Abstract
In the present study we used human breast cancer cell lines to assess the influence of ceramide and glucosylceramide (GC) on expression of MDR1, the multidrug resistance gene that codes for P-glycoprotein (P-gp), because GC has been shown to be a substrate for P-gp. Acute exposure (72 h) to C8-ceramide (5 µg/ml culture medium), a cell-permeable ceramide, increased MDR1 mRNA levels by 3-and 5-fold in T47D and in MDA-MB-435 cells, respectively. Acute exposure of MCF-7 and MDA-MB-231 cells to C8-GC (10 µg/ml culture medium), a cell-permeable analog of GC, increased MDR1 expression by 2-and 4-fold, respectively. Chronic exposure of MDA-MB-231 cells to C8-ceramide for extended periods enhanced MDR1 mRNA levels 45-and 390-fold at passages 12 and 22, respectively, and also elicited expression of P-gp. High-passage C8-ceramide-grown MDA-MB-231 (MDA-MB-231/C8cer) cells were more resistant to doxorubicin and paclitaxel. Incubation with [1-14C]C6-ceramide showed that cells converted short-chain ceramide into GC, lactosylceramide, and sphingomyelin. When challenged with 5 µg/ml [1-14C]C6-ceramide, MDA-MB-231, MDA-MB-435, MCF-7, and T47D cells took up 31, 17, 21, and 13%, respectively, and converted 82, 58, 62, and 58% of that to short-chain GC. Exposing cells to the GCS inhibitor, ethylenedioxy-P4, a substituted analog of 1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol, prevented ceramide’s enhancement of MDR1 expression. These experiments show that high levels of ceramide and GC enhance expression of the multidrug resistance phenotype in cancer cells. Therefore, ceramide’s role as a messenger of cytotoxic response might be linked to the multidrug resistance pathway.
Keywords: Multidrug resistance, Ceramide, Glucosylceramide, P-glycoprotein, Breast cancer
1. Introduction
Cancer cells develop various mechanisms to escape the cytotoxic impact of chemotherapy drugs. These cellular changes present a formidable barrier to the successful treatment of cancer. One of the most consistent biochemical alterations in drug resistance is the overexpression of P-glycoprotein (P-gp). P-gp is a membrane-resident protein that functions as an energy-dependent pump reducing the intracellular concentration of anticancer drugs, chiefly hydrophobic, natural product agents such as anthracyclines, Vinca alkaloids, and the taxane, paclitaxel [1]. The MDR1 gene, responsible for multidrug resistance, codes for P-gp [2].
P-gp and related transporter proteins reduce the intracellular concentration of antitumor, antiviral, and antibacterial agents and thus impede treatment of cancer, HIV/AIDS, and bacterial infections. ABC transporter proteins also play a role in Plasmodium falciparum resistance to antimalarials [3], and resistance of Leishmania to antimonials [4]. It is thus imperative not only to develop and test novel ABC-transporter antagonists such as Biricodar (VX710) and LY335979, but also explore and identify regulatory junctures of these proteins.
Several reports have surfaced documenting the interesting but curious association of multidrug resistance with glycolipids [5– 9]. Other works have demonstrated that glucosylceramide synthase (GCS) regulates cytotoxic response to chemotherapy [10–13]. GCS catalyzes glucosylceramide (GC) formation, using ceramide and UDP-glucose as reactants. In wild-type cancer cells, it was shown that pressure for resistance to natural product chemotherapy selected for enhanced GCS expression and activity, in addition to enhanced P-gp expression [5]; a complementary study in multidrug resistant cells showed that inhibition of GCS suppressed the expression of MDR1 [12]. These studies indicate that GCS serves a regulatory role in expression of the multidrug resistance phenotype.
The present study was undertaken to determine whether lipids of the ceramide metabolic pathway affect a cell’s proclivity towards a multidrug-resistant phenotype. Here we show that ceramide (8-carbon and 6-carbon cell-permeable analogs) and a glycosylated counterpart, glucosyl-C8:0 ceramide (C8-GC), upregulate MDR1 expression in human breast cancer cell lines when supplemented to the growth medium; aliphatic lipids such as octanoic acid and oleic acid were not active. All cell lines examined readily glycosylated short-chain ceramide forming, in abundance, the corresponding short-chain GC, and the introduction of a GCS inhibitor, which abolished GC synthesis, prevented ceramide-enhanced MDR1 mRNA increases. Of particular significance to our study is work showing that hexanoyl-glucosylceramide (C6-GC) inhibits P-gp activity, and it is known that inhibitor/substrates of MDR1 can upregulate MDR1 expression [14]. Further, because GC has been shown to be a substrate for the flipase action of P-gp [15], our work suggests that build-up of cellular GC may lead to the induction of MDR1/P-gp. While the role of ceramide as a cellular messenger of apoptosis has been extensively studied [16,17], the possibility of simultaneous induction of MDR1 via GC is novel.
2. Materials and methods
2.1. Cell culture
MCF-7, T47D, MDA-MB-231 and MDA-MB-435 human breast cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in Gibco™ RPMI-1640 medium (Invitrogen Corp., Grand Island, NY) containing 10% FBS (HyClone, Logan, UT), 50 units/ml penicillin, 50 µg/ml streptomycin, and 584 mg/l-glutamine (in addition to the glutamine already in the medium formulation); cells were subcultured using Gibco’s 0.05% trypsin/0.53 mM EDTA solution. Cells were grown in a humidified atmosphere, 95% air, 5% CO2, at 37 °C and subcultured at confluence. K-B-ChR-8-5 (colchicine-resistant human epidermoid carcinoma) cells, used as a P-gp-positive control, were a gift from Dr. Michael M. Gottesman, Laboratory of Cell Biology, National Cancer Institute (Bethesda, MD). Cells were cultured in high glucose DMEM plus 10% FBS, with glutamine, penicillin and streptomycin as above, and colchicine (10 ng/ml).
[1-14C]C6-ceramide (N [1-14C]hexanoyl-sphingosine) was synthesized as described previously [18], and upon purification had a radiospecific activity of 135,118 cpm/nmol. The GCS inhibitor ethylenedioxy-P4 [19] is a phenyl ring substituted analog of parent P4, d-threo-1-phenyl-2-hexadacanoylamino-3-pyrrodilino-1-propanol, and was a gift from Dr. James Shayman, University of Michigan, Ann Arbor.
2.2. Cell supplements
C8-ceramide (made from d-erythro-sphingosine), C6-ceramide, d-erythro-sphingosine, and glucosyl-C8-ceramide (d-glucosyl-β1-1′-N-octanoyl-d-erythro-sphingosine) (Avanti Polar Lipids, Alabaster, AL) were dissolved in ethanol and added in microliter amounts to 37 °C complete culture medium to achieve the desired final concentration of lipid. [1-14C]C6-ceramide was diluted with unlabeled C6-ceramide to achieve a radiospecific activity of 6756 cpm/nmol (16,890 cpm/µg), for cell metabolism experiments. Ethanol stock solutions of these lipids were stored at −20 °C. All lipid-containing media were prepared freshly and not reused. Exposure regimens are given in the figure legends.
2.3. Continuous growth of MDA-MB-231 cells with C8-ceramide
MDA-MB-231 cells (1.5×106) were seeded in T-75 flasks and grown to confluence (15 × 106 cells) over two passages in medium containing 2.5 µg/ml C8-ceramide before being switched to medium containing 5.0 µg/ml C8-ceramide. Cells were then continually passaged at 1:10 in medium containing 5.0 µg/ml C8-ceramide and used in experiments at various passages. Cells grown continuously in medium containing C8-ceramide were termed MDA-MB-231/C8cer cells. In one set of experiments, MDA-MB-231/C8cer cells, at passage 17, were grown without C8-ceramide for 10 passages and used in subsequent assays.
2.4. Lipid analysis
Total lipids were extracted from cells as previously described [11].C8-GC and C6-GC (these lipids co-migrate), and natural cellular GC were resolved from other lipids by thin-layer chromatography (TLC) on Silica Gel G plates (Analtech, Newark, DE) in a solvent system containing chloroform/methanol/ammonium hydroxide (70:35:4, v/v). In this solvent system certain GC species containing hydroxylated aliphatic moieties and galactosylceramide will also migrate with or near GC. C8-lactosylceramide (LacCer) (Avanti Polar Lipids) was resolved in a solvent system containing chloroform/methanol/water (60:40:8, v/v), and C6-sphingomyelin (C6-SPM) (Matreya, Pleasant Gap, PA) was resolved in a solvent system containing chloroform/methanol/ammonium hydroxide (60/35/8, v/v). Short-chain and natural ceramides were resolved in a solvent system containing chloroform/acetic acid (90:10, v/v). Visualization of lipids was by sulfuric acid charring [12], or by iodine staining, when quantitative analysis was by liquid scintillation counting (LSC). In some instance with radiolabeled lipids, TLC analysis was done by zonal profile scan [20,21] by scraping 2–3 mm zones of silica from the plate, followed by LSC for analysis of radioactivity.
2.5. RNA isolation and RT-PCR
Total RNA was isolated from cultured cells as previously described [12], and analysis of gene expression by real-time RT-PCR (qPCR) was conducted as previously described [12]. We used a Rotor-Gene RG-3000 analyzer (Corbett Research, Westborough, MA). Primers and probe sequences for MDR1 were as follows: MDR1 forward 5′-GGTTTATAGTAGGATTTACACGTGGTTG-3′, MDR1 reverse 5′-AAGATAGTATCTTTGCCCAGACAGC-3′, and MDR1 probe 5′ FAM CTAACCCTTGTGATTTTGGCCATCAGTCC Tamra 3′. Human β-actin was used as endogenous control. Both systems employed SuperScript III Platinum One-Step qRT-PCR kits (Invitrogen, Carlsbad, CA). To quantitate the abundance of each MDR1 mRNA, all qPCR runs were conducted with a standard curve comprising a dilution series of qPCR plasmid standards (Invitrogen, Carlsbad, CA) containing open reading frame from start to stop codon for the β-actin gene, ranging from 2 × 102 to 2 × 106 copies/ml. Data were collected from threshold cycle values using the automatic function of the Rotor-Gene software program. The total amount of mRNA of each gene in each sample was calculated as the mean of triplicate samples. Levels of MDR1 mRNA were expressed as the ratio of the target gene to the control gene. Cellular RNA was also analyzed by thermocycling (Bio-Rad, MyCycler) as described previously [12], and the RT-PCR products were analyzed by 1% agarose gel electrophoresis stained with ethidium bromide.
2.6. Rhodamine and [3H]paclitaxel uptake/ efflux assay
The rhodamine assay, a functional test for P-gp efflux activity, was performed as described previously [11]. Briefly, MDA-MB-231 cells and high passage MDA MB-231/C8cer cells were harvested using trypsin and washed with RPMI-1640 medium. To halt P-gp activity during uptake, cells (2×106) were pretreated with cyclosporine A (10 µM) for 2 h. Cells were then exposed to 5 µM rhodamine 123 in 1.0 ml 5% FBS RPMI-1640 medium, with cyclosporine A present, for 60 min at 37 °C. After centrifugation at 500×g for 10 min, supernatants were discarded, and the cells were further washed twice in RPMI-1640 medium. Cellular uptake of rhodamine-123 was measured by adding 200 µl culture medium containing 0.02% SDS to the washed cells, and fluorescence was measured at λexcitation 485 nm/ λemission 530 nm using the FL600 Fluorescent Microplate Reader (BioTek, Winooski, VT). For efflux measurements, 1.0 ml of 5% FBS RPMI-1640 medium without or with cyclosporine A was added, and the cells were incubated at 37°C for another 60 min. After three washes, cell fluorescence was measured using SDS as above. The efflux was calculated by the difference in cell fluorescence after the 60-min incubation compared with initial cell uptake.
Cellular uptake and efflux of paclitaxel was conducted as previously described [12] using [3H]paclitaxel, 25.2 Ci/mmol (Moravek Biochemical, Brea, CA). Unlabeled and radiolabeled paclitaxel were mixed to achieve the desired radiospecific activity of 600 cpm/pmol.
2.7. Chemotherapy cytotoxicity assay — cell viability
Assays were performed as described previously [11]. MDA-MB-231 and MDA-MB-231/C8cer cells were seeded in 96-well plates (perimeter wells contain water) at 3,000 cells/0.1 ml/well, in 10% FBS RPMI-1640 medium and grown at 37 °C for 24 h before adding drug. Doxorubicin and paclitaxel, obtained from LKT Laboratories (St. Paul, MN), were added in 0.1 ml FBS-free medium, and the cells were cultured at 37 °C for 72 h. Cytotoxicity was determined using the CellTiter 96 Aqueous One Solution cell proliferation assay kit (Promega, Madison, WI). Absorbance at 490 nm was recorded using a Microplate Fluorescent Reader FL600.
2.8. Sphingomyelinase treatment
Cellular conversion of [14C]C6-ceramide to SPM was confirmed by sphingomyelinase treatment. Following exposure (24 h) of cells to [14C]C6-ceramide (84,500 cpm/ml medium) plus 4.75 µg/ml unlabeled C6-ceramide, total cellular lipids were extracted and chromatographed (TLC). The area of the plate corresponding to SPM (natural SPM from egg and commercial C6-SPM have similar Rf values) was scraped, and the radiolabeled lipid was removed from the Silica Gel by extraction with organic solvents [11]. After evaporation of solvent, the resultant lipid was suspended by sonication in 0.5 ml Tris–HCl buffer (pH 7.4, 0.1 M) containing 10 mM MgCl2, and 0.2 U B. cereus sphingomyelinase was added and incubated with shaking in a 37 °C water bath for 30 min. The reaction mixture was extracted [11] and the presence of [14C]C6-ceramide, the sphingomyelinase product, was verified by TLC.
2.9. Western blot for P-gp
Western blots were performed as previously described [12], using C219 murine monoclonal antibody (2.5 µg/ml) (Calbiochem, Pasadena, CA) against human P-gp.
3. Results
3.1. Ceramide and GC influence on MDR1 expression — acute exposure
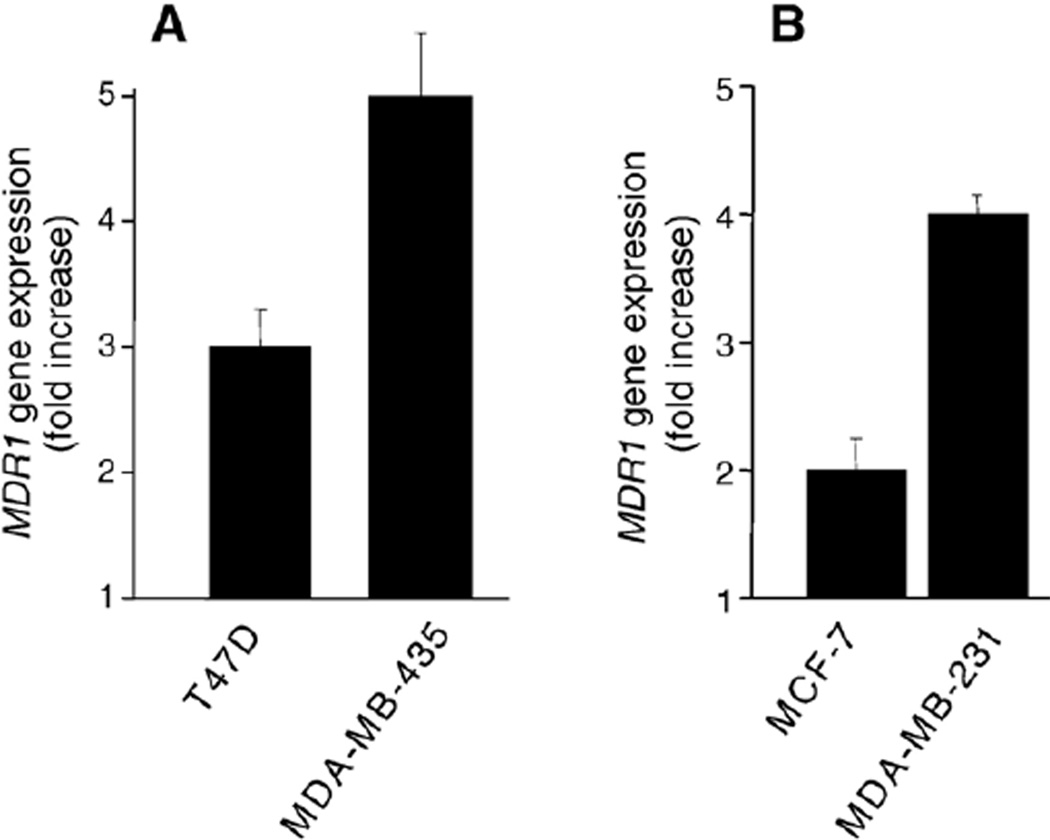
Exposure of estrogen receptor-positive T47D cells, in mid-log phase growth, to C8-ceramide (5 µg/ml, 11.7 µM) for 72 h resulted in a 3-fold increase in MDR1 mRNA levels, over the control, ceramide-naïve cells (Fig. 1A). C8-ceramide also elicited an increase in MDR1 mRNA levels in estrogen receptor-negative MDA-MB-435 cells; a 72 h exposure resulted in a near 5-fold enhancement (Fig. 1A). Acute exposure to C8-ceramide did not alter MDR1 expression in MCF-7 cells, whereas exposure of MDA-MB-231 cells to C8-ceramide (5 µg/ml) was cytotoxic and lowered in subsequent experiments. We then investigated whether GC, a known P-gp substrate [15], would upregulate MDR1 expression. Acute exposure of MCF-7 and MDA-MB-231 cells to C8-GC (72 h) resulted in 2-and 4-fold increases, respectively, in MDR1 mRNA levels (Fig. 1B). T47D and MDA-MB-435 cells did not respond to C8-GC (5 and 10 µg/ml) (data not shown).
Fig. 1.
Influence of C8-ceramide and C8-GC on MDR1 expression in human breast cancer cells. Cells (500,000) were seeded into 6-cm dishes and the following day supplemented with (A) C8-ceramide (5 µg/ml medium) or (B) C8-GC (10 µg/ml medium). Controls received ethanol vehicle (0.1% final concentration). After 72 h, total RNA was extracted and analyzed by qPCR. Fold increases in MDR1 were calculated using actual number of gene copies per unit β-actin expression. Results are the average of triplicate experiments, and experiments were repeated several times.
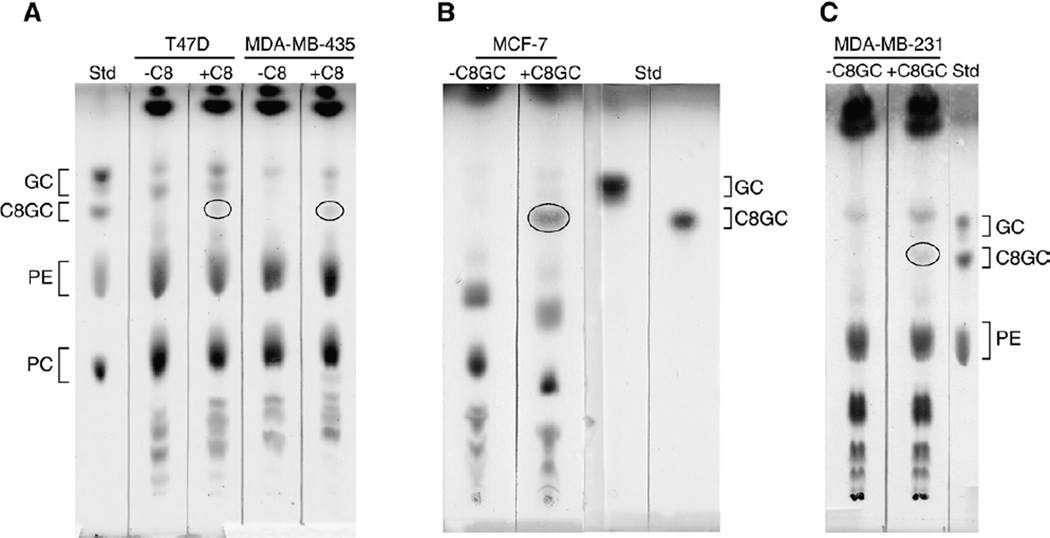
Uptake and conversion of C8-ceramide to C8-GC was evidenced by TLC analysis of T47D and MDA-MB-435 cells that had been exposed to C8-ceramide (Fig. 2A). As shown by the chromatogram, both cell lines supplemented with C8-ceramide contained C8-GC, which migrated slightly below the natural, endogenous GC doublet. C8-GC was absent in control (−C8) T47D and MDA-MB-435 cells. Of note, both T47D and MDA-MB-435 cells responded to C8-ceramide with increased MDR1 mRNA levels (Fig. 1A); however, these cell lines did not respond to C8-GC when given as a supplement. Experiments to evaluate uptake of C8-GC revealed that all cell lines took up the short-chain cerebroside analog, and this is shown using two cell lines, MCF-7 and MDA-MB-231 as examples (Fig. 2B, C).
Fig. 2.
Conversion of C8-ceramide to C8-GC and uptake of G8-GC when supplemented to breast cancer cells. (A) C8-ceramide conversion. Cells were grown in the absence and presence of C8-ceramide (5 µg/ml medium) for 72 h. (B, C) Uptake. Cells were grown in the absence and presence of C8-GC (10 µg/ml medium) for 48 h. Total lipids were extracted from washed cells and analyzed by TLC (200 µg total lipid/lane). Lipids were resolved using a solvent system containing chloroform/methanol/ammonium hydroxide (70:35:4, v/v), and visualization was by sulfuric acid char. Commercial standards (Std) used were GC, glucosylceramide (brain), C8-GC, phosphatidylethanolamine (PE), phosphatidylcholine (PC). In (A) the oval denotes C8-GC synthesized from C8-ceramide supplement. In (B, C) the ovaldenotes C8-GC that was found intracellularly after cells were incubated with C8-GC supplement.
In order to determine whether responses were lipid-specific, cells were exposed to oleic acid and to octanoic acid, the fatty acid hydrolysis product of C8-ceramide. MDA-MB-435 cells, which were responsive to C8-ceramide (Fig. 1), were used in these experiments. Exposure of cells to 5 µg/ml of either fatty acid (72 h) failed to significantly alter MDR1 mRNA levels (qPCR), compared to unsupplemented cells. For example, using the actual number of gene copies per unit β-actin, octanoic acid and oleic acid exposure yielded no increase and a 1.4-fold increase, respectively, in MDR1 expression, over control.
3.2. Ceramide influence on MDR1 phenotype — chronic exposure
During testing of C8-ceramide influence on MDR1 mRNA levels in the various cell lines, we noted that MDA-MB-231 cells, after a 3-day exposure, were more sensitive to the cytotoxicity imparted by this ceramide analog compared to the other cell lines. We chose this cell line to study the influence of chronic exposure to C8-ceramide, the intent being to derive a ceramide tolerant cell line with enhanced capacity for ceramide clearance via glycosylation. This pathway would presumably enhance the levels of cellular C8-GC, and provide an alternate model for evaluating the impact of GC on the multidrug resistance phenotype. MDA-MB-231 cells were first grown with 2.5 µg/ml doses of C8-ceramide for two passages, after which the dose was increased to 5.0 µg/ml, with minimal cytotoxicity.
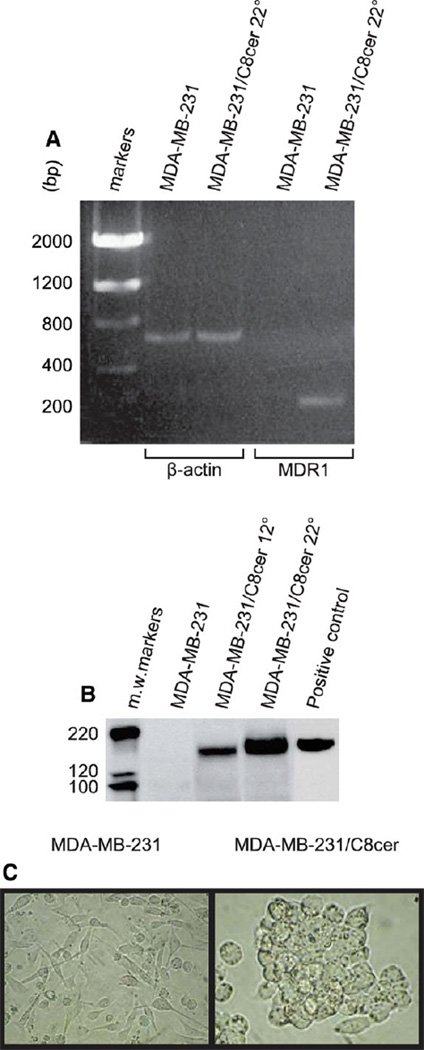
Prolonged growth of MDA-MB-231 cells in C8-ceramide-containing medium caused a robust increase in MDR1 mRNA levels. As measured by qPCR, after 5, 12, and 22 passages with C8-ceramide, MDR1 mRNA amounts increased by 22-, 44-, and 390-fold, compared to ceramide-naïve cells. Fig. 3A demonstrates, by gel electrophoresis of RT-PCR products, the dramatic increase in the level of MDR1 expression in MDA-MB-231/C8cer cells (at passage 22) compared to wild-type MDA-MB-231 cells. In order to determine if upregulation of MDR1 mRNA levels resulted in enhanced expression of P-gp protein, Western blot analyses were conducted. The results of Fig. 3B show that under the conditions employed, P-gp was undetectable in wild-type MDA-MB-231 cells; however, P-gp levels increased with increased C8-ceramide exposure time (passages 12 and 22). These data show that C8-ceramide exposure upregulates both MDR1 mRNA and protein. Prominent morphology changes also accompanied extended C8-ceramide exposure. Fig. 3C shows that MDA-MB-231/C8cer cells were rounder, larger, and tended to grow in islands consisting of clumped, multilayered cells compared to wild-type MDA-MB-231 cells which were spindle-shaped with long stellate cytoplasmic extensions.
Fig. 3.
The influence of chronic exposure to C8-ceramide on MDR1 mRNA, P-gp levels, and cell morphology in MDA-MB-231 cells. (A) MDR1 mRNA levels by RT-PCR in MDA-MB-231 cells and in high passage C8-ceramide cells (MDA-MB-231/C8cer, passage 22). Samples were subjected to RT-PCR analysis (0.5 µg RNA/tube) and products were resolved on 1% agarose gels. β-actin was employed as housekeeping gene. (B) P-gp levels by Western blot in MDA-MB-231 and in MDA-MB-231/C8cer cells at passages 12 and 22. Aliquots (100 µg cell protein) were electrophoresed for Western blot analysis of P-gp (C219 antibody). KB-ChR-8-5 (colchicine-resistant human epidermoid carcinoma) cell protein (50 µg) was used as a positive control for P-gp. For comparative purposes, 0.25 µg protein from MCF-7-AdrR cells was sufficient to detect P-gp in this highly drug-resistant cancer cell line (data not shown). (C) Cellular morphology. MDA-MB-231 cells, after first being exposed to 2.5 µg/ml C8-ceramide were grown continuously in the presence of 5 µg/ml C8-ceramide. MDA-MB-231/C8cer cells shown on right are from passage 19. Photomicrographs at 200× magnification.
Chemotherapy sensitivity status of MDA-MB-231/C8cer cells was evaluated and compared with wild-type MDA-MB-231 cells. Doxorubicin and paclitaxel, natural product chemotherapy drugs that are substrates for P-gp, were used. Firstly, MDA-MB-231/C8cer cells were not more resistant to C8-ceramide, when compared to MDA-MB-231 cells. The IC50 of C8-ceramide in MDA-MB-231 and MDA-MB-231/C8cer cells was 7.2 (3.1 µg/ml) and 7.6 µM (3.2 µg/ml), respectively. Because of the similar 1C50 values, we assessed GCS expression. Results from qPCR showed that chronic exposure of MDA-MB-231 cells to C8-ceramide (17 passages) downregulated GCS expression by approximately 12-fold, compared to wild-type MDA-MB-231 cells (based on number GCS gene copies/ copies β-actin). MDA-MB-231/C8cer cells were more resistant to anticancer drugs. As shown by dose-response/cell viability curves (Fig. 4), MDA-MB-231/C8cer cells were approximately 3- and 9-fold more resistant to doxorubicin and paclitaxel, respectively, compared to MDA-MB-231 cells. Computer-calculated IC50 values were 88 and 270 nM for doxorubicin in MDA-MB-231 and MDA-MB-231/C8cer cells, respectively, and 36 and 331 nM for paclitaxel in MDA-MB-231 and MDA-MB-231/C8cer cells, respectively.
Fig. 4.
Doxorubicin and paclitaxel sensitivity in MDA-MB-231 (■) and MDA-MB-231/C8cer cells (●). MDA-MB-231/C8cer cells (passage 17) were used in the chemosensitivity assays, (A) doxorubicin and (B) paclitaxel. C8-ceramide was not in the medium during the experiment. Cell viability was conducted as described in Materials and methods. Data are the mean ± S.D. of six replicates. Experiments were repeated giving similar results.
In order to determine if the presence of P-gp in MDA-MB-231/C8cer cells correlated with higher efflux potential, we tested the cells using rhodamine, a heterocyclic fluorescent compound that is a substrate for MDR1 protein. These experiments were conducted using cyclosporine A, a pump inhibitor, during rhodamine loading. This step eliminates efflux that occurs during uptake. Under these conditions, MDA-MB-231 and MDA-MB-231/C8cer cells took up approximately 1125 and 373 F.U., respectively, and retained 607 and 268 F.U., respectively, after the efflux period (Fig. 5). Further, the absence of cyclosporine A during uptake reduced rhodamine retained by only 22 and 21% in MDA-MB-231 and MDA-MB-231/C8cer cells, respectively. Therefore, the presence of P-gp in MDA-MB-231/C8cer cells did not impact extracellular effluxing of rhodamine. We further investigated the efflux potential of MDA-MB-231/C8cer cells using paclitaxel, a chemotherapy drug is a P-gp substrate. After 90 min, MDA-MB-231 and MDA-MB-231/C8cer cells took up 270±6 and 63±5 pmol [3H]paclitaxel, respectively. Following a 90-min efflux period, MDA-MB-231 and MDA-MB-231/C8cer cells lost 120 ± 5 (44%) and 28 ± 2 (44%) pmol of [3H]taxol, respectively.
Fig. 5.
Rhodamine uptake and efflux in MDA-MB-231 and MDA-MB-231/C8cer cells. The experiments were conducted as detailed in Materials and methods. Cyclosporine A was present during rhodamine uptake. F.U., fluorescence unites. Uptake refers to intracellular F.U. after 60 min rhodamine exposure. Efflux refers to intracellular F.U. remaining after 60 min reincubation.
3.3. Cellular metabolism of short-chain ceramides
In order to gain insight into the nature of the lipid causal in modifying MDR1 expression, we conducted in depth metabolism studies. The data in Table 1 show the influence of C8-ceramide on cellular ceramide synthesis from [3H]palmitic acid in the breast cancer cell lines. Exposure to C8-ceramide enhanced synthesis of 3H-ceramide in two cell lines, MDA-MB-231 and MCF-7, by 83 and 50%, respectively, whereas C8-ceramide supplements did not alter incorporation of radiolabeled palmitate into ceramide in T47D and MDA-MB-435 cells. We next assessed the capacity of the various cell lines to glycosylate C8-ceramide to C8-GC, by simultaneous incubation with C8-ceramide and [3H]galactose. Using zonal profile analyses of the thin-layer chromatograms, the data of Fig. 6 demonstrate that each cell line has the capacity to glycosylate C8-ceramide, albeit to different degrees. In Fig. 6, the left-most peak corresponds to tritium migrating with commercial C8-GC standard and the right-most peak corresponds to lipid counts migrating with natural GC (glucocerebroside, Gaucher spleen). Of the total counts in monohexosylceramides, C8-GC accounted for 68, 26, 50, and 28% in MDA-MB-231, MDA-MB-435, MCF-7, and T47D, respectively. Notably, in some cell lines, the presence of C8-ceramide in the medium increased the synthesis of natural GC; for example, radiolabel in GC was increased by 25 and 255% in T47D and MCF-7 cells, respectively, (Fig. 6B, C), compared to cells in the absence of supplement.
Table 1.
Influence of C8-ceramide on cellular ceramide synthesis from [3H]palmitic acid
| Cell line |
3H-Ceramide (cpm/100,000 cpm total lipid) |
|
|---|---|---|
| minus C8-cer | plus C8-cer | |
| MDA-MB-231 | 347 ± 25 | 635 ± 19 |
| T47D | 1,470 ± 178 | 1,478 ± 122 |
| MCF-7 | 1,142 ± 51 | 1,722 ± 94 |
| MDA-MB-435 | 227 ± 57 | 233 ± 30 |
Cells (70% confluent) were incubated in 6-well plates without and with C8-ceramide (5 µg/ml medium) in culture medium containing 1.0 µCi/ml [3H] palmitic acid for 24 h. Ceramide was resolved from total lipid extract by TLC.
Fig. 6.
Metabolism of and influence of C8-ceramide on GC formation in breast cancer cells. Cells in 6-well plates were grown in medium containing [3H]galactose (10 µCi/ml 5% FBS medium) in the absence and presence of C8-ceramide (5 µg/ml) for 24 h. Cellular total lipid extracts were analyzed by TLC. Zonal analysis (incremental scraping) was done by LSC only on the GC area of the chromatogram. Results are the mean ± S.D. from triplicate cultures.
To obtain a more detailed analysis of short-chain ceramide metabolism, including possible conversion to SPM and LacCer, we exposed cells to [1-14C]C6-ceramide. The [14C]C6-ceramide was diluted with unlabeled C6-ceramide to obtain a concentration in the medium that mimicked the C8-ceramide exposure level wherein MDR1 expression was enhanced (Fig. 1). The data of Fig. 7 confirm the capacity of the various breast cancer cell lines to glycosylate short-chain ceramides (see Fig. 6 for comparison), but more clearly demonstrate the high extent to which this conversion occurs. MDA-MB-231 cells converted 80% of the counts taken up to [14C]C6-GC in a 24 h period, and MDA-MB-435, MCF-7, and T47D cells had approximate conversion rates of 58, 62, and 58%, respectively. All cell lines synthesized [14C]C6-LacCer, with MDA-MB-435 cells demonstrating the highest conversion rate, approximately 10% of total counts. All cell lines synthesized C6-SPM from [14C]C6-ceramide, with counts ranging from 8 to 18% of total radioactivity. The presence of SPM was verified by TLC purification of the radioactivity migrating with commercial C6-SPM. This isolate was treated with SPMase (see Materials and methods). The radiolabeled lipid from the SPMase reaction, when assessed by TLC, migrated with commercial C6-ceramide. Metabolism of [1-14C]C6-ceramide was also evaluated in MDA-MB-231/C8-cer cells (passage 17). As shown in Fig. 7, right panel, ceramide radioactivity accounted for >60% of the intracellular lipids, and GC counts, in contrast to MDA-MB-231 wild-type cells, amounted to only 20% of total. Therefore, MDA-MB-231/C8-cer cells, compared to wild-type, sluggishly convert [14C]C6-ceramide to [14C]C6-GC.
Fig. 7.
Metabolism of [14C]C6-ceramide by breast cancer cell lines. Cells in 6-well plates were grown with [14C]C6-ceramide (500,000 cpm/ml, 5 µg/ml) for 24 h, after which total lipids were extracted and analyzed by TLC for radiolabeled free C6-ceramide, C6-GC, C6-SPM and C6-LacCer by LSC. For MDA-MB-231/C8cer cells (see right panel), the C8-ceramide was removed from the medium at seeding (6-well plates) and was also absent during the [14C]C6-ceramide exposure period. Results are the mean±S.D. from triplicate cultures.
3.4. MDA-MB-231/C8cer cell stability
In order to determine whether removal of C8-ceramide from the growth medium of MDA-MB-231/C8cer cells would cause the cells to revert, C8-ceramide was removed for 10 passages. Experiments were initiated using MDA-MB-231/C8cer cells at passage 17. Cellular morphology remained identical with that of MDA-MB-231/C8cer cells (see Fig. 3C) after C8-ceramide was removed for 10 passages. Further, the high levels of MDR1 mRNA did not decrease upon removal of C8-ceramide from the medium of MDA-MB-231/C8cer cells. The low GCS expression (mRNA) remained low in MDA-MB-231/C8cer cells cultured without C8-ceramide. Sensitivity to C8-ceramide was slightly enhanced when cells were cultured for 10 passages in the absence of ceramide supplement. For example, the IC50 values were 7.6 and 3.0 µM in MDA-MB-231/C8cer and MDA-MB-231/C8cer minus cells, respectively. Lastly metabolism of [14C]C6-ceramide by MDA-MB-231/C8cer cells after removal of supplement was not different from that depicted in Fig. 7, right panel, for MDA-MB-231/C8cer cells.
3.5. Blocking conversion of C6-ceramide to GC
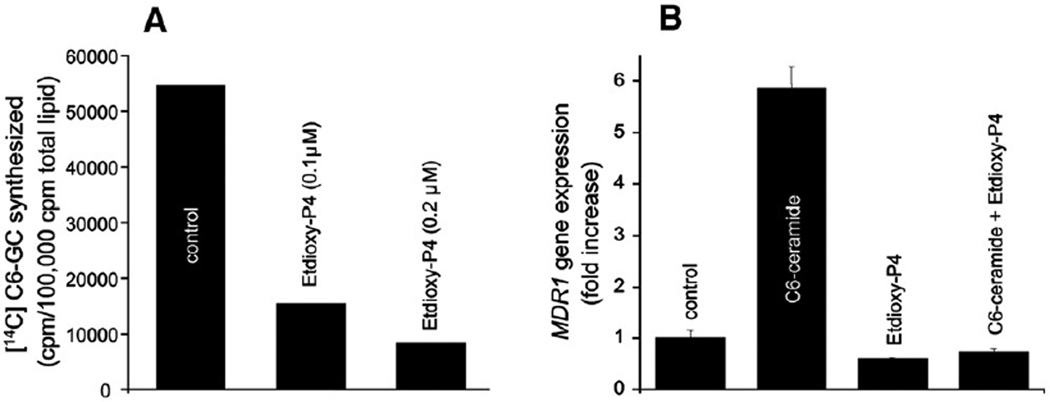
We have shown that exposure of cells to C8-ceramide and C8-GC increases the expression of MDR1 (Fig. 1), and specifically with regard to ceramide metabolism, all cell lines examined robustly metabolized ceramide to GC (Fig. 7). Using MDA-MB-435 cells, which respond well to ceramide (Fig. 1A), we tested whether interfering with the conversion of ceramide to GC would impede upregulation of MDR1. The experiment depicted in Fig. 8 shows that the GCS inhibitor ethylenedioxy-P4 at low concentrations, effectively inhibits [14C]C6-ceramide conversion to [14C] C6-GC in MDA-MB-435 cells (Fig. 8A); for example, at a concentration of 0.2 µM, ethylenedioxy-P4 inhibited GC synthesis by nearly 90%. Fig. 8B demonstrates that whereas C6-ceramide exposure resulted in a near 6-fold enhancement of MDR1 expression, coadministration of ethylenedioxy-P4 and C6-ceramide completely blocked MDR1 upregulation. These data suggest that GC could participate in upregulating MDR1 gene expression.
Fig. 8.
Influence of GCS inhibition on ceramide-induced MDR1 upregulation in MDA-MB-435 cells. (A) Influence of ethylenedioxy-P4 (Etdioxy-P4) on cellular GC synthesis. Monolayer cultures (6-cm dishes) were incubated with [14C]C6-ceramide mixed with unlabeled C6-ceramide (84,500 cpm/ml, 5 µg/ml) for 24 h in the absence or presence of ethylenedioxy-P4. [14C]CG-GC was quantitated in the total lipid extract by TLC and LSC. (B) Influence of GCS inhibitor, ethylenedioxy-P4, on MDR1 expression. Cells in 10-cm dishes were exposed to the agents indicated for 24 h. Total RNA was extracted and MDR1 mRNA was measured by qPCR. C6-ceramide, 5.0 µg/ml; ethylenedioxy-P4, 0.2 µM. Fold increase was calculated as in Fig. 1.
4. Discussion
We and others have shown that multidrug-resistant cancer cells, compared to drug-sensitive cells, contain high levels of GC [8,22,23]. Glucosylceramides and derivatives, found abundantly in nature, are involved in a myriad of processes including proliferation [24], oncogenic transformation [25,26], differentiation [27], and tumor metastasis [26,28]. In skin, GC production is responsible for normalized keratinocyte proliferation wherein upregulation of GCS protects against excess ceramide levels provoked by stress [29]. Other studies have demonstrated that LacCer synthase is upregulated in MDR1-overexpressing tumor cells [30] and that selection pressure for resistance to natural product chemotherapy selects for enhanced ceramide metabolism through GCS, in addition to upregulated P-gp expression [5]. These works draw a curious parallel between glycolipids and multidrug resistance caused by P-gp.
P-gp is expressed in some normal tissues such as kidney, pancreas, intestinal mucosa, adrenal gland, testis, and capillaries of brain. Cancers derived from these tissues may therefore be intrinsically drug-resistant. However, acquired drug resistance is common and occurs during chemotherapy in ovary, lung, bladder, and tumors of the breast. It is well known that anticancer agents, doxorubicin, vinblastine, etoposide, cytarabine, methotrexate, and paclitaxel for example, induce multidrug resistance through direct activation of the MDR1 gene [31,32]. To date, ceramide has not been listed as a MDR1 activator. More recent studies have demonstrated transcriptional activation of the MDR1 gene by UV irradiation [33] and by heat-shock transcription factor 1 [34]. A heat-shock independent induction of multidrug resistance by heat-shock factor 1 has also been described [35]. Although considerable progress has been made, the complexity of the ABC transporter family of proteins and their role in obstructing therapeutic goals necessitates that we improve our understanding of the mechanisms underlying their expression.
To our knowledge, the present work is the first showing that ceramide can lead to upregulated expression of MDR1. Upregulation of MDR1 was based on: (i) increased message as quantitated by both qPCR and gel electrophoresis, (ii) increased P-gp protein levels. The small increase in resistance to natural product anticancer agents (Fig. 4), while a characteristic of multidrug resistance, appears not to be the result of effluxing to the extracellular milieu, because uptake and efflux dynamics (Fig. 5) were not in line with drug pumping. The rhodamine efflux capacity of the P-gp-containing model (see Fig. 5) did not differ appreciably from wild-type MDA-MB-231 cells. Bates and Fojo and colleagues [36] have shown that although expression of the MDR1 gene and gene product can be increased by various agents, this change is not necessarily associated with a decrease in cytotoxic drug accumulation, as was assessed by vinblastine uptake. Several other factors may account for low efflux capacity in MDA-MB-231/C8cer cells. Firstly, when compared to MCF-7-AdrR cells, MDA-MB-231/C8cer cells exhibited an approximate 800-fold lower amount of P-gp; this was based on Western blot analysis of P-gp per unit total cell protein (unpublished data, investigators laboratory). Secondly, although MDA-MB-231/C8cer cells contain P-gp (see Fig. 3), whether it is plasma membrane-localized is not known, and plasma membrane localization is required for effluxing to the extracellular milieu. Wojtal et al. [37] have recently shown that trafficking of P-gp to apical canalicular plasma membrane requires GC.
Many studies have shown that P-gp is present in cancer cells and brain in the Golgi apparatus, rough ER, and the nuclear envelope [38–42]. Maraldi et al. [39] suggest, from work with MDR1 gene-transfected osteosarcoma, that in vitro drug resistance is largely dependant on expression of cell surface P-gp. Molinari et al. [40], in studying cultured tumor cells, show that cytoplasmic P-gp regulates intracellular drug traffic and thus impacts cellular drug targeting. Lastly, it is clear that MDA-MB-231/C8cer cells take up less rhodamine compared to wild-type (see Fig. 5), and less paclitaxel. Altered membrane lipid composition is known to influence cellular response to chemotherapy and the activity of membrane-bound enzymes, which in turn has been shown to impact uptake and sensitivity to anticancer agents [43–46]. These factors may underlie the physiochemical response of MDA-MB-231/C8cer cells to rhodamine, paclitaxel, and doxorubicin. However, it is important to note that the small increase in resistance observed is more in line with what is seen clinically, as many of the established multidrug resistant cancer cell lines, MCF-7-AdrR for example, demonstrate extreme drug resistance and have high P-gp levels that are not clinically relevant [47]. For example, cells selected in culture overexpress P-gp as much as 100-fold, whereas human cancers overexpress P-gp 2–5-fold [48], which is adequate to confer resistance [49,50].
In this study, some cell lines responded to short-chain GC supplements whereas other cell lines responded to short-chain ceramides (C8 and C6). These disparate responses may be linked to distinct physicochemical behavior of the analogs as well as distinct neighborhood topology. It is possible that C8-GC is poorly active in T47D and MDA-MB-435 cells because the GC must be synthesized in situ to be active in MDR1 modulation. GC is synthesized on the cytoplasmic surface of early Golgi and converted to LacCer, in the lumen of the Golgi presumably after being “flipped in” [51,52]. Along these lines, Tepper et al. [53] have noted specific differences in cellular ceramide metabolism related to topological segregation, in particular with GCS access. It is possible that ceramide, because of the proximity of the enzymatic machinery, is physiologically favored, after conversion to GC, as a substrate of MDR1 modulation.
The metabolic studies using [14C]C6-ceramide were conducted using concentrations that mimicked the MDR1 induction experiments (see Fig. 1). This was done to challenge the cells and determine how GCS, would metabolize high concentrations of ceramide. All cell lines readily converted ceramide to GC (see Fig. 7); SPM and LacCer were generated, but to a lesser extent. It is well known that P-gp can serve as a flipase for glycol-sphingolipids including GC [54]. Other ABC transporters also have broad specificity for lipid translocation [15,55,56]. Hence, supplying the cell with an abundance of substrate, in this case GC, could induce enzyme upregulation, in this case, P-gp. Our experiments show that introduction of a GCS inhibitor to ceramide-supplemented cells eliminated MDR1 increases (see Fig. 8), suggesting that GC influences MDR1 expression. However, at this point it is not possible to determine which lipid is responsible for MDR1 upregulation. The association of GCS with MDR1 was recently demonstrated by experiments showing that inhibition of GCS severely limits the expression of MDR1 and its product, P-gp (12). It should be noted, however, that increased MDR1 expression is not always accompanied by enhanced conversion of ceramide to GC, as gleaned from Fig. 7, right panel. Thus, complex mechanisms likely account for MDR1 upregulation.
In similar studies on the metabolism of sphingolipid analogs, Radin, Shayman, and colleagues [57] showed, in Madin–Darby canine kidney cells, that supplementation with C8-GC and C8-ceramide produced increases in natural GC, ceramide, and free sphingosine, and that radiolabeled C8-ceramide yielded radiolabeled GC and sphingomyelin; hydrolysis of lipids was also noted. Ogretmen et al. [58] have shown that short-chain ceramide can be enzymatically cleaved and the backbone reacylated to form long-chain ceramide counterpart. We do not know whether these ceramides participate in the MDR1 response; however, results in Table 1 suggest little cleavage of C8-ceramide occurred.
The role of lipids in multidrug resistance has been the subject of a recent review [59], and although lipids can impact drug transport and membrane fluidity, and perhaps P-gp’s integrity and stability in the plasma membrane, little is known about the influence of lipids on MDR1 gene expression. The relevance of sphingolipids to transcriptional regulation of ABC drug transporters [60,61] could be major, and with ceramide and metabolites, the intracellular signaling pathways that participate, including protein kinase C [62], are vast. Perhaps ceramide’s role as a messenger of cytotoxic response to chemotherapy is linked to the multidrug resistance pathway. We hope these results inspire new studies in the area of lipids and multidrug resistance.
Acknowledgements
This work was supported by a National Institutes of Health PHS grant (GM77391); the AVON Foundation, The Susan G. Komen Breast Cancer Foundation (grant number BCTR0Y02450); Department of the Army DAMD04-1-0491 (Postdoctoral Traineeship in breast cancer research); Associates for Breast and Prostate Cancer Studies, Los Angeles, and the Fashion Footwear Association of New York Charitable Foundation (Shoes on Sale/QVC).
The authors thank June Kawahara, Vicky Norton, Jessica Alvarez and Eric Morrison for assembling the manuscript, and Karen Hirsch, PhD, and Adam Blackstone of Creative Services for assembling the figures.
Abbreviations
- ABC
ATP-binding cassette protein
- CTP
cytidine triphosphate
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- GC
glucosylceramide
- GCS
glucosylceramide synthase
- IC50
concentration of agent that inhibits 50% of cell growth
- MDR1 gene
multidrug resistance gene 1
- P-gp
P-glycoprotein
- ethylenedioxy-P4
ethylenedioxy-1-phenyl-2-hexadecanoy-lamino-3-pyrrolidino-1-propanol
- qPCR
quantitative real-time RT-PCR
- RT-PCR
reverse transcriptase polymerase chain reaction
- TLC
thin-layer chromatography
- UDP
uridine diphosphate
References
- 1.Gottesman MM. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem. Biophys. Res. Commun. 1986;141:956–962. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 3.Peel SA. The ABC transporter genes of Plasmodium falciparum and drug resistance. Drug Resist. Updat. 2001;4:66–74. doi: 10.1054/drup.2001.0183. [DOI] [PubMed] [Google Scholar]
- 4.Ouellette M, Legare D, Haimeur A, Grondin K, Roy G, Brochu C, Papadopoulou B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist. Updat. 1998;1:43–48. doi: 10.1016/s1368-7646(98)80213-6. [DOI] [PubMed] [Google Scholar]
- 5.Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol. Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 6.Sietsma H, Veldman RJ, Kok JW. The involvement of sphingolipids in multidrug resistance. J. Membr. Biol. 2001;181:153–162. doi: 10.1007/s00232-001-0033-1. [DOI] [PubMed] [Google Scholar]
- 7.Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism—a strategy for overcoming drug resistance. J. Natl. Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 8.Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J. Biol. Chem. 1996;271:19530–19536. doi: 10.1074/jbc.271.32.19530. [DOI] [PubMed] [Google Scholar]
- 9.Sietsma H, Dijkhuis AJ, Kamps W, Kok JW. Sphingolipids in neuroblastoma: their role in drug resistance mechanisms. Neurochem. Res. 2002;27:665–674. doi: 10.1023/a:1020228117739. [DOI] [PubMed] [Google Scholar]
- 10.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim. Biophys. Acta. 2002;1585:172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 12.Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 13.Chan SY, Hilchie AL, Brown MG, Anderson R, Hoskin DW. Apoptosis induced by intracellular ceramide accumulation in MDA-MB-435 breast carcinoma cells is dependent on the generation of reactive oxygen species. Exp. Mol. Pathol. 2007;82:1–11. doi: 10.1016/j.yexmp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hu XF, Slater A, Rischin D, Kantharidis P, Parkin JD, Zalcberg J. Induction of MDR1 gene expression by anthracycline analogues in a human drug resistant leukaemia cell line. Br. J. Cancer. 1999;79:831–837. doi: 10.1038/sj.bjc.6990133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 16.Kok JW, Sietsma H. Sphingolipid metabolism enzymes as targets for anticancer therapy. Curr. Drug Targets. 2004;5:375–382. doi: 10.2174/1389450043345452. [DOI] [PubMed] [Google Scholar]
- 17.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev., Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 18.Bielawska A, Hannun YA. Merrill AH, Hannun YA. Methods in Enzymology: Sphingolipid metabolism and cell signaling, Parts A and B. Academic Press; San Diego: 2000. Radiolabeled sphingolipids, part II. Preparation of radiolabeled ceramides and phosphosphingolipids; pp. 499–518. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J. Biol. Chem. 1999;274:14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 20.Snyder F, Kimble H. An automatic zonal scraper and sample collector for radioassay of thin-layer chromatograms. Anal. Biochem. 1965;11:510–518. doi: 10.1016/0003-2697(65)90069-2. [DOI] [PubMed] [Google Scholar]
- 21.Snyder F. Thin-layer chromatographic behavior of glycerolipid analogs containing ether, ester, hydroxyl, and ketone groupings. J. Chromatogr. 1973;82:7–14. doi: 10.1016/s0021-9673(01)80070-4. [DOI] [PubMed] [Google Scholar]
- 22.Kok JW, Veldman RJ, Klappe K, Koning H, Filipeanu CM, Muller M. Differential expression of sphingolipids in MRP1 overexpressing HT29 cells. Int. J. Cancer. 2000;87:172–178. doi: 10.1002/1097-0215(20000715)87:2<172::aid-ijc3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levade T, Manfait M. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int. J. Cancer. 2001;94:157–165. doi: 10.1002/ijc.1449. [DOI] [PubMed] [Google Scholar]
- 24.Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 25.Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- 26.Morton DL, Ravindranath MH, Irie RF. Tumor gangliosides as targets for active specific immunotherapy of melanoma in man. Prog. Brain Res. 1994;101:251–275. doi: 10.1016/s0079-6123(08)61954-8. [DOI] [PubMed] [Google Scholar]
- 27.Takami Y, Abe A, Matsuda T, Shayman JA, Radin NS, Walter RJ. Effect of an inhibitor of glucosylceramide synthesis on cultured human keratinocytes. J. Dermatol. 1998;25:73–77. doi: 10.1111/j.1346-8138.1998.tb02353.x. [DOI] [PubMed] [Google Scholar]
- 28.Thurin J, Thurin M, Herlyn M, Elder DE, Steplewski Z, Clark WH, Jr, Koprowski H. GD2 ganglioside biosynthesis is a distinct biochemical event in human melanoma tumor progression. FEBS Lett. 1986;208:17–22. doi: 10.1016/0014-5793(86)81522-8. [DOI] [PubMed] [Google Scholar]
- 29.Uchida Y, Murata S, Schmuth M, Behne MJ, Lee JD, Ichikawa S, Elias PM, Hirabayashi Y, Holleran WM. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J. Lipid Res. 2002;43:1293–1302. [PubMed] [Google Scholar]
- 30.Hummel I, Klappe K, Kok JW. Up-regulation of lactosylceramide synthase in MDR1 overexpressing human liver tumour cells. FEBS Lett. 2005;579:3381–3384. doi: 10.1016/j.febslet.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary PM, Roninson IB. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J. Natl. Cancer Inst. 1993;85:632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- 32.Kohno K, Sato S, Takano H, Matsuo K, Kuwano M. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem. Biophys. Res. Commun. 1989;165:1415–1421. doi: 10.1016/0006-291x(89)92761-7. [DOI] [PubMed] [Google Scholar]
- 33.Hu Z, Jin S, Scotto KW. Transcriptional activation of the MDR1 gene by UV irradiation. Role of NF-Y and Sp1. J. Biol. Chem. 2000;275:2979–2985. doi: 10.1074/jbc.275.4.2979. [DOI] [PubMed] [Google Scholar]
- 34.Vilaboa NE, Galan A, Troyano A, de Blas E, Aller P. Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1) J. Biol. Chem. 2000;275:24970–24976. doi: 10.1074/jbc.M909136199. [DOI] [PubMed] [Google Scholar]
- 35.Tchenio T, Havard M, Martinez LA, Dautry F. Heat shock-independent induction of multidrug resistance by heat shock factor 1. Mol. Cell. Biol. 2006;26:580–591. doi: 10.1128/MCB.26.2.580-591.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates SE, Mickley LA, Chen YN, Richert N, Rudick J, Biedler JL, Fojo AT. Expression of a drug resistance gene in human neuroblastoma cell lines: modulation by retinoic acid-induced differentiation. Mol. Cell. Biol. 1989;9:4337–4344. doi: 10.1128/mcb.9.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtal KA, de Vries E, Hoekstra D, van Ijzendoorn SC. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol. Biol. Cell. 2006;17:3638–3650. doi: 10.1091/mbc.E06-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molinari A, Calcabrini A, Meschini S, Stringaro A, Del Bufalo D, Cianfriglia M, Arancia G. Detection of P-glycoprotein in the Golgi apparatus of drug-untreated human melanoma cells. Int. J. Cancer. 1998;75:885–893. doi: 10.1002/(sici)1097-0215(19980316)75:6<885::aid-ijc11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Maraldi NM, Zini N, Santi S, Scotlandi K, Serra M, Baldini N. P-glycoprotein subcellular localization and cell morphotype in MDR1 gene-transfected human osteosarcoma cells. Biol. Cell. 1999;91:17–28. [PubMed] [Google Scholar]
- 40.Molinari A, Calcabrini A, Meschini S, Stringaro A, Crateri P, Toccacieli L, Marra M, Colone M, Cianfriglia M, Arancia G. Subcellular detection and localization of the drug transporter P-glycoprotein in cultured tumor cells. Curr. Protein Pept. Sci. 2002;3:653–670. doi: 10.2174/1389203023380413. [DOI] [PubMed] [Google Scholar]
- 41.Calcabrini A, Meschini S, Stringaro A, Cianfriglia M, Arancia G, Molinari A. Detection of P-glycoprotein in the nuclear envelope of multidrug resistant cells. Histochem. J. 2000;32:599–606. doi: 10.1023/a:1026732405381. [DOI] [PubMed] [Google Scholar]
- 42.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies CL, Loizidou M, Cooper AJ, Taylor I. Effect of gamma-linolenic acid on cellular uptake of structurally related anthracyclines in human drug sensitive and multidrug resistant bladder and breast cancer cell lines. Eur. J. Cancer. 1999;35:1534–1540. doi: 10.1016/s0959-8049(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 44.Escriba PV, Ferrer-Montiel AV, Ferragut JA, Gonzalez-Ros JM. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990;29:7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- 45.Holleran WM, DeGregorio MW, Ganapathi R, Wilbur JR, Macher BA. Characterization of cellular lipids in doxorubicin-sensitive and-resistant P388 mouse leukemia cells. Cancer Chemother. Pharmacol. 1986;17:11–15. doi: 10.1007/BF00299859. [DOI] [PubMed] [Google Scholar]
- 46.Das UN, Madhavi N, Sravan Kumar G, Padma M, Sangeetha P. Can tumour cell drug resistance be reversed by essential fatty acids and their metabolites? Prostaglandins Leukot. Essent. Fat. Acids. 1998;58:39–54. doi: 10.1016/s0952-3278(98)90128-4. [DOI] [PubMed] [Google Scholar]
- 47.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker RJ, Fruehauf JP. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin. Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 48.Maitra R, Halpin PA, Karlson KH, Page RL, Paik DY, Leavitt MO, Moyer BD, Stanton BA, Hamilton JW. Differential effects of mitomycin C and doxorubicin on P-glycoprotein expression. Biochem. J. 2001;355:617–624. doi: 10.1042/bj3550617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biedler JL. Drug resistance: genotype versus phenotype-thirty-second G. H. A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:666–678. [PubMed] [Google Scholar]
- 50.Kerbel RS, Rak J, Kobayashi H, Man MS, St Croix B, Graham CH. Multicellular resistance: a new paradigm to explain aspects of acquired drug resistance of solid tumors. Cold Spring Harbor Symp. Quant. Biol. 1994;59:661–672. doi: 10.1101/sqb.1994.059.01.076. [DOI] [PubMed] [Google Scholar]
- 51.Lannert H, Bunning C, Jeckel D, Wieland FT. Lactosylceramide is synthesized in the lumen of the Golgi apparatus. FEBS Lett. 1994;342:91–96. doi: 10.1016/0014-5793(94)80591-1. [DOI] [PubMed] [Google Scholar]
- 52.Lannert H, Gorgas K, Meissner I, Wieland FT, Jeckel D. Functional organization of the Golgi apparatus in glycosphingolipid biosynthesis. Lactosylceramide and subsequent glycosphingolipids are formed in the lumen of the late Golgi. J. Biol. Chem. 1998;273:2939–2946. doi: 10.1074/jbc.273.5.2939. [DOI] [PubMed] [Google Scholar]
- 53.Tepper AD, Diks SH, van Blitterswijk WJ, Borst J. Glucosylceramide synthase does not attenuate the ceramide pool accumulating during apoptosis induced by CD95 or anti-cancer regimens. J. Biol. Chem. 2000;275:34810–34817. doi: 10.1074/jbc.M005142200. [DOI] [PubMed] [Google Scholar]
- 54.Eckford PD, Sharom FJ. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem. J. 2005;389:517–526. doi: 10.1042/BJ20050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raggers RJ, van Helvoort A, Evers R, van Meer G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J. Cell Sci. 1999;112(Pt 3):415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 56.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim. Biophys. Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 57.Abe A, Wu D, Shayman JA, Radin NS. Metabolic effects of short-chain ceramide and glucosylceramide on sphingolipids and protein kinase C. Eur. J. Biochem. 1992;210:765–773. doi: 10.1111/j.1432-1033.1992.tb17479.x. [DOI] [PubMed] [Google Scholar]
- 58.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 59.Argiles JM, Costelli P, Carbo N, Pallares-Trujillo J, Lopez-Soriano FJ. Tumour growth and nitrogen metabolism in the host (Review) Int. J. Oncol. 1999;14:479–486. [PubMed] [Google Scholar]
- 60.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 61.Yague E, Armesilla AL, Harrison G, Elliott J, Sardini A, Higgins CF, Raguz S. P-glycoprotein (MDR1) expression in leukemic cells is regulated at two distinct steps, mRNA stabilization and translational initiation. J. Biol. Chem. 2003;278:10344–10352. doi: 10.1074/jbc.M211093200. [DOI] [PubMed] [Google Scholar]
- 62.Uchiumi T, Kohno K, Tanimura H, Hidaka K, Asakuno K, Abe H, Uchida Y, Kuwano M. Involvement of protein kinase in environmental stress-induced activation of human multidrug resistance 1 (MDR1) gene promoter. FEBS Lett. 1993;326:11–16. doi: 10.1016/0014-5793(93)81750-t. [DOI] [PubMed] [Google Scholar]