Abstract
Spectroscopic techniques have demonstrated that in the microscopically normal mucosa, there is an increase in mucosal micro-circulation in patients harboring neoplasia elsewhere in the colon (i.e. marker of field carcinogenesis). However, the physiological and molecular basis of this early increase in blood supply (EIBS) has not been elucidated. We, therefore, investigated the microvessel density (MVD) and angiogenic gene expression in the premalignant colonic mucosa from the well-validated azoxymethane (AOM)-treated rat experimental model of colon carcinogenesis. Fisher 344 rats were treated with AOM (15 mg/kg i.p.) or saline and euthanized 14 weeks later (a time-point that precedes carcinoma development). Colon sections were studied for MVD via immunohistochemical assessment for CD31 and location was compared with optical assessment of mucosal hemoglobin with low-coherence enhanced backscattering spectroscopy (LEBS). Finally, we performed a pilot real-time PCR angiogenesis microarray (84 genes) from the microscopically normal colonic mucosa of AOM and age-matched saline treated rats. AOM treatment increased MVD in both the mucosa and submucosa of the rats (125% increase in mucosa; p<0.007, and 96% increase in submucosa; p<0.02) but the increase was most pronounced at the cryptal base consistent with the LEBS data showing maximal hemoglobin augmentation at 200-225μm depth. Microarray analysis showed striking dysregulation of angiogenic and anti-angiogenic factors. We demonstrate, for the first time, that neo-angiogenesis occurs in the microscopically normal colonic mucosa and was accentuated at the bottom of the crypt. This finding has potential implications as a biomarker for risk-stratification and target for chemoprevention.
1. Introduction
Colorectal carcinogenesis is characterized by sequential progression through various morphological stages (aberrant crypt foci, small adenoma, large adenoma, carcinoma-in-situ, invasive cancer). These are orchestrated by a well established series of mutational/epigenetic events initiated by loss of either adenomatous polyposis coli (APC) tumor suppressor gene or DNA mismatch repair (e.g. hMLH1 or hMSH2) function[1]. It has been estimated that for CRC to develop approximately 15 signaling pathways are required to be altered[2]. Given that the colonocyte is relatively short-lived (3-7 days before becoming shed into the fecal stream), it is becoming increasingly clear that dysregulation of apoptosis and proliferation are prerequisites for the formation of dysplastic lesions. These alterations in cell growth/death occur throughout the colon reflecting the diffuse “field of injury” as a consequence of endogenous (e.g. genetic, diabetes) and exogenous (diet, smoking etc) risk factors[3,4]. Thus, neoplastic transformation in the colon epitomizes the field carcinogenesis concept. This has numerous well recognized clinical implications such as an elevated risk for both synchronous and metachronous lesions[5]. In this regard, current guidelines mandate that patients with a distal adenoma (i.e. detected on flexible sigmoidoscopy) require full colonic evaluation (colonoscopy). Furthermore, since patients with one adenoma are at higher risk of developing future lesions, their colonoscopic interval is usually shortened (e.g. 3 years if an advanced adenoma is detected versus 10 years for no adenoma detection)[5].
Given the important clinical ramifications, there is an emerging interest in accurately identifying and elucidating the biological nature of colonic field carcinogenesis. For instance, epigenetic, genomic, proteomic and micro-architectural biomarkers have been demonstrated to be altered in the microscopically normal mucosa during field carcinogenesis[6-9]. On a cellular note, it has long been recognized that the mucosa is hyperproliferative in patients who harbor neoplasia elsewhere in their colon [10]. Indeed, the proliferative indices from rectal biopsies have been shown to correlate with proximal neoplasia[11]. The corollary to this is that the hyperproliferative mucosa would be expected to be hypermetabolic. The gene expression consequences of the “relative hypoxia” have been recently demonstrated through a series of elegant microarray studies using APC mutations[12]. As would be predicted, the gene expression profile suggested a relative hypoxia in the APC mutated mouse model.
Our group was the first to confirm existence of the phenomena of colonic early increase in blood supply (EIBS) using an optical technology, four dimensional elastic light scattering fingerprinting (4D-ELF) (1). This novel technique allows highly accurate depth selective quantification of the microvascular blood supply. We demonstrated that in the well-validated model of colon carcinogenesis, the azoxymethane (AOM) treated rat. Importantly, the alterations in microvascular blood flow was demonstrated in microscopically normal mucosa at a premalignant time-point (2-15 weeks after AOM treatment) and the magnitude mirrored risk of future development of colonic neoplasia[13]. These were replicated in the MIN mouse, a genetic model of intestinal tumorigenesis[13]. We then demonstrated EIBS clinically using developed an endoscopically-compatible 4D-ELF fiber-optic probe in patients undergoing colonoscopy (2). Importantly, EIBS was detectable in the visually normal rectum in patients harboring advanced adenomas elsewhere in the colon (3)[14], thus suggesting applications as a minimally-intrusive risk-stratification.
While the potential clinical significance of EIBS is clear, the biological underpinnings behind EIBS have been largely unexplored. It is logical to postulate angiogenesis may be a major factor in EIBS given its well-established role in colon carcinogenesis. Indeed, suppression of angiogenesis is a mainstay of CRC therapy (5). However, to our knowledge, no previous studies have evaluated angiogenesis at pre-malignant stages (histologically-normal) mucosa where the phenomenon of EIBS is apparent. We, therefore, wanted to assess angiogenesis in the microscopically normal mucosa as the mechanisms of EIBS. We used the AOM-treated rat model because of its well-validated nature, defined time-frame for carcinogenesis (adenomas start developing in 20 weeks and carcinomas require 35-40 weeks) and the fact that it recapitulates many of the genetic and epigenetic features of human field carcinogenesis[13,15-17].
2. Materials and Methods
2.1 Animal studies
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee for NorthShore University HealthSystem. Twenty-four Fisher 344 rats (150–200 g; Harlan, Indianapolis, IN) were treated with either two weekly injections (i.p.) of 15 mg/kg AOM (Midwest Research Institute, Kansas City, MO) or saline. The rats were kept on a standard AIN76a diet. Rats were euthanized after 14 weeks of second AOM injection, colons were removed, rinsed with 1mM dithiothreitol in normal saline and presence of adenoma was ruled out. For the analysis, the distal colon was utilized given our previous data that in the AOM-treated rat model, EIBS (and also future neoplasia) was most marked in this region of the colon.
2.2 Mucosal RNA Isolation
Briefly, after euthanizing rats (both AOM/saline treated), the colons were isolated, washed and opened longitudinally. The distal colonic mucosa was inspected to assure no neoplastic lesions and then isolated through gentle scraping with a microscope slide as previously described[18]. Total RNA was extracted using TRI Reagent (Sigma) and stored at –80°C.
2.3 Spectroscopic analysis of Hb content at different depths of the colonic wall
In order to study the depths at which blood content is altered in field carcinogenesis we employed a depth resolved optical technique known as Low-coherence Enhanced Backscattering Spectroscopy (LEBS) [19-21]. The LEBS instrumentation has been described in full detail in other publications[22,23]. In brief, the LEBS system employs spatial coherence gating to interrogate the optical scattering and absorption spectrum at different depths within a tissue specimen. Using of an algorithm based on Beer's law, the absorption spectrum can be used to quantify the hemoglobin concentration at each depth within the colonic wall. Due to ex vivo nature of the study, hemoglobin was rapidly deoxygenated and thus it was not possible to accurately determine contributions between oxy-hemoglobin and deoxy-hemoglobin. Therefore, we present all data as total hemoglobin. LEBS measurements were obtained from 9 AOM treated and 9 saline control animals at 10 distinct sites on a fresh distal colonic segments.
2.4 Immunohistochemical staining
Formalin fixed, paraffin embedded distal colonic segments from 14 weeks post AOM treated rats and their age matched controls were subjected to immunohistochemical analysis as described previously[18,24]. Briefly, 5μm paraffin-embedded sections was mounted on Superfrost + slides (Vector Laboratories, Burlingame, CA), heated at 60°C for 1 hour and then deparaffinized with two 5 minute washes of xylene followed by a series of graded ethanol washes. Antigen retrieval for CD31 and Col18a1 were accomplished by pressure microwaving (NordicWare, Minneapolis, MN) in antigen unmasking reagent (Vector Laboratories) at high power setting for 9 minutes. Endogenous peroxidase activity was quenched by a 5 minute wash in 3% hydrogen peroxide while the non-specific binding was blocked by 30 minute incubation with 5% horse serum at room temperature. Sections were then incubated overnight with primary antibodies anti-CD31 (1:200) from Cell Signaling Technology; Anti-Col18a1 (1:100) from Santa Cruz Biotechnology] followed by incubation with appropriate biotinylated secondary antibodies. After three 5 min washings in PBS, the specimen was further developed using Vectastatin Elite ABC kit (Vector Laboratories).
2.5 RT2 Profiler PCR Array
Rat angiogenesis array was performed using RNA isolated from rat mucosal scrapings and RT2 Profiler PCR Array kit from SA Biosciences (Maryland, USA), which quantitatively assessed a panel of 84 genes implicated in angiogenesis. This was a pre-specified commercially available array that includes both pro- and anti-angiogenic factors known to modulate different facets of angiogenesis either directly or through intermediates. The 84 genes included in the array encompassed growth factors/receptors, adhesion molecules, matrix proteins, proteases (along with their inhibitors); cytokines/chemokines and transcription factors. (the complete list of the genes is provided in the supplementary material for this article). RT2 First strand kit from SA Biosciences was used for cDNA synthesis and RT2 SYBER® Green qPCR Master Mix and compatible BioRad® CFX-384 Cycler and 384-well array plates (96×4 format) were used for the gene expression profiling. The assay was performed as per manufacturer's directions. The expression normalization was done with 5 constitutively expressed genes (Rplp1, Hprt1, Rpl13a, Ldha and Actb).
2.6 Statistical analysis
Micro-vessel density (MVD) was quantified by counting microvessel number in 5 randomly selected fields looking at mucosal and submucosal regions under 40X in distal colon tissue sections from AOM and saline treated Fischer 344 rats. The pathologist (S.C.) was blinded to the treatment group. The comparison of MVD in saline and AOM treated rats was performed by averaging out the numbers of micro-vessels in 5 random fields in each colonic section from AOM and saline treated rats and p value was calculated by students t-test. Staining intensity for Col18a1 protein expression in tissue sections was measured on a five-point intensity scale (0, none; 1, equivocal; 2, low; 3, moderate; 4 and 5; high) by a pathologist blinded to the treatment group and standard averaging and t test was performed for statistical analysis. Micro-array analysis was performed by using array analysis web portal tool available at manufacturer's (SA Biosciences) website and fold change above 1.5 and below 0.67 was considered significant.
3. Results
3.1 Angiogenesis occurs at pre-adenoma stage and is predominantly pericryptal in location
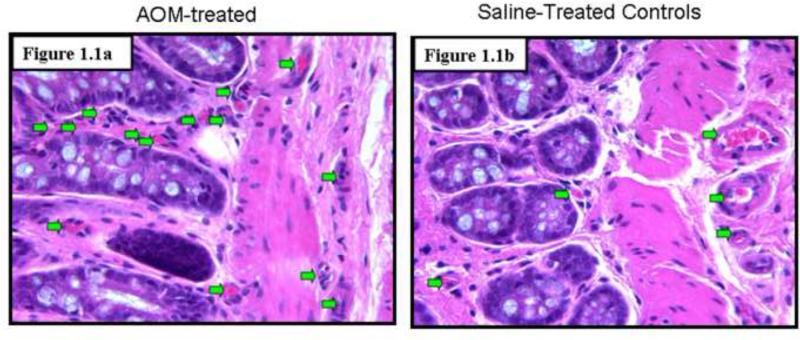
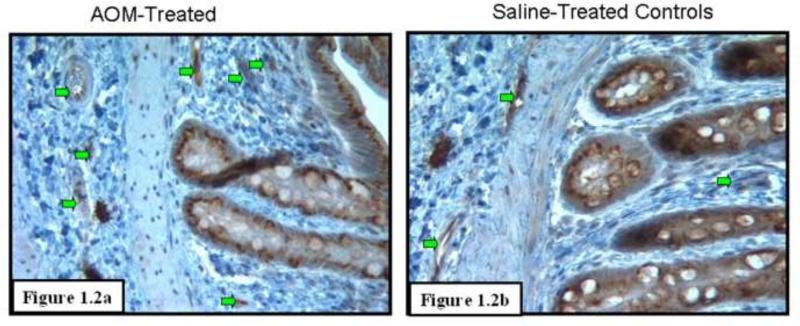
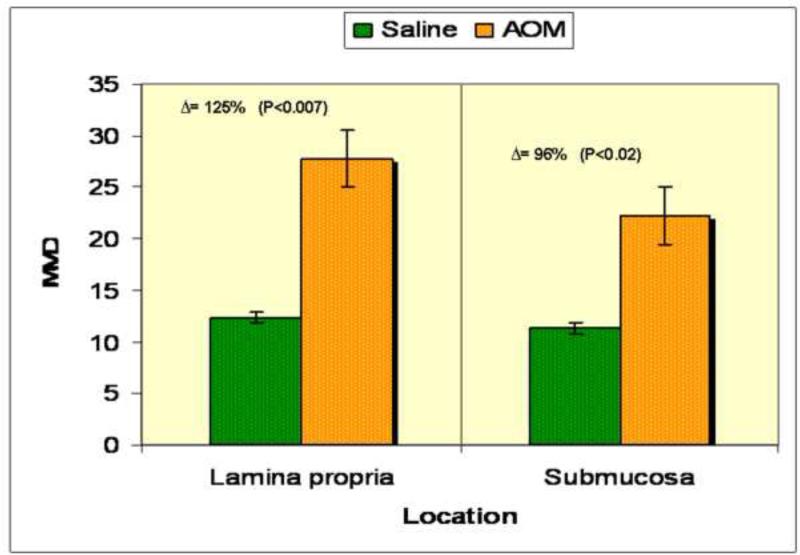
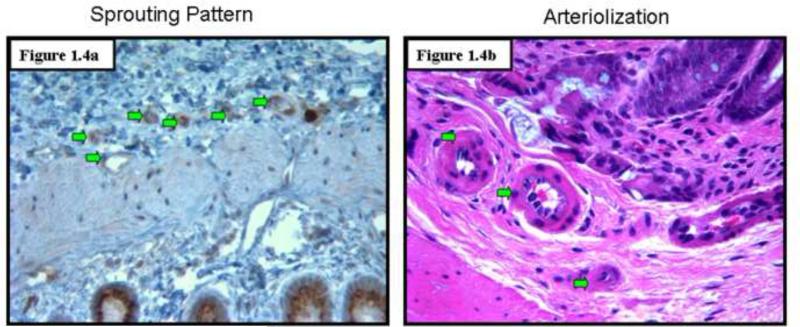
To assess angiogenesis in the premalignant mucosa, we utilized both H&E sections (Figs. 1.1) and those where endothelial cells were highlight with CD31 immunostaining (Figs 1.2) from paraffinized sections taken from rats after 14 weeks of AOM treatment and their age matched controls. We carefully examined the colon under magnification to demonstrate that there were no tumors. Moreover, samples were also evaluated by light microscopy by a trained pathologist (SEC) which confirmed lack of overt dysplasia. There was a marked increase in the mucosal microvessels that was easily observed. In our calculation of microvessel density (MVD), we only scored capillaries, arterioles and venules with larger vessels being excluded. We noticed 125% increase (p<0.007) in MVD in the mucosal lamina propria and 96% increase (p<0.02) in the submucosa of these AOM-treated animals (Fig. 1.3). Aside from the increase in vessel number, there were also qualitative alterations that were consonant with carcinogenesis-related neo-angiogenesis. For instance, the sprouting pattern of micro-vessels (Fig. 1.4a) was observed, which in itself, is an indicator of angiogenesis. This was accompanied by significant vasodilation and arteriolarization (Figure 1.4b) in mucosal and submucosal regions (more pronounced in the submucosal micro-vessels. The arteriolarization in the pre-existing micro-vessels is suggestive of higher pressure reflecting the augmented colonic blood supply early in neoplastic transformation (when the epithelium is histologically normal).
Figure 1.
Increased Microvessel Density in the Premalignant Mucosa of the AOM-treated Rat: These studies were performed on paraffin embedded colon tissue sections from rats euthanized after 14 weeks of second AOM injection and their age-matched saline treated rats. Necropsies confirmed lack of evidence of neoplasia.
Fig 1.1: Examination of hematoxylin and eosin (H & E) stained slides noted a profound increase in microvessels (green arrows) in the AOM-treated rat (Fig 1.1a) versus the age-matched saline treated controls (Fig 1.1b). Of note, the microscopic examination of the slides did not reveal any evidence of dysplastic alterations in the mucosa.
Fig 1.2: Microvessel density assessment utilizing CD31 as a marker of endothelial cells to highlight stromal microvessels (green arrows) (CD 31 also cross-reacted with epithelial cells). There was a dramatic induction in microvessels with the AOM-treated animals (Fig. 1.2a) when compared to age-matched saline controls (Fig. 1.2b).
Figure 1.3: Quantification of microvessel density (MVD) was calculated by examining 5 random fields under 40X in both AOM and age matched saline treated rats after CD31 staining. Large vessels were excluded from analysis. Examiners were blinded to treatment group, There was a significant increase in MVD in the AOM treated rats and while this occurred diffusely it was more pronounced in the mucosal lamina propria (125% increase in mucosa; p<0.007 and 96% increase in submucosa; p<0.02)].
Figure 1.4: Qualitative alterations in microvessels: AOM-treatment resulted not simply in the doubling of MVD but also qualitative changes that are hallmarks of neo-angiogenesis. These include the “sprouting” blood vessel pattern (Fig. 1.4a) and arteriolization (Fig 1.4b).
3.2 Microvascular changes are most pronounced at the base of the crypts
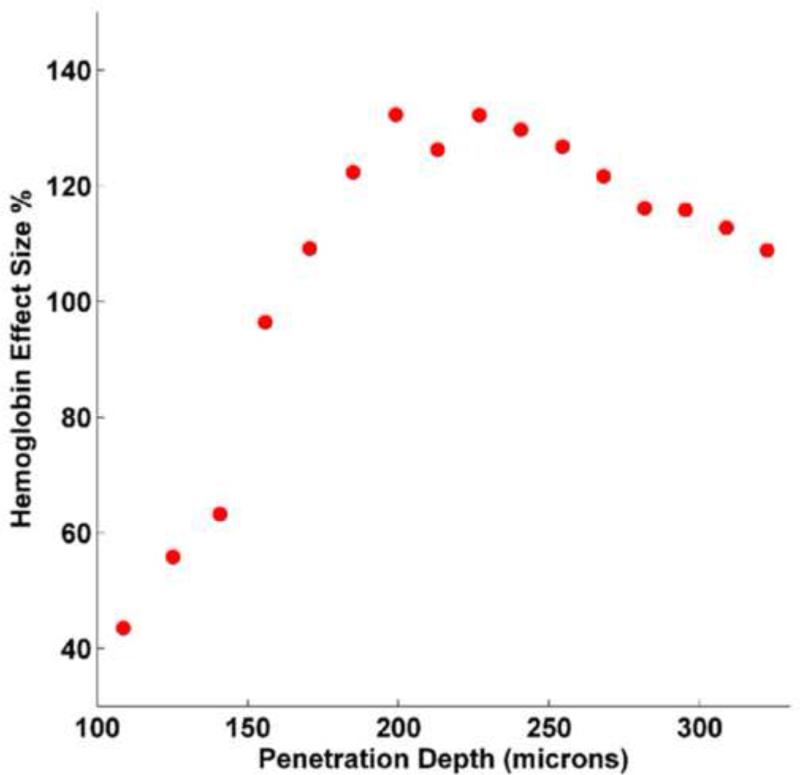
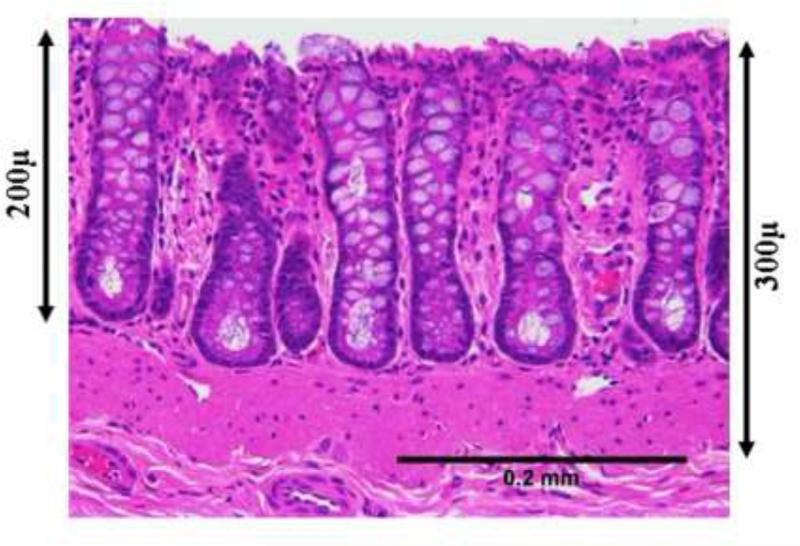
Although significant increase in microvascular blood content was noticed throughout the mucosa and the submucosa, there appeared to be a predilection for the region around the cryptal bases (Figs 1.1 and 1.2). To correlate the augmentation in MVD to the EIBS phenomena detected biophotonically, we utilized depth-resolved LEBS spectroscopy. Figure 2.1 shows the difference between Hb levels in the saline and the AOM-treated rats at different penetration depths as measured by the LEBS system. The effect size which is defined as , (where μ is the mean and σ is the standard deviation) shows the relative increase of Hb in the AOM treated rats over the age matched saline control rats). These results indicate an overall increase in Hb content around the crypts with a maximum effect occurring at around 200-225μm depth. This depth corresponds to the base of the crypts as demonstrated by the representative tissue sections (Fig 2.2). These measurements were confirmed by confocal microscopy (data not shown). This is where the proliferative compartment of the colonic crypt resides thus supporting the hypothesis that greater metabolic demands may be the physiological driving factor to microcirulatory augmentation. Furthermore, these studies demonstrate that the microcirculatory abnormalities detected with optical techniques appeared to co-localize with MVD bolstering the proposition that the increased MVD is responsible for the phenomena of EIBS.
Figure 2.
The depth of maximal mucosal hemoglobin concentrations co-localizes with the increase in MVD.
Fig 2a. Mucosal hemoglobin concentration was measured biophotonically with LEBS. The measures are the differential between AOM-treated and age-matched controls expressed as “effect size” expressed as a percentage. As can be seen, while at all depths there was a positive effect size with AOM (more hemoglobin), the maximal differential appeared to be at ~200-225 microns from tissue surface. As one probes deeper, there is a modest diminution in the AOM versus saline differences in hemoglobin.
Figure 2b: In order to understand the histological correlates for this ~200-225 microns optimal effect size in Hb we analyzed H&E stained sections of the colonic crypts were imaged at 20x magnification using an Olympus BH-2 brightfield microscope equipped with a SPOT camera. Scale bars were added using ImageJ software (NIH) to show various depths of the colonic mucosa. It appears that~200-225 microns from the tissue surface corresponds to the base of the crypts consistent with the hypothesis related to increased mucosal oxygen demand.
3.3 Altered expression profile of angiogenic and anti-angiogenic factors at pre-adenoma stage
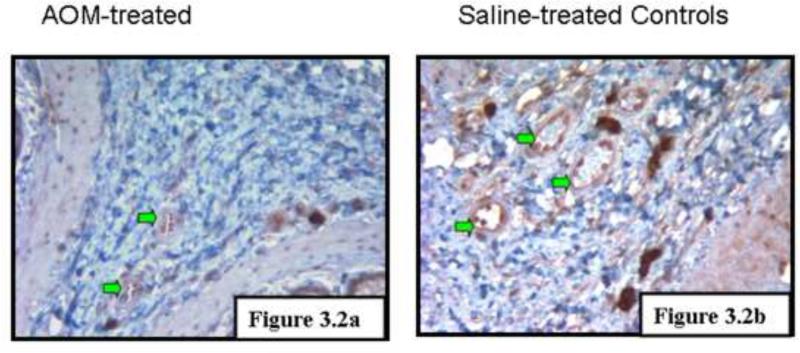
RT2 Profiler Angiogenesis PCR Array comprised of an 84 gene panel encompassing both angiogenic and anti-angiogenic factors was applied to the premalignant mucosa of AOM treated rats (14 weeks post carcinogen injection). With regards to downregulation, there were several genes that were decreased including tissue inhibitor of metalloproteinases 2 (timp2, 0.67 fold), matrix metalloproteinases 3 (0.46 fold), pro-collagen type XVIII, α1 (Col18a1, 0.61 fold), sphingosine kinase (Sphk1, 0.58 fold), coagulation factor 2 (F2, 0.67 fold) and interferon γ (Ifng, 0.49 fold). With regards to genes induced, these included chemokine (c-x-c motif) ligand 9 (Cxcl9, 1.81 fold), chemokine (c-c motif) ligand 2 (Ccl2 1.75 fold) and connective tissue growth factor (Ctgf, 1.50 fold). In general there was a shift in balance with increased angiogenic and decreased anti-angiogenic factors. However, there were some exceptions underscoring the complexity/cross-talk in these angiogenic regulatory pathways. The expression profile also suggested of pathological angiogenesis as seen with tumors and not with the angiogenesis associated with physiological processes[25]. An intriguing case in point is Col18a1 is a strong anti-angiogenic factor associated with tumor angiogenesis but does not appear to have a role during the angiogenesis associated with wound healing or reproduction [26]. We corroborated the microarray findings with immunohistochemical detection of Col18a1 (figure 3.2 a).
Figure 3.
RT2 Profiler PCR array performed with the rat colon samples from 14 weeks post AOM treated rats and their age matched controls looked at the expression of 84 genes implicated directly or indirectly in angiogenesis.
Fig 3.1: The heat map (saline treated animals left, AOM-treated animals right) indicates significant alterations in levels of both angiogenic and anti-angiogenic factors consonant with the induction of neo-angiogenesis.
Fig 3.2 Col18a1 immunostaining. Col18a1 was noted to be downregulated in the AOM-treated rats by the real time microarray. To assess at a protein level, we performed immunohistochemical evaluation and noted that saline treated controls had markedly higher expression than AOM-treated animals. This provides pilot corroboration of the array findings that anti-angiogenic factors decreased and pro-angiogenic factors increased with AOM.
4. Discussion
We report, herein, for the first time the occurrence of angiogenesis in the premalignant mucosa of the colon during colorectal carcinogenesis prior to adenoma formation. Moreover, not only was the micro-vessel density increased, but there was evidence of vasodilation and arteriolarization. All of these could contribute to the phenomena of early increase in blood supply (EIBS) that we have observed through the utilization of novel spectroscopic techniques[13]. Importantly, the LEBS data shows the physiological measurement of microcirculation co-localized with the anatomic findings of increased MVD supporting the role of neo-angiogenesis in EIBS. We developed some potential mechanistic insights by demonstrating upregulation of a number of pro-angiogenic factors along with inhibition of several anti-angiogenic mediators during colorectal carcinogenesis.
It bears emphasis that these changes in the microvasculature occur in the premalignant (histologically normal) mucosa. We chose 14 week post-AOM injection rats because this is a pre-adenomatous time-point (in this model it takes ~20 weeks for adenomas to start developing and 35-40 weeks for carcinomas) and recapitulate many of the genetic and epigenetic features of human field carcinogenesis[15,16]. We had previously shown that the 14 week time-point manifested robust induction in blood supply and the changes correlated well with changes in field carcinogenesis in humans. Indeed, our clinical studies (both ex vivo and in situ) have shown an excellent correlation. From a teleological perspective, this increase in microvascular blood flow is needed to support the diffuse mucosal hyperproliferation, which is a hallmark of field carcinogenesis. It is intriguing that the maximal effect size (differences in concentration between AOM-treated and saline-treated rats) from both optical Hb concentration and MVD was in the region of the bottom third of the crypt corresponding to the proliferative compartment (the cells in the middle third typically are not proliferating but undergoing differentiation while the top third are in the process of apoptosis)[22]. Thus, the base of the crypts would be expected would to have a higher metabolic rate. In support of this concept is the demonstration that APC mutations induced alterations in a various metabolic genes in the microscopically-normal intestinal mucosa suggesting a reactive pattern to the relative hypoxia which would be anticipated to lead to augment the micro-circulation[12]. From a neoplastic perspective, it is intriguing to note that this area is also where the colonic stem resides, which are the putative initiators of colon carcinogenesis and dysregulated in field carcinogenesis[27].
While there has been evidence from other systems that angiogenesis mediates the hyperplasia-dysplasia transition[28], this is the first demonstration of increased MVD in the microscopically normal colon at the premalignant (hyperproliferative)[24] time-point. MVD is a well-established technique to assess angiogenesis in CRCs and has prognostic significance. It is clear that MVD increases earlier at the small adenoma stage [29]. The role of MVD in the earliest lesion in colon carcinogenesis, the aberrant crypt foci (ACF), is controversial. On one hand, Shiptz and colleagues (13) noted a marked upregulation in MVD in ACFs, whereas a recent study by Cho et al. (14) using similar methodology failed to confirm the marker. It is important to note that the majority of these studies addressed MVD between the lesion (ACF and adenoma) versus the uninvolved mucosa. To our knowledge, this is the first study to compare the pre-malignant microscopically normal mucosa of colon versus those without any carcinogenesis. This is, we believe, the most relevant comparison for understanding the role of increased micro-circulation in field carcinogenesis. Indeed, this has been our comparators for all studies to date demonstrating EIBS in rat models (comparing AOM versus saline), mice models (comparing APC mutated versus wildtype animals) and humans (comparing patients harboring neoplasia elsewhere in their colon versus those who are neoplasia-free)[13]. In this regard, there have been microarray studies showing that putative vascular modulating agents such as osteopontin and cyclooxygenase from the microscopically normal mucosa of patients with CRC were 10-20 fold upregulated compared to the colonic mucosa taken from patients without neoplasia[30].
With regards to molecular mechanisms, we performed a pilot study to demonstrate that numerous angiogenic regulators are modulated in the premalignant colonic mucosa. The overall expression pattern is consonant with the multi-step process of angiogenesis including loss of integrity of basement membranes of micro-vessels, endothelial cell proliferation, migration, vessel tube formation and stabilization. For example, loss of endogenous inhibitor Col18a1, an integral part of micro-vessels basement membranes leads to a wide array of effects ranging from endothelial cell proliferation (mediated through upregulation of many angiogenic stimulators and downregulation of inhibitor (15)) to extracellular matrix (ECM) remodeling (mediated through the loss of blockage of MMP2, 3 and 19 activity[31]). Additionally, the altered expression of MMP3 and TIMP2 also points towards ongoing ECM remodeling during carcinogenesis. Interestingly, we observed altered expression of several secreted factors (both epithelial and stromal) like Ifng, Cxcl2, Cxcl9 and CTGF, which suggests the possibility of epithelial-stromal interaction and their effects on micro-vasculature, especially endothelial cells migration as seen with tumor angiogenesis [32][33]. The gradient nature of the effect of such secreted factors could actually be responsible for the relatively higher increase in MVD locally near the cryptal bases. However, it needs to be mentioned that this model, while teleologically compelling, is conjecture. In this case, these genes, in conjunction with factors already identified in AOM-induced EIBS such as iNOS[34] and factors that have been shown to be modulated in human field carcinogenesis (COX2, VEGF, osteopontin)[30] create a picture that is complex with interplay between various factors, and requires much further investigation. Finally, it bears emphasis that the study was conducted exclusively on premalignant mucosa so no data is available on dysplastic tissue. However, the other reports have supported the progressive modulation in angiogenicmodulatory factors (VEGF, COX 2 etc.) during the transition from premalignant to frank colorectal cancers (30)).
There are a number of potential implications of this work. Firstly, we have demonstrated that biophotonic detection of increased micro-vascular blood content correlates with increased angiogenesis during carcinogenesis. Since this is a diffuse phenomenon, we have been able to use the rectum to predict lesions elsewhere in the colon. The clinical need is that only 5-6% of the currently recommended screening colonoscopy yields significant neoplasia and thus imparts a cancer preventive benefit[23]. Thus, developing a minimally intrusive pre-screening technique could substantially undermine the need for colonoscopy. We have published in situ data from over 700 subjects that confirms the promise of this approach (for advanced adenomas the area under the receiver operator curve was ~0.90)[14,35]. We envision simplifying our probes to the extent that they could be used at the primary care physician's office during annual rectal examination to effectively risk-stratify the population for colorectal cancer. This work is important in further solidifying the biological underpinnings for this novel pre-screening technique for clinical applications. Secondly, it may have implications for cancer prevention. This supports the emerging field of angioprevention—targeting vasculature[36,37]. And finally, this provides important insights into the early events in colorectal cancer biology.
On the other hand, it is important to acknowledge some of the limitations of this work. Firstly, the study is conducted solely in the AOM-treated rat model. However, AOM is a specific colorectal carcinogen and is widely accepted model for colorectal field carcinogenesis studies[16,17]. Moreover, the observation that MVD and optically derived EIBS appeared intimately related in the AOM-treated rat suggests that it may be reasonable to speculate that MVD may also be noted in human colonic field carcinogenesis. Secondly, while MVD is a standard indicator of angiogenesis, this is only a surrogate marker of blood supply[38]. But this concern is ameliorated by our rigorous optical quantification of mucosal hemoglobin concentration (a more physiological parameter). Other aspects of micro-circulatory abnormalities that we noted included arteriolization and vasodilation but these are more difficult to quantify and, thus, the true magnitude of the microvascular abnormalities in early colon carcinogenesis may be underestimated by our MVD quantification. Thirdly, our RT2 Profiler PCR Array was designed to yield a high-level overview of the molecular changes in angiogenesis but it needs to be emphasized that the genes in the array (84 genes) are not exhaustive with regards to mediators of angiogenesis. Although this study has provided us with many putative novel candidate genes, future studies are required for both corroboration and elucidation of the roles of individual factors. Furthermore, the interactions of these novel genes with the more established angiogenic modulatory factors that have been demonstrated to be dysregulated in field carcinogenesis (e.g. cyclooxygenase, osteopontin, vascular endothelial growth factor, inducible nitric oxide synthase)[39] needs to be thoroughly investigated. Therefore, the array studies simply demonstrate the alterations of angiogenic factors in field carcinogenesis and the results of individual genes should be considered hypothesis generating.
In conclusion, we demonstrate herein that angiogenesis occurs in premalignant mucosa prior to adenoma formation, is mostly located towards the cryptal bases and together with vasodilation, it is responsible for augmentation of vascular content at pre-adenoma stage. Additionally, we report a net shift of balance between the angiogenic and anti-angiogenic factors in the premalignant mucosa leading to angiogenesis during colorectal carcinogenesis. This study provides further mechanistic insights into the EIBS phenomenon and the biology of colorectal carcinogenesis. Our observations have the potential for biomarker development for minimally intrusive and effective risk stratification of CRC as well as formulate novel CRC chemoprevention strategies by altering expression of angiogenesis related genes (angioprevention).
Supplementary Material
Acknowledgements
We thank Ms. Beth Parker for assistance in manuscript preparation and the support of the Duckworth and Lefkofsky family foundations.
Drs. Roy and Backman are co-founders and shareholders of American BioOptics LLC. The funding source had no role in the design or execution of the study, data analysis or manuscript preparation.
This works was supported in part by National Institutes of Health (grants U01CA111257, R01CA128641, R01CA109861, R01CA118794, R01CA156186, 5R42CA130508, and 5R21CA140936)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All aspects of the study and manuscript preparation were done under the supervision of the conflict of interest committee at Northwestern University.
References
- 1.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 2.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 3.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331–7. [PubMed] [Google Scholar]
- 4.Steiling K, Ryan J, Brody JS, Spira A. The field of tissue injury in the lung and airway. Cancer Prev Res (Phila) 2008;1:396–403. doi: 10.1158/1940-6207.CAPR-08-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551–7. doi: 10.1056/NEJMcp063038. [DOI] [PubMed] [Google Scholar]
- 6.Paun BC, Kukuruga D, Jin Z, Mori Y, Cheng Y, Duncan M, Stass SA, Montgomery E, Hutcheon D, Meltzer SJ. Relation between normal rectal methylation, smoking status, and the presence or absence of colorectal adenomas. Cancer. 2010;116:4495–501. doi: 10.1002/cncr.25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao CY, Moore DH, Chiu YS, Wong P, Bennington JL, Smith AP, Chen LC, Lee NM. Altered gene expression in normal colonic mucosa of individuals with polyps of the colon. Dis Colon Rectum. 2005;48:2329–35. doi: 10.1007/s10350-005-0153-2. [DOI] [PubMed] [Google Scholar]
- 8.Polley AC, Mulholland F, Pin C, Williams EA, Bradburn DM, Mills SJ, Mathers JC, Johnson IT. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006;66:6553–6562. doi: 10.1158/0008-5472.CAN-06-0534. [DOI] [PubMed] [Google Scholar]
- 9.Alberts DS, Einspahr JG, Krouse RS, Prasad A, Ranger-Moore J, Hamilton P, Ismail A, Lance P, Goldschmid S, Hess LM, Yozwiak M, Bartels HG, Bartels PH. Karyometry of the colonic mucosa. Cancer Epidemiol Biomarkers Prev. 2007;16:2704–16. doi: 10.1158/1055-9965.EPI-07-0595. [DOI] [PubMed] [Google Scholar]
- 10.Ahnen DJ, Byers T. Proliferation happens. Jama. 1998;280:1095–6. doi: 10.1001/jama.280.12.1095. [DOI] [PubMed] [Google Scholar]
- 11.Anti M, Marra G, Armelao F, Percesepe A, Ficarelli R, Ricciuto GM, Valenti A, Rapaccini GL, De Vitis I, D'Agostino G. Rectal epithelial cell proliferation patterns as predictors of adenomatous colorectal polyp recurrence. Gut. 1993;34:525–30. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Pezo RC, Corner G, Sison C, Lesser ML, Shenoy SM, Mariadason JM, Singer RH, Augenlicht LH. Altered dynamics of intestinal cell maturation in Apc1638N/+ mice. Cancer Res. 2010;70:5348–57. doi: 10.1158/0008-5472.CAN-09-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wali RK, Roy HK, Kim YL, Liu Y, Koetsier JL, Kunte DP, Goldberg MJ, Turzhitsky V, Backman V. Increased Microvascular Blood Content is an Early Event in Colon Carcinogenesis. Gut. 2005;54:654–660. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy HK, Gomes AJ, Ruderman S, Bianchi LK, Goldberg MJ, Stoyneva V, Rogers JD, Turzhitsky V, Kim Y, Yen E, Jameel M, Bogojevic A, Backman V. Optical measurement of rectal microvasculature as an adjunct to flexible sigmoidosocopy: gender-specific implications. Cancer Prev Res (Phila) 2010;3:844–51. doi: 10.1158/1940-6207.CAPR-09-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy HK, Liu Y, Wali RK, Kim YL, Kromine AK, Goldberg MJ, Backman V. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology. 2004;126:1071–1081. doi: 10.1053/j.gastro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee A, Quirke P. Experimental models of colorectal cancer. Dis Colon Rectum. 1998;41:490–505. doi: 10.1007/BF02235764. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther. 2009;8:1313–7. doi: 10.4161/cbt.8.14.8983. [DOI] [PubMed] [Google Scholar]
- 18.Kunte DP, Wali RK, Koetsier JL, Roy HK. Antiproliferative effect of sulindac in colonic neoplasia prevention: role of COOH-terminal Src kinase. Mol Cancer Ther. 2008;7:1797–806. doi: 10.1158/1535-7163.MCT-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YL, Liu K, Wali RK, Roy HK, Goldberg MJ, Kromine AK, Chen K, Backman V. Detection of the initial stages of colorectal carcinogenesis using polarization light scattering spectroscopy with multivariate statistical analysis. Lasers in Surgery and Medicine. 2003:13–13. [Google Scholar]
- 20.Kim YL, Liu Y, Turzhitsky VM, Roy HK, Wali RK, Backman V. Coherent backscattering spectroscopy. Optics Letters. 2004;29:1906–1908. doi: 10.1364/ol.29.001906. [DOI] [PubMed] [Google Scholar]
- 21.Kim YL, Liu Y, Wali RK, Roy HK, Backman V. Low-coherent backscattering spectroscopy for tissue characterization. Applied Optics. 2005;44:366–377. doi: 10.1364/ao.44.000366. [DOI] [PubMed] [Google Scholar]
- 22.Roy HK, Kim YL, Liu Y, Wali RK, Goldberg MJ, Turzhitsky V, Horwitz J, Backman V. Risk stratification of colon carcinogenesis through enhanced backscattering spectroscopy analysis of the uninvolved colonic mucosa. Clinical Cancer Research. 2006;12:961–968. doi: 10.1158/1078-0432.CCR-05-1605. [DOI] [PubMed] [Google Scholar]
- 23.Roy HK, Turzhitsky V, Kim Y, Goldberg MJ, Watson P, Rogers JD, Gomes AJ, Kromine A, Brand RE, Jameel M, Bogovejic A, Pradhan P, Backman V. Association between rectal optical signatures and colonic neoplasia: potential applications for screening. Cancer Res. 2009;69:4476–83. doi: 10.1158/0008-5472.CAN-08-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy HK, Kunte DP, Koetsier JL, Hart J, Kim YL, Liu Y, Bissonnette M, Goldberg M, Backman V, Wali RK. Chemoprevention of colon carcinogenesis by polyethylene glycol: suppression of epithelial proliferation via modulation of SNAIL/beta-catenin signaling. Mol Cancer Ther. 2006;5:2060–9. doi: 10.1158/1535-7163.MCT-06-0054. [DOI] [PubMed] [Google Scholar]
- 25.Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–55. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 29.Aotake T, Lu CD, Chiba Y, Muraoka R, Tanigawa N. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res. 1999;5:135–42. [PubMed] [Google Scholar]
- 30.Chen L, Hao C, Chiu Y, Wong P, Melnick J, Brotman M, Moretto J, Mendes F, Smith A, Bennington J, Moore D, Lee N. Alteration of Gene Expression in Normal-Appearing Colon Mucosa of APCmin Mice and Human Cancer Patients. Cancer Research. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 31.Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, Pihlajaniemi T, Salo T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J Biol Chem. 2003;278:22404–11. doi: 10.1074/jbc.M210325200. [DOI] [PubMed] [Google Scholar]
- 32.Gerber PA, Hippe A, Buhren BA, Muller A, Homey B. Chemokines in tumor-associated angiogenesis. Biol Chem. 2009;390:1213–23. doi: 10.1515/BC.2009.144. [DOI] [PubMed] [Google Scholar]
- 33.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–62. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 34.Roy HK, Wali RK, Kim Y, Liu Y, Hart J, Kunte DP, Koetsier JL, Goldberg MJ, Backman V. Inducible nitric oxide synthase (iNOS) mediates the early increase of blood supply (EIBS) in colon carcinogenesis. FEBS Lett. 2007;581:3857–62. doi: 10.1016/j.febslet.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomes AJ, Roy HK, Turzhitsky V, Kim Y, Rogers JD, Ruderman S, Stoyneva V, Goldberg MJ, Bianchi LK, Yen E, Kromine A, Jameel M, Backman V. Rectal mucosal microvascular blood supply increase is associated with colonic neoplasia. Clin Cancer Res. 2009;15:3110–7. doi: 10.1158/1078-0432.CCR-08-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menakuru SR, Brown NJ, Staton CA, Reed MW. Angiogenesis in pre-malignant conditions. Br J Cancer. 2008;99:1961–6. doi: 10.1038/sj.bjc.6604733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albini A, Noonan DM, Ferrari N. Molecular pathways for cancer angioprevention. Clin Cancer Res. 2007;13:4320–5. doi: 10.1158/1078-0432.CCR-07-0069. [DOI] [PubMed] [Google Scholar]
- 38.Nico B, Benagiano V, Mangieri D, Maruotti N, Vacca A, Ribatti D. Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol. 2008;23:601–7. doi: 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KJ, Nielson KR, Albertine KH. Defining a molecularly normal colon. J Histochem Cytochem. 2001;49:667–8. doi: 10.1177/002215540104900516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.