 0.0001 |
| Trunk fat (%) | 7.0 ± 5.01 | 8.4 ± 6.01 | 25.2 ± 8.4 | 0.0001 |
| Lean body mass (Kg) | 51.7±4.81,2 | 59.2±5.0 | 59.9±8.8 | 0.0001 |
All values are means ± SD.
Significantly different from WD group, P≤0.0001
Significantly different from EX group, P≤0.001
Sex endogenous steroid hormones
Serum total testosterone and the free androgen index were significantly lower in the CR group than in the EX and WD groups (Table 2). Serum SHBG concentration was higher in the CR group than in the EX and WD groups, but the difference was statistically different only between the CR and the WD group (Table 2). Serum 17-β-estradiol concentrations and the estradiol:SHBG ratio were significantly lower in the CR and EX groups than in the WD group (Table 2). Serum DHEA-S concentrations were not significantly different between groups (Table 2).
Table 2.
Serum sex hormone concentrations of the study subjects
| CR group (n=24) | EX group (n=24) | WD group (n=24) | Among group P | |
|---|---|---|---|---|
| Total testosterone (nmol/L) | 12.0 ± 5.31,2 | 18.8 ± 3.6 | 17.6 ± 6.3 | 0.0001 |
| 17β-estradiol (pg/ml) | 13.9 ± 7.51 | 16.4 ± 3.73 | 21.8 ± 8.2 | 0.001 |
| SHBG (nmol/L) | 238 ± 1001 | 193 ± 62 | 142 ± 79 | 0.001 |
| Free androgen index | 5.9 ± 3.51,4 | 10.9 ± 4.7 | 15.8 ± 9.7 | 0.0001 |
| Estradiol:SHBG ratio | 33.8 ± 44.93 | 35.6 ± 20.13 | 62.7 ± 28.5 | 0.005 |
| DHEA-S (ng/ml) | 832 ± 378 | 829 ± 497 | 932 ± 468 | NS |
All values are means ± SD.
1,3Significantly different from WD group
P≤0.004
P≤0.05
2,4Significantly different from EX group
P≤0.001
P≤0.05
Serum SHBG concentration correlated linearly with the Matsuda and DeFronzo insulin sensitivity index (r = 0.348, P = 0.003) and serum adiponectin concentration (r = 0.406, P = 0.001) (Figure 1). Serum SHBG concentration correlated inversely with BMI (r = 0.515, P = 0.0001) and serum IGF-1 concentration (r = 0.312, P = 0.008) (Fig.1). Serum total testosterone concentration correlated linearly with BMI (r = 0.413, P = 0.0001), lean mass (r = 0.348, P = 0.001), systolic blood pressure (r = 0.387, P = 0.001), and serum concentration of triiodothyronine (r = 0.387, P = 0.001) (Figure 2). Data on the Matsuda and De Fronzo ISI (Matsuda & DeFronzo, 1999), systolic blood pressure, and on serum concentration of triiodothyronine, IGF-1, and adiponectin have been reported previously (Meyer et al., 2006; Fontana et al., 2006; Fontana et al., 2009; Fontana et al., 2008), and have been used here for correlation with the new data (i.e. serum SHBG and testosterone concentrations) in figure 1 and 2.
Figure 1.
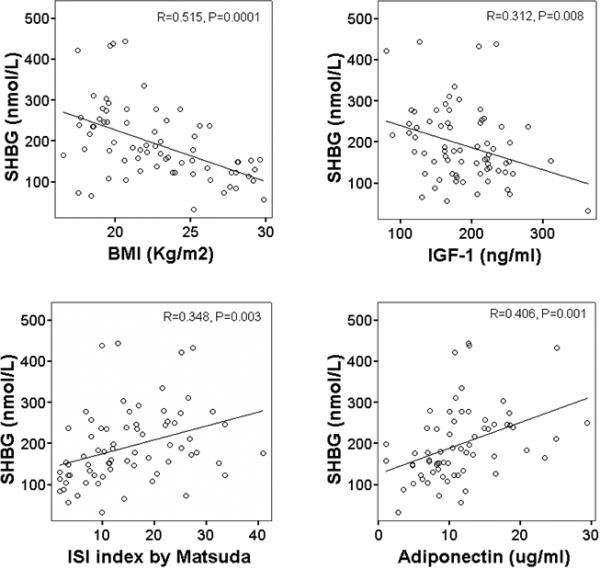
Relationship between serum SHBG concentration and BMI (upper left panel), ISI by Matsuda and De Fronzo (upper right panel), serum IGF-1 concentration (lower left panel), and serum adiponectin concentration (lower right panel) in the study subjects. Pearson correlation was used to assess associations between continuous variables.
Figure 2.
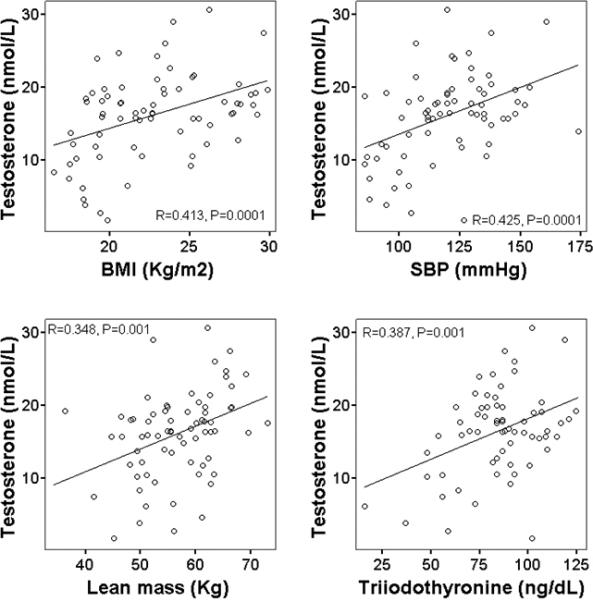
Relationship between serum total testosterone concentration and BMI (upper left panel), systolic blood pressure (upper right panel), lean mass measured by DXA (lower left panel), and serum triiodothyronine concentration (lower right panel) in the study subjects. Pearson correlation was used to assess associations between continuous variables.
DISCUSSION
The data from the present study show that long-term CR with adequate nutrition is associated with lower circulating sex hormones in healthy lean men. Serum concentrations of total and free testosterone were significantly lower in CR participants than in body fat-matched endurance runners and non-obese sedentary individuals who were consuming Western diets. Serum concentration of 17-β-estradiol was significantly lower in the CR and EX group than in the WD group. In addition, circulating levels of SHBG, a glycoprotein that binds to sex hormones, was significantly higher in the CR group, and tended to be higher in the EX group than in the WD group. This CR-mediated reduction in sex hormones is consistent with that seen in long-lived CR rodents, and further support the concept that many of the hormonal adaptations to CR are remarkably well conserved among species (Grewall et al., 1971; Howland, 1975; Merry & Holehan, 1981; Govic et al., 2008). However, no differences in serum DHEA-s concentrations were detected between groups.
Whether the CR-mediated reduction in anabolic sex hormones plays an independent role in modulating the biology of aging and lifespan extension is not known. However, it is well known that in experimental animals CR reduces the production of anabolic hormones and growth factors (Weindruch & Walford, 1988; Fontana & Klein, 2007; Kalant et al., 1988; Grewall et al., 1971; Breese et al., 1991). Anabolic/mitogenic processes require energy to synthesize large complex molecules from small simple molecules, promote cell proliferation and growth. When food intake is reduced, cell growth and proliferation decrease as a result of lower availability of substrate and energy. It appears from the data on CR animals and our findings on CR humans that there are feedback mechanisms by which decreased availability of substrates and/or ATP bring about a decrease in production of anabolic hormones that normally stimulate cell growth and proliferation. The results of our study show that long-term CR with adequate nutrition cause a chronic decrease in serum total and free testosterone concentrations in men, independent of body fat mass. It is interesting in this context that body mass index and lean mass were both directly correlated with serum testosterone concentrations. Moreover, circulating levels of triiodothyronine, a hormone typically reduced by CR that regulates cellular metabolism and growth (Braverman & Utiger, 2004), was also directly correlated with serum testosterone concentrations, further suggesting an integrated hormonal/metabolic control of anabolic processes during CR. The mechanism(s) responsible for the relationship between reduced intake of calories and lower serum concentration of sex hormones is not completely understood, but a decreased production of gonadotrophins by the pituitary is clearly involved (Howland, 1975; Pugeat et al., 1991).
Calorie restriction without malnutrition is also the most potent and reproducible physiological intervention for protecting against cancer in mammals (Longo & Fontana, 2010). Accumulating evidence suggests that chronically elevated concentrations of growth factors and anabolic hormones exert direct powerful mitogenic effects through several signalling transduction pathways (e.g. phosphatidylinositol-3 kinase/Akt/Tor/S6kinase, and p66Shc ) in many cell types, especially in pre-neoplastic cells (Heinlein & Chang, 2004; Lee et al., 2004; Longo & Fontana, 2010). CR likely exerts its anti-cancer effects by down-regulating a number of anabolic/growth factor molecules and pathways, and by up-regulating several genes and signal transduction molecules that promote DNA and cellular repair, resistance against oxidative stress and apoptosis of damaged cells (Longo and Fontana, 2010). Whether the CR-mediated reduction in anabolic sex hormones has an independent role in the prevention of cancer is not clear. In women, higher concentrations of estrogens and testosterone are associated with an increased risk of breast and endometrial cancer (Yager & Davidson., 2006; Davidson et al., 1981). Recent data from the Baltimore Longitudinal Study of Aging suggest that elevated circulating levels of serum free testosterone are associated with an increased risk of aggressive prostate cancer among older men, but with a reduced risk in men younger than 65 years (Pierorazio et al., 2009). More studies are needed to elucidate the role of a reduction in anabolic hormones (e.g. testosterone and 17-β-estradiol) in combination with the reduction of other growth factors (e.g. insulin and IGF-1) and inflammatory cytokines (e.g. IL6) in mediating the anti-cancer effects of CR. Further studies are warranted to understand the long-term effects of the CR-induced reduction in sex hormones concentrations on skeletal and bone mass and strength.
The data from the present study also show that long-term CR with adequate nutrition is associated with a marked increase in SHBG, a circulating protein that binds to sex hormones and inhibits the function of these hormones (Pugeat et al., 1991). SHBG is mainly, but not exclusively, produced by liver cells and is released into the bloodstream. Several nutritional and hormonal factors regulate the hepatic production of SHBG, including monosaccharides, insulin, and IGF-1(Selva et al., 2007; Plymate et al., 1990). Weight loss in obese men is associated with an increase in SHBG levels, improvement in insulin sensitivity, and a significant decrease in plasma insulin levels (Niskanen et al., 2004; Kaukua et al., 2003). In contrast, overfeeding (1000 kcal energy excess/day) for a period of 100 days was associated with a fall in SHBG in a study of young, sedentary, male monozygotic twins (Pritchard et al., 1998). In this study, we found that body mass index and serum IGF-1 concentration were both inversely correlated with serum SHBG concentration, whereas serum adiponectin concentration and the Matsuda and DeFronzo insulin sensitivity index were directly correlated with serum SHBG concentration. More studies are needed to understand if this increase in serum SHBG concentration induced by reduced energy intake has clinical implications (e.g. prevention of cardiovascular disease and cancer) or is simply a biological marker of energy status (Davidson et al., 1981; Laaksonen et al., 2004; Kalme et al., 2005).
In gorillas, chimps and human primates, the sex steroid hormone DHEAS, which is produced by the adrenal cortex, circulates in far higher concentrations than any of the other endogenous sex steroid hormones in serum (Hornsby, 1995). In contrast, rodents secrete little or no DHEA-S. Data from epidemiological studies have shown that serum concentration of DHEA-S rapidly declines with age so that by age 75 yr serum DHEA-S concentration is ~80% lower than at 20 yr (Orentreich et al., 1992). It has been reported that CR protects against the decrease in serum DHEA-s concentration with advancing age in rhesus monkeys. Serum DHEA-S concentration declined more than 30% over 4 yr in adult male rhesus monkeys eating an “ad libitum” diet, whereas the decline was only 3% in the CR monkeys (Lane et al., 1997). In the present study we found that long-term CR without malnutrition does not protect against a decline in DHEAS in humans as there were no differences in serum DHEAS concentration between the individuals practicing CR for an average of 7 years and age-matched controls.
Our study has limitations. First, because of the cross-sectional design of our study, we are only able to show associations with diet, physical activity and circulating sex hormones, and cannot determine true causal relationships. A long-term randomized controlled trial would be needed to determine cause-and-effect relationships. However, this type of trial is extremely difficult to perform, because of the difficulty in achieving long-term dietary and exercise compliance, and because of the cost. In contrast, the ability to utilize specimens from human volunteers engaged in long-term CR (and the comparison with equally lean endurance athletes) is providing valuable information regarding the maximal effects of CR on several important metabolic and hormonal factors that are attainable in response to prolonged, severe CR by highly motivated, disciplined lean individuals. A multicenter randomized controlled trial of the effects of 2 yr of 25% CR in middle-aged non-obese (body mass index ≥ 22 and <28 kg/m2) women and men, currently in progress (CALERIE phase 2), might provide some of this information regarding a cause-and-effect relationship, and the time-course of these CR-mediated hormonal adaptations. Second, women were not included in this study because at this time we have been able to identify and study only four women who are practicing severe CR, and are willing to participate in our study.
Our data show that long-term CR with adequate protein and micro-nutrient intake is associated with low serum total and free testosterone, independent of body fat mass, while serum concentrations of SHBG are elevated in CR men. More studies are needed to elucidate whether this CR-mediated reduction in sex hormones has beneficial or detrimental effects in modulating aging and the pathogenesis of age-associated chronic diseases such as cancer.
EXPERIMENTAL PROCEDURES
Study Subjects
Twenty-four men who had been practicing severe CR with adequate nutrition for an average of ~7 years (range 3-20 yrs) were recruited through the Calorie Restriction Society; four were from the St. Louis area and the others came to the Washington University Medical Center from other cities in the USA and Canada. Twenty-four men, who are long-term endurance runners (EX), matched with the CR group in terms of age, height, and body fat were used as a lean comparison group. The endurance runners ran an average 48 miles/week (range 20 to 90 miles/week), and had been training regularly for an average of 21 years (range 5-35 yrs). Twenty-four untrained (regular exercise <1 h per week) men eating typical Western diets matched with the CR and EX groups in terms of age, and height, served as a sedentary comparison group. The characteristics of the study participants are shown in Table 1. None of the participants had evidence of chronic disease, including cardiovascular, lung, gastrointestinal and autoimmune diseases, type 2 diabetes, or cancer. None were smokers. In addition, none of the participants were taking medications that could have affected the outcome variables. All of the study participants were weight stable, i.e. less than 2 kg weight change in the preceding 6 months. This study was approved by the Human Studies Committee of Washington University School of Medicine, and all participants gave informed consent.
Dietary Assessment
Participants were instructed by a research dietician to record all food and beverages consumed, preparation methods, and portion sizes for 7 consecutive days. To assist with portion size determinations, measuring spoon and cup sets were provided to the participants, and the food diaries had a ruler imprinted on the back cover. Food records were analysed by using the NDS-R program (version 4.03_31), which is the Nutrition Data System for research from the Nutrition Coordinating Center at the University of Minnesota.
Study protocol
Subjects were admitted to the outpatient facilities of Washington University School of Medicine General Clinical Research Center in the morning after they had fasted for 12 hours overnight. Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale in the morning after a 12-hour fast. Body mass index (BMI) was calculated by dividing body weight (in kilograms) by the square of height (in meters). Total body fat mass and lean body mass were determined by dual-energy X-ray absorptiometry (DEXA) (QDR 1000/w, Hologic, Waltham, MA). Blood pressure was measured with a mercury sphygmomanometer, with the patient in the sitting position after 5 minutes of rest in a quiet environment, according to the recommendations of the American Hypertension Society. Four measurements of systolic and diastolic blood presure were made at ~5-min intervals and averaged. Systolic blood pressure from 21 of 24 CR male volunteers and 21 of 24 WD male subjects were reported previously in a study that evaluated the effects of CR on diastolic function (Meyer et al., 2006). A venous blood sample was obtained to determine plasma glucose and serum total testosterone, total 17-β-estradiol, SHBG, DHEA-S, insulin, triiodothyronine, adiponectin and IGF-1 concentrations.
Sample analyses
Circulating endogenous sex hormones were measured using commercial kits from Diagnostic Systems Laboratories (Webster, TX): ultra-sensitive RIA kits for 17-β-estradiol and steroid hormone-binding globulin; and EIA kits for testosterone and DHEA-S. The free androgen index (FAI) is calculated from total testosterone and SHBG: FAI = (100 × total testosterone)/SHBG, with both total testosterone and SHBG expressed in nanomoles per liter (Mathur et al., 1981). Commercial available ELISA and radioimmunoassay kits were used to measure serum adiponectin (B-Bridge International, Sunnyvale, CA), insulin (Linco Research), and insulin-like growth factor 1 (Diagnostic Systems Laboratories Inc, Webster, Tex). Serum triiodothyronine concentrations were determined by using microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, IL). Plasma glucose was measured by the glucose oxidase method (YSI Stat Plus, Yellow Springs, OH). The index of insulin sensitivity (ISI) was calculated according to the method of Matsuda and Defronzo (Matsuda & DeFronzo, 1999). Serum IGF-1, adiponectin, and T3 concentrations and the insulin sensitivity index of Matsuda and DeFronzo from 24 of 28 CR volunteers were reported previously (Fontana et al., 2006; Fontana et al., 2009; Fontana et al., 2008).
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare group variables followed by Tukey post-hoc testing where indicated. One-way ANOVA by ranks was performed for non-normally distributed data. Statistical significance was set at P < 0.05 for all tests. All data were analyzed by using SPSS FOR WINDOWS software, version 12.0 (SPSS Inc, Chicago). Pearson correlation was used to assess associations between continuous variables. Statistical significance was set at P < 0.05 for all tests. All values are expressed as means ± SD.
ACKNOWLEGEMENTS
Funding/Support: Supported by Grant Number UL1 RR024992 from the National Center for Research Resources (a component of the National Institutes of Health and NIH Roadmap for Medical Research), by Istituto Superiore di Sanità/National Institutes of Health Collaboration Program Grant, a grant from the Longer Life Foundation (an RGA/Washington University Partnership), and a donation from the Scott and Annie Appleby Charitable Trust.
Role of the Sponsor: The funding agencies had no role in the analysis or interpretation of the data or in the decision to submit the report for publication.
Footnotes
Financial Disclosures: The author had no conflicts of interest.
References
- Bartke A, Turyn D. Mechanisms of Prolonged Longevity: Mutants, Knock-Outs, and Caloric Restriction. Journal of Anti-Aging Medicine. 2001;4:197–203. [Google Scholar]
- Brverman LE, Utiger RD, editors. A fundamental and clinical text. 9th edn. Lippincott; New York: 2004. Werner & Ingbar's The Thyroid. [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–7. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allsion DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Calorie restriction delays morbidity and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BJ, Gambone JC, Lagasse LD, Castaldo TW, Hammond GL, Siiteri PK, Judd HL. Free estradiol in postmenopausal women with and without endometrial cancer. J. Clin. Endocrinol. Metab. 1981;52:404–408. doi: 10.1210/jcem-52-3-404. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–5. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–7. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action and adipokine production. Age. 2009 doi: 10.1007/s11357-009-9118-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govic A, Levay EA, Hazi A, Penman J, Kent S, Paolini AG. Alterations in male sexual behaviour, attractiveness and testosterone levels induced by an adult-onset calorie restriction regimen. Behav Brain Res. 2008;190:140–6. doi: 10.1016/j.bbr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Grewall T, Mickelsen O, Hafs HD. Androgen secretion and spermatogenesis in rats following semistarvation. Proc Soc Exp Biol Med. 1971;138:723–7. doi: 10.3181/00379727-138-35976. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Mechanisms of Disease: Production and Actions of Estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen Receptor in Prostate Cancer. Endocrine Reviews. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann.N.Y.Acad.Sci. 1995;774:29–46. doi: 10.1111/j.1749-6632.1995.tb17370.x. [DOI] [PubMed] [Google Scholar]
- Howland BE. The influence of feed restriction and subsequent re-feeding on gonadotrophin secretion and serum testosterone levels in male rats. J Reprod Fertil. 1975;44:429–36. doi: 10.1530/jrf.0.0440429. [DOI] [PubMed] [Google Scholar]
- Kalant N, Stewart J, Kaplan R. Effect of diet restriction on glucose metabolism and insulin responsiveness in aging rats. Mech Ageing Dev. 1988;46:89–104. doi: 10.1016/0047-6374(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Kalme T, Seppälä M, Qiao Q, Koistinen R, Nissinen A, Harrela M, Loukovaara M, Leinonen P, Tuomilehto J. Sex hormone-binding globulin and insulin-like growth factor-binding protein- as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J. Clin. Endocrinol. Metab. 2005;90:1550–1556. doi: 10.1210/jc.2004-0762. [DOI] [PubMed] [Google Scholar]
- Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–6. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Lee MS, Igawa T, Chen SJ, Van Bemmel D, Lin JS, Lin FF, Johansson SL, Christman JK, Lin MF. p66Shc protein is upregulated by steroid hormones in hormone-sensitive cancer cells and in primary prostate carcinomas. Int J Cancer. 2004;108:672–8. doi: 10.1002/ijc.11621. [DOI] [PubMed] [Google Scholar]
- Longo VD, Fontana L. Calorie restriction and cancer: metabolic and molecular mechanisms. Trends in Pharmacological Sciences. 2010 doi: 10.1016/j.tips.2009.11.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mathur RS, Moody LO, Landgrebe S, Williamson HO. Plasma androgens and sex hormone-binding globulin in the evaluation of hirsute females. Fertil Steril. 1981;35:29–35. doi: 10.1016/s0015-0282(16)45254-4. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol. Reprod. 1996;54:70–75. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- Merry BJ, Holehan AM. Onset of puberty and duration of fertility in rats fed a restricted diet. J Reprod Fertil. 1979;57:253–9. doi: 10.1530/jrf.0.0570253. [DOI] [PubMed] [Google Scholar]
- Merry BJ, Holehan AM. Serum profiles of LH, FSH, testosterone and 5 alpha-DHT from 21 to 1000 days of age in ad libitum fed and dietary restricted rats. Exp Gerontol. 1981;16:431–44. doi: 10.1016/0531-5565(81)90025-5. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term Caloric Restriction Ameliorates the Decline in Diastolic Function in Humans. Journal of American Collage Cardiology. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SC. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–215. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J.Clin.Endocrinol.Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and Death: What Is the Connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Pierorazio PM, Ferrucci L, Kettermann A, Longo DL, Metter EJ, Carter HB. Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore Longitudinal Study of Aging. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08853.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Hoop RC, Jones RE, Matej LA. Regulation of sex hormone-binding globulin production by growth factors. Metabolism. 1990;39:967–70. doi: 10.1016/0026-0495(90)90309-z. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Després JP, Gagnon J, Tchernof A, Nadeau A, Tremblay A, Bouchard C. Plasma adrenal, gonadal, and conjugated steroids before and after long-term overfeeding in identical twins. J Clin Endocrinol Metab. 1998;83:3277–3284. doi: 10.1210/jcem.83.9.5136. [DOI] [PubMed] [Google Scholar]
- Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, Déchaud H, Tourniaire J. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40:841–9. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nature Reviews Molecular Cell Biology. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117:3979–87. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Charles C Thomas Publisher; Springfield, IL: 1988. [Google Scholar]
- Yager JD, Davidson NE. Mechanisms of disease: Estrogen Carcinogenesis in Breast Cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]


