Abstract
Selective attention experimental designs have shown that neural responses to stimuli in primary somatosensory cortex are stronger when the sensory stimuli are task relevant. Other studies have used animals under no task demands for data collection. The relationship between neural responses in the brain during behavior, and while an animal has no task demands, remains underexplored. We trained two animals to perform somatosensory detection for several weeks, followed by somatosensory discrimination for several weeks. Data in response to physically identical stimuli were collected from cortical implants while the animal was under no task demands before each behavioral session and also during that behavioral session. The Fourier spectra of the field potentials during detection or discrimination compared with the no task condition demonstrated suppression of the somatosensory μ-rhythm that is associated with readiness and anticipation of cognitive use of somatosensory and motor inputs. Responses to the task target were stronger during detection and discrimination than in the no task condition. The amplitude normalized time course of the target evoked response was similar in both cases. Evoked responses to the task distractor were not significantly stronger during behavior than in recordings under no task demands. The normalized time course of the distractor responses showed a suppression that peaks 30–35 ms after the onset of the response. The selectivity of this within trial suppression is the same as the selectivity of enduring suppression evident in studies of sensory cortical plasticity, which suggests the same neural process may be responsible for both.
Keywords: somatosensory cortex, learning-induced plasticity, attention and arousal, awake monkey
learning-induced change in the neural representations in sensory cortex can be driven by associations with reinforcement. If an animal makes a positive association between a stimulus and a reward, then the neural response to that stimulus increases. If an animal associates a sensory stimulus with omission of reward, e.g., a task distractor, then the neural response to that sensory stimulus weakens. These changes have been observed many times in the somatosensory system (Jenkins et al. 1990; Recanzone et al. 1992a,b; Xerri et al. 1994, 1999; Wang et al. 1995; Blake et al. 2005; Spingath et al. 2011), in the auditory system (Recanzone et al. 1993; Beitel et al. 2003; Polley et al. 2004, 2006; Weinberger 2004; Blake et al. 2002, 2006), and in the visual system (Schoups et al. 2001; Ghose et al. 2002; Yang and Maunsell 2004; Raiguel et al. 2006), but the changes in neural activity that occur each day between a period before engaging in behavior, and during that behavior, are less well documented. These within-day changes plausibly contain clues for the mechanisms that guide learning-dependent cortical plasticity in primary sensory cortex. In the current study, our animal model was changed from the owl monkey (Blake et al. 2005) to the macaque monkey to achieve a greater number of rewarded trials per day. The larger number of behavioral trials reduced the variability in measuring physiological responses during the behavior, which increased the statistical power in comparisons between the physiological responses before, and during, the behavioral session.
This experimental approach enables sampling of cortical responses before behavioral sessions under no task demands and during behavioral sessions while attention is required for task performance. In a prior study, flutter responses to 17 SI neurons in one monkey under no task demands were compared with 25 SI neurons in a different, behaving, animal, and it was reported that arousal effects are minimal (Mountcastle et al. 1990). Other work in 53 neurons in SI cortex found enhancement of responses when task irrelevant conditions were compared with somatosensory attending conditions (Chapman and Meftah 2005). In work in pattern and texture discrimination, it was reported that 50% (Hsiao et al. 1993; Burton and Sinclair 2000) and 23% (Meftah et al. 2002) of the neurons in SI cortex are impacted by directing attention to somatosensory cortex compared with directing attention to a different brain region. Our work seeks to associate the differences between neural responses in the “No Task” condition animal and the behaving animal to the longer term changes in brain responses called neuroplasticity.
The anticipation of, or input of, somatosensory inputs has been related to decreases in EEG energy in specific frequency bands localized to the somatosensory strip or desynchronization of the somatosensory μ-rhythm. The μ-rhythm has bands close to 10 Hz, close to 20 Hz, and possibly at 30 Hz (Pfurtscheller et al. 2006). Some studies have reported that low or higher frequency μ-rhythm in near threshold detection behavior can predict single trial outcomes (Jones et al. 2007; Kulics 1982) and even made the observation that prestimulus μ can predict subsequent detection (Zhang and Ding 2010). In the present study, the Fourier spectra of the prestimulus field potentials is compared between the No Task condition and the detection or discrimination condition.
METHODS
Ethics statement.
Animal welfare was regulated by the Institutional Animal Care and Use Committee at the Medical College of Georgia under Animal Use Protocol nos. 05-12-753 and 08-11-128. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Behavioral training was accomplished via food reinforcement without altering the weekly average of the daily intake of food. Surgeries were performed using aseptic technique in approved surgical suites, and anesthesia and analgesia were carried out under the direct supervision of the clinical veterinarian. Animals were provided with environmental enrichment designed by the clinical veterinarian.
Experimental methods, behavior, and analyses used here were the same as previously published (Spingath et al. 2011), where they are described in detail. Briefly, an implant holding 64 microelectrodes was implanted into SI. Two adult, male Rhesus macaques were used in these experiments. Electrode depths were optimized in the 6 wk after implantation surgery to maximize the usable recording yield in the following months. Optimization entailed raising and lowering electrodes to find more suitable receptive fields or to find an audibly stronger evoked spiking field. After optimization, the electrodes were left stationary throughout all experiments to record from a consistent population of neurons. Electrodes were never raised or advanced on a daily basis to isolate single units. The reported single units (for example, see Fig. 3) consisted of recordings with high signal-to-noise ratios. The single unit recordings were not common enough to conduct this class of study on single units alone. As in all implant studies, many electrodes never yielded viable data. Each electrode on each experimental day was counted as one recording for the purposes of this study, as long as it had a response to the target or distractor tap. A paragraph in the discussion analyzes the impacts of this form of sampling on the conclusions drawn. The implant surgery has been previously described in detail (deCharms et al. 1999; Spingath et al. 2011).
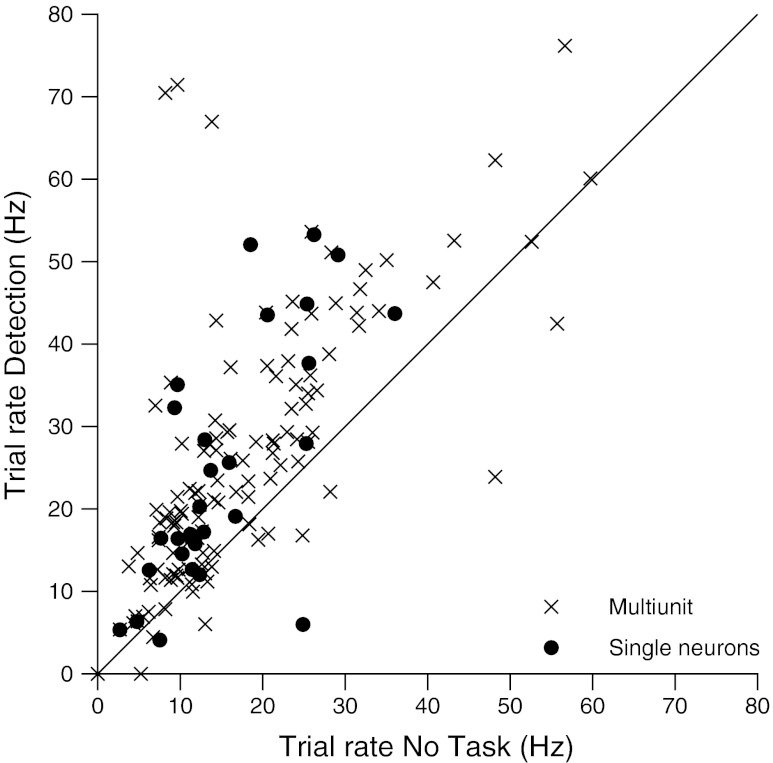
Fig. 3.
Mean trial rate No Task condition vs detection. For each data point, the mean trial rate during No Task condition animal data collection is the x coordinate, and the mean rate during detection performance is the y coordinate. Single units are plotted with filled circles. The 96 multiunit and 28 single unit recordings are plotted.
Spike detection thresholds were manually set on a daily basis for multiunit data such that spontaneous rates were roughly 10–20 Hz, which generally meant thresholds were close to 3.75 noise standard deviations. Electrodes were only used for recording if they had clear receptive fields in manual mapping. Each electrode was checked daily for responses and receptive field maps.
Somatosensory stimuli were delivered via custom-built tactile motors under linear variable displacement transformer displacement-feedback control. The stimulus contact was made with a 1-mm cylindrical rod with a rounded spherical end. Neural responses to motorized taps were collected before, during, and after each day's behavior. The responses before each day's behavior are compared with the responses during the behavior in the present study. Recording sessions before the behavioral session, or the No Task condition, consisted of delivering a series of taps to each skin site while the monkey was passively and quietly seated. During the behavioral recordings, neural responses to the behaviorally relevant taps were recorded. Receptive fields were manually mapped before and after each day's behavior. A thin, rounded glass probe was used to define receptive fields. Images of the receptive fields were drawn and stored using Reconstruct software (Synapse Web, Austin, TX).
Stimulus presentation.
Tap stimuli were presented to the animal's digits in two basic contexts: to collect spiking data quantitatively outside of the rewarded behavioral context, and to present behaviorally relevant stimuli during the behavior. Throughout the study, these cognitive states are referred to as the No Task condition, detection, and discrimination. The animal's hand and fingers were immobilized with a cast mold to ensure that the stimuli were presented and received in a consistent manner. The motorized tip was always lowered until barely touching the skin and then indented 500 μm into the skin before delivery of any taps.
In the No Task recording sessions, 50 taps were each presented at tap displacements of 100, 200, and 400 μm. Data from the 200-μm taps were used in the No Task analyses, as these taps were physically identical to the taps that were used in the operant behavior. Each tap had the shape of one period of a 40-Hz sinusoid, with zero first derivative at its start, end, and midpoint. Data were collected daily by presenting the series of taps to each site on a grid of sites. To maintain spatial consistency throughout the experiments, the grid was marked on the digits in permanent ink and refreshed periodically. In the present study, only tap responses at the grid locations that were used as the task target, or task distractor, were used.
During the behavior, the target or distractor tap was a single 200-μm tap. Displacements were continuously monitored via the linear variable displacement transformer sensor displayed on an oscilloscope. Human discriminative thresholds for longer 40-Hz stimulation are <20 μm (Talbot et al. 1968), and the stimuli were perceived as clearly discernable to experimenters.
Animal behavior.
Data were collected from animals under passive restraint, a condition referred to as the No Task condition. Animals sat quietly through this condition. On the infrequent trials in which animals did not sit quietly through a data collection period, it was repeated. These sessions preceded the daily operant training session.
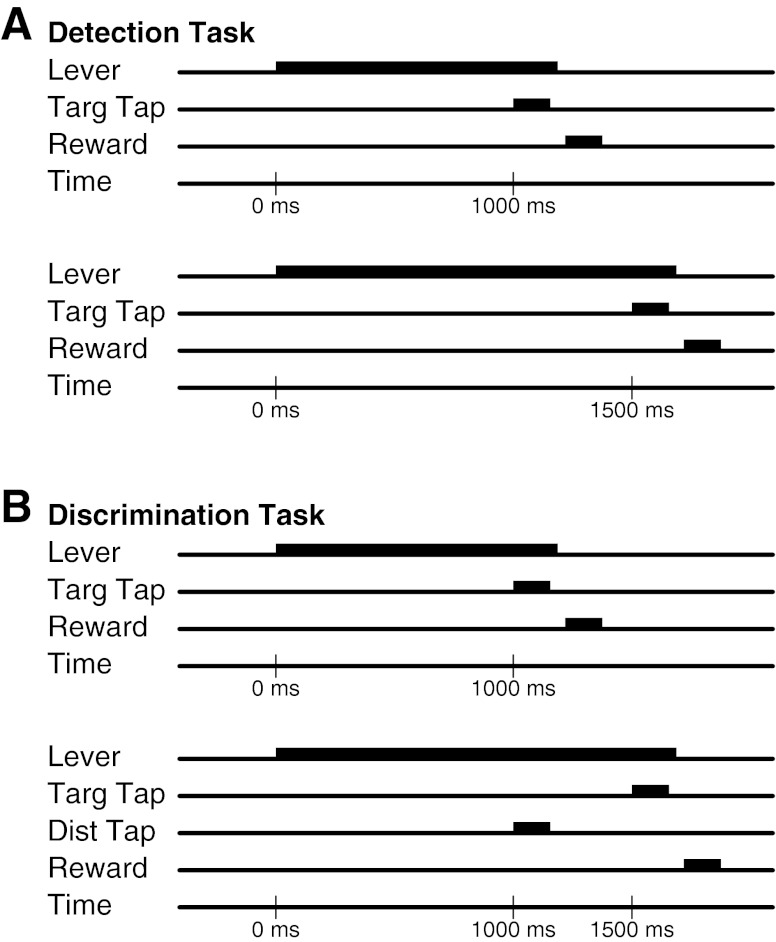
Before beginning experimental behavioral tasks, animals were pretrained to perform a lever holding task constructed to mimic the operant component of the detection and discrimination tasks. This timed task consisted of holding a lever down for at least 1,000 ms, releasing the lever, and then receiving a food reward triggered by the lever release. A 1,000-ms intertrial interval prevented the animal from beginning the next trial immediately after completion of the previous trial. During the lever holding task, no taps were presented to the animal's digits in conjunction with any part of the behavior.
The detection task consisted of presenting a target tap at a predetermined target site to which the animal could respond by releasing the lever for a food reward. The target tap was randomly presented at one of two time points, 1,000 or 1,500 ms. Randomized timing of the target tap presentation prevented the animal from being able to perform the task solely by timing the behavioral response. The animal learned to detect the target tap by trial and error. Throughout this task, a second motor tip was present at a 500-μm indentation at the future distractor site. This second motor never presented any tap stimuli during the detection task.
Hits were categorized as lever release within 500 ms after target tap presentation. False alarms were categorized as lever release between 1,000 and 1,500 ms when the target would have been presented at 1,500 ms. Misses were categorized as lever release that was later than 500 ms after target tap presentation. Misses and false alarms were followed by a brief timeout. Early errors were categorized as lever release before 1,000 ms. Early errors were not used to calculate d'. The trials in which the target was presented at the later time were used only to classify false alarms, because once the target was not presented at the earlier time, the rest of the trial was not random.
The discrimination task only differed from the detection task in the addition of a distractor tap before presentation of the target tap. The two trial types, randomly interleaved, were a target tap presentation at 1,000 ms or a distractor tap presentation at 1,000 ms followed by the target tap at 1,500 ms. The animal only needed to continue responding to the target tap and ignore any distractor taps to receive a reward. Distinguishing the target tap from the distractor tap was learned by trial and error. The hits, misses, and early errors are the same as in the detection task. False alarms were defined as lever release within 500 ms after presentation of the distractor tap. Misses and false alarms were always followed by a brief 800-ms timeout, during which the animal would be unable to initiate another trial. Hits on trials on which a distractor tap was delivered were not counted as hits for the calculation of hit rate, because once the target is not delivered at the earlier time, the rest of the trial is deterministic. Second window hits were used in the calculation of false alarm rate.
An experimental run consisted of a series of three behaviors: lever holding, detection, and discrimination. Each was run in series, and in all but one case, at least 2 wk of data were collected for each one. Each experimental run took a minimum of 6 wk in one animal. Target and distractor sites were determined at the beginning of each experimental run and remained the same until the end of the run. When beginning a new experimental run, a different set of target and distractor sites were independently chosen. Each target and distractor tap caused a tap response in at least one implanted electrode before the behavioral series. These skin sites could be on any digit, regardless of whether a digit had a target or distractor site on it in previous runs or in the run to be executed. The present study includes data from one experimental run in animal 1 and three experimental runs in animal 2. Accordingly, four target locations were used, and four distractor locations were used. Data were collected for 2 wk (8–10 behavioral sessions) under all conditions except for the discrimination condition in animal 1, which consisted of four behavioral sessions. Each experimental run averaged >800 rewarded trials, each of which included a presentation of the task target. Half of the trials in discrimination included a presentation of the task distractor.
The d' was calculated using hit rate for trials in which the target tap was presented at 1,000 ms, and the false alarm rate in which the target was presented at 1,500 ms. The d' is calculable as the difference in the cumulative normal distribution corresponding to these two probabilities, which was calculated with the norminv function in Matlab (Mathworks, Natick, MA).
Data analysis.
In all population plots, multiunit tap responses were plotted in response to the target tap for all recorded electrodes on all days, and only electrodes that had significant responses to the target tap on at least one day were used to construct the population analysis. Similarly, only electrodes that had significant responses to the distractor tap on at least 1 day were included in the distractor population plots. Such electrodes were used on all days if possible. On some occasions, spike detection thresholds on a channel were no longer usable because rates increased dramatically. In that case, all data from that electrode on that day were discarded. Otherwise, all electrode recordings were used on all days, and recordings were always paired between the prebehavioral No Task condition and the detection or discrimination condition.
Fourier power spectral analysis.
Local field potential data were collected at 1 kHz in experiments from the same electrodes that were used for action potential based recordings but with different filters on the data. All Fourier analysis used 256 data points in a discrete Fourier transform (DFT) instantiated by the FFTW library (www.fftw.org). Before the Fourier transform was calculated, the 256 data points had the mean subtracted off, and the data were windowed by a Hamming window that softens the ends of the data sequence to prevent ringing in the spectral transform. The DFT was calculated and multiplied by its complex conjugate to give the power spectrum. Units for the power spectrum are mV2. Data are plotted in 4-Hz increments representing the rounding of the DFT to the nearest 4 Hz. Data were cleaned by plotting the distribution of the means of each sample from 4 to 40 Hz. A tight unimodal distribution existed for units under 4,000 mV2, and there were scattered noise artifacts at sometimes much larger amplitudes. These trials were discarded from analysis in all cases equivalently.
RESULTS
Rhesus monkeys were implanted in SI cortex with microelectrode arrays and responses to the glabrous surfaces of digits were isolated. Recordings were taken before and during behavior each day while monkeys serially learned somatosensory detection and spatial discrimination tasks.
Detection task.
Behavioral learning and cross-condition plasticity effects are the topic of a prior manuscript (Spingath et al. 2011). Briefly, monkeys learned a tactile detection task, and then learned a tactile discrimination task which are shown in Fig. 1. Prior work presented the psychophysical learning results and compared them to responses to the stimuli used as the task target and task distractor when those stimuli were presented to the animal in the No Task condition before each behavioral session. The current work details the differences among the No Task, prebehavioral neural activity, and the activity during the detection and discrimination sessions.
Fig. 1.
Behavioral tasks. A: detection. Animals pressed a lever to initiate trials. Lever hold was released after a target tap was delivered at either of two potential target times. B: discrimination. Animal again holds the lever until the target is presented. On half the trials, a distractor tap is delivered.
Monkeys learned to detect the suprathreshold taps with a large stepwise improvement in behavioral performance metrics as detailed in prior work (Spingath et al. 2011). Of the 24 detection sessions detailed here, all had d' >1, and all but three had d' >2, with an average of 3.1. Responses to target stimuli were collected in half hour long No Task recording sessions before each day's detection session, and also while the animal attempted to detect the target. Figure 2 shows the averaged responses to the stimuli under these two conditions. Each peristimulus time histogram (PSTH) compares responses collected from the same electrodes to the same stimulus on the same day. Data were used from every electrode that showed a response to the target tap in any behavioral session and are paired across the two PSTHs, which means that every recording that contributes to the No Task PSTH average is paired with a recording taken the same day during the behavioral session. One of the four behavioral datasets from prior work (Spingath et al. 2011) was not includable because data during the behavioral session lacked synchronizing pulses. The data presented here are a subset of those data for that reason.
Fig. 2.
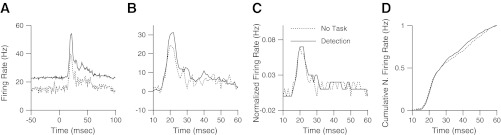
A: population responses to the target tap. Each peristimulus time histogram shows the population average neural response to a 200 micron tap delivered at time zero. Responses recorded from the No Task condition monkey are plotted with the dotted line, and responses during detection performance are plotted with the solid line. All data are paired, and one recording from a location during the No Task condition is paired with a recording from the same location during behavior. Data are averaged over all pairs that had a response to the target. B: normalized responses to the target tap. Same data are replotted, and the average prestimulus rate is subtracted. C: data are divided by the sum of all rates between 10 and 60 ms after tap onset to compare the shape of the responses. D: cumulative sum of the normalized responses are shown. A group of 124 paired recordings was used and averaged in all plots.
The responses during detection performance were significantly stronger than those recorded from the animal in the No Task condition, as shown in the population average target responses in Fig. 2A (P < 0.00001, paired t-test). A second plot of the same data normalizes the recordings by subtracting the pretap baseline. The evoked responses from 10 to 60 ms after tap onset, shown in Fig. 2B, are significantly stronger during the behavior than in No Task recordings, with an average ratio between the two conditions of 1.26 (P < 0.00001, paired t-test). The responses from 10 to 60 ms after tap onset were normalized so that the sum of all activity in that time period was 1. This normalization was performed to compare the time course of the response without regard to the background rate or evoked rate, as shown in Fig. 2C. A cumulative sum of this plot is shown in Fig. 2D, and no significant differences between these two datasets in the time course of the response were found [P > 0.10, Kolmogorov-Smirnoff (KS) test].
The No Task condition responses were collected in a series of 50 taps at an interval of 300 ms, while the responses during detection typically had several seconds between target taps. To test whether adaptation to the stimulus could have caused the shift between the detection responses and the responses in the No Task condition, the number of action potentials per tap trial was regressed against the trial number during data collection from the No Task condition animal. However, no significant effects were found (correlation between tap number and spikes per trial, r = 0.027, t = 0.003, P > 0.4).
The responses from the individual recordings are plotted during the detection behavior compared with the No Task condition recordings in Fig. 3. A total of 124 recordings in the No Task condition are compared with recordings from the same locations roughly 1 h later while the animal performed detection. The No Task condition data collection occurred back to back with the detection session, and the No Task data were collected over 30 min, while the detection session required 1–2 h. The mean trial rates are compared, and in 107 of the 124 cases, the mean trial rate is larger for the recordings during the detection behavior (P < 0.0000001, sign test). A subset of the data had action potential waveforms with higher signal to noise ratios. The population of 28 single units had significantly higher mean rates during the detection behavior compared with the No Task condition recordings (P < 0.001, paired t-test). All action potential data in this study are multiunit unless otherwise specified.
The change in responsiveness during detection behavior is compared with responsiveness in the animal under No Task demands. The monkeys are trained to sit quietly through the No Task condition data collection because food is withheld until the behavior begins, and data collection is repeated if the animals are not calm. However, there is no control of behavior. This method of data collection is common in the fields of somatosensory and auditory nonhuman primate electrophysiology (Lu et al. 2001; Barbour and Wang 2003; Spingath et al. 2011; Pei et al. 2011; Thakur et al. 2012). However, it is clearly not equivalent to a physiological setup in which the animal is attending to, and anticipating, the target stimulus. To gain insight into other changes that may accompany the move from the No Task condition animal to an animal performing detection or discrimination, local field potential spectral analysis was performed. The field potential near somatosensory cortex has a μ-rhythm that is desynchronized during active sensory or motor use in a topographic manner. Field potentials were collected from the same electrodes used to record action potential traces.
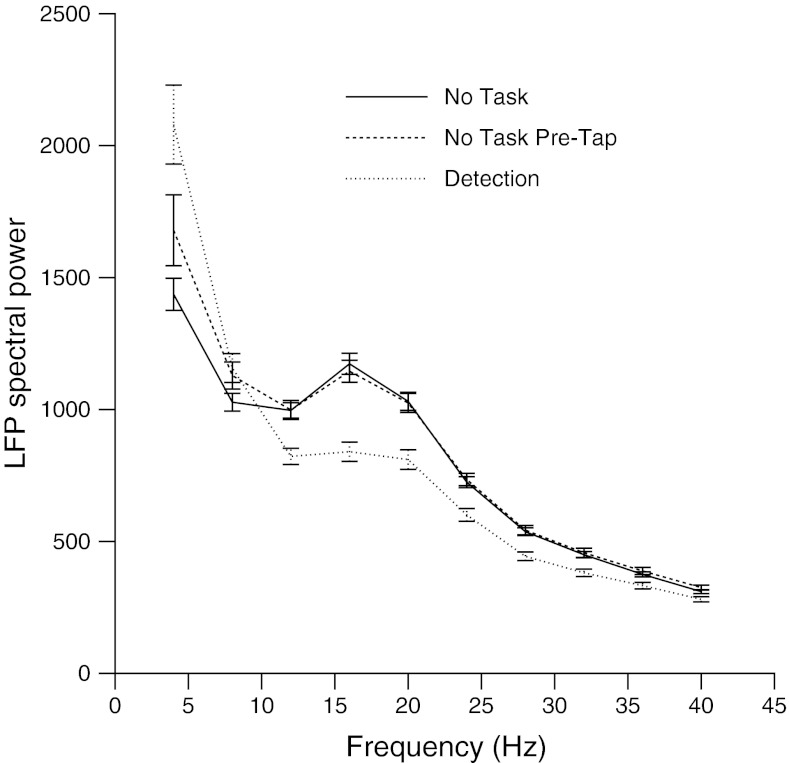
As shown in Fig. 4, substantial differences in spectral content are observed. An ANOVA was used to compare responses under the No Task condition to those during detectio, and found group differences with significant post hoc effects at 4 Hz favoring the detection group and from 12 to 28 Hz favoring the No Task condition animal. A concern exists that the tap stimuli, which are delivered every 300 ms to the No Task condition animal, would have an impact on the local field potential spectral energy collected in the 256 ms before each tap. To control for this, the local field potential spectral power was analyzed in the few seconds before any tap delivery to the No Task condition animal. The results, also shown in Fig. 4 as the No Task PreTap data, did not find any significant shift caused by the intermittent delivery of tap stimuli.
Fig. 4.
Spectral power during data collection. Spectral powers are compared during detection (dotted line), during the No Task condition (solid line), and immediately before the No Task condition data collection (No Task PreTap, dashed line). LFP, local field potential.
Discrimination task.
After 2 wk of detection task performance with d' averaging 3.1, animals were transitioned to the discrimination task. In this task, for half of the trials a tap is delivered to a distractor spatial location at the earliest possible target time relative to lever holding onset. The monkey is required to hold through the distractor and wait for the target, as shown in Fig. 1. As described in prior work (Spingath et al. 2011), d' on average drops from 4 on the last day of detection to 2 on the first day of discrimination performance and thereafter climbs back towards 3 as the animals more successfully withhold responses to the task distractor each day. Again, responses to taps were collected from the No Task condition animal before the day's discrimination session and also during the discrimination session. Recordings were paired from all electrodes that exhibited a significant neural response the target in any behavioral session to make the population plot in Fig. 5.
Fig. 5.
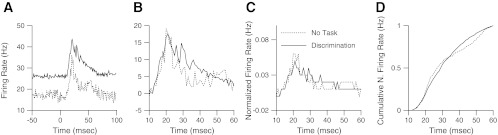
Target population average peristimulus time histogram during discrimination. A: population average firing rate in response to the task target was recorded during the No Task condition before each day's behavioral session and during that session. B: prestimulus firing rate was subtracted from each plot. C: evoked response 10 to 60 ms after tap onset was normalized to a sum of 1 and replotted to compare shape. D: cumulative sum of the normalized responses is plotted. A group of 168 paired recordings was used to make these plots.
Neural responses to the target tap were analyzed, and the statistical findings were replicated those found during detection. Population average responses during the behavior shown in Fig. 5A were stronger than responses from the No Task condition animal (P < 0.00001), and data were normalized by subtracting the pretap response rate to compare only the evoked responses from 10 to 60 ms after tap onset, as shown in Fig. 5B. Again, the evoked responses to the target during discrimination behavior were stronger than the No Task condition recorded responses (P < 0.00001), with a ratio between the two conditions of 1.33. The evoked responses were normalized to a cumulative sum of 1 and plotted against time in Fig. 5C and as a cumulative integral in Fig. 5D. Again, no significant difference in the time course of the responses were noted (P > 0.1, KS test).
The responses to the task distractor were similarly compared in Fig. 6. Significant differences in rate were again observed over the course of the tap response (P < 0.00001) as shown in Fig. 6A. The plots were normalized by subtracting the prestimulus rate and plotted in Fig. 6B. The differences in evoked response were not significant (P > 0.2), with a ratio between the discrimination and No Task condition responses of 1.15 favoring the responses during the behavior. The evoked responses were normalized to a cumulative sum of 1 and replotted in Fig. 6C to compare time course of response independently of differences in rate. The time courses were significantly different as shown in Fig. 6D (P < 0.01, KS test), and this statistical finding means there was a larger fraction of the evoked response in the discrimination condition before 45 ms after the tap onset, and there was a larger fraction of the evoked response in the No Task condition after 45 ms after the tap onset. If the analysis is carried back to the evoked responses in Fig. 6B, the discrimination condition response is 29% weaker than the No Task condition after 45 ms and 53% stronger than the No Task condition before 45 ms.
Fig. 6.

Distractor population peristimulus time histogram during discrimination. A: population average firing rate in response to the task distractor was recorded under the No Task condition before each day's behavioral session and during that session. B: prestimulus firing rate was subtracted from each plot. C: evoked response 10 to 60 ms after tap onset was normalized to a sum of 1 and replotted to compare shape. D: cumulative sum of the normalized responses is plotted. An arrow indicates the location of the maximal difference between distributions used in the Kolmogorov-Smirnov test. A group of 113 paired recordings was used to make these plots.
In the discrimination data, asserting that the target tap response was elevated and the distractor response was not does not imply a difference between those two groups, but a two tailed t-test comparing the differences between the group phasic responses from 10 to 60 ms after tap onset was significant (P < 0.03). To perform this t-test, the data were transformed. First, in each recording, the prestimulus mean rate was subtracted, and the average rate from 10 to 60 ms was calculated. In each pair of recordings, the difference between the No Task condition and the discrimination condition was then calculated. Then, the group of 168 paired recordings for the target responses was tested against the group of 113 paired recordings for the distractor responses in a two tailed t-test, with the null hypothesis that the means were not different.
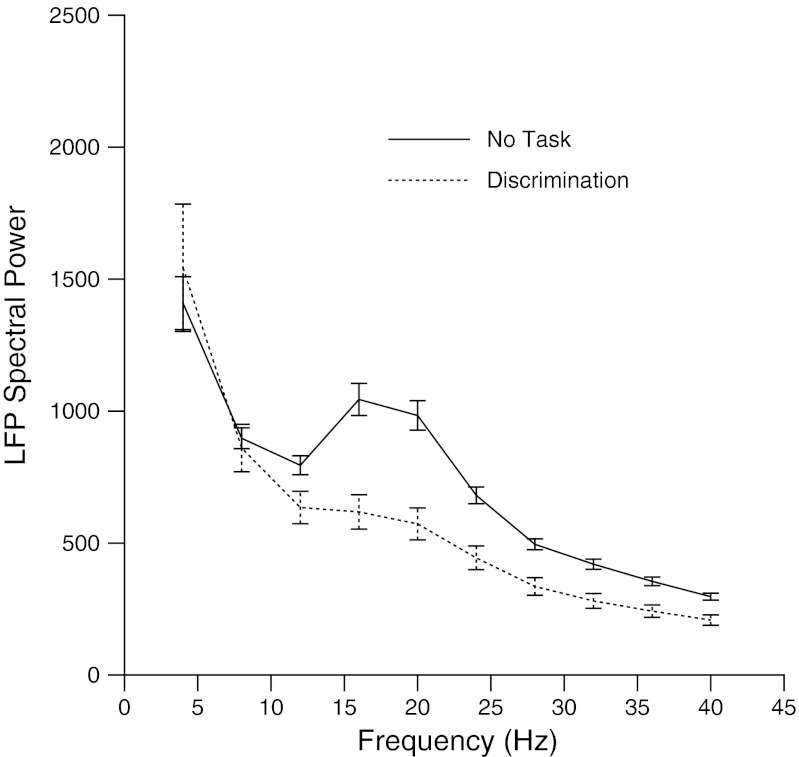
To examine changes in the local field potentials, low frequency voltage was sampled from every electrode used in the population PSTH analysis that contained a clean low frequency signal before the delivery of a tap, and then averaged over all recordings, and compared across conditions. As seen in Fig. 7, the power spectra were comparable to those examined during detection performance, with significant increases in Fourier spectral power from 16 to 40 Hz during No Task condition recordings. As the data in detection and discrimination were all paired, the differences between Fourier power during the No Task condition and active behavior were calculated and compared between detection and discrimination using unpaired t-tests at each sampling point or every 4 Hz. The points at 20 and 40 Hz were significant at the P < 0.01 level, with the active suppression being greater during discrimination than during detection.
Fig. 7.
Local field potentials during discrimination. Field potentials were sampled for a quarter second before delivery of taps in the No Task condition session (dotted line) and during discrimination (solid line). Squared amplitude of the Fourier transform of the field potential data was averaged over all recordings and all channels used for tap peristimulus time histograms in the Figs. 5 and 6. Significant differences were assessed at P < 0.001 at each data point, with differences from 16 to 40 Hz.
DISCUSSION
Overview.
SI responses recorded before the behavior in the No Task condition animal were compared with responses recorded during detection and discrimination to identical stimuli. The main findings were threefold. First, responses were stronger during behavioral sessions than before behavioral sessions. Second, the local field potential power spectra were desynchronized throughout the 12- to 40-Hz band during the behavior compared with before the behavior, and this difference cannot be attributed to delivery of sensory inputs. Third, the task dependent increase in response to the task distractor in discrimination is significantly weaker than the task-dependent increase in response to the task targets.
Stronger responses during behavior.
The enhanced responses during detection and discrimination compared with during the No Task condition are entirely analogous to recordings performed in the auditory system (Miller et al. 1972; Ryan et al. 1984). In those studies, monkeys detected acoustic stimuli, and enhanced auditory neuron onset, and offset, responses to those acoustic stimuli were noted. In such a study, as in the present study, the cognitive state during detection is controlled and motivated by reinforcement, while the comparison cognitive state is uncontrolled. These effects also appear qualitatively similar to those shown in visual spatial attention tasks (Moran and Desimone 1985; Desimone and Duncan 1995; Treue and Maunsell 1996) in studies in which spatial selective attention either is, or is not, focussed on the stimulus most responsible for evoking the neural response being recorded. Effects that occur between No Task and detection conditions in visual studies are not similarly well studied, possibly because of confounds by the impacts of visual fixation during operant conditions, and saccades outside operant conditions. The range of attentional enhancements noted, however, suggest that the modulation of stimuli by selective attention in visual studies is comparable to the modulation in auditory and somatosensory studies by changing from a No Task condition to a detection behavior.
Distractor suppression.
Two possible roles exist for the suppression observed in the task distractor responses. First, the same neural activity that leads to this suppression may also lead to the enduring suppression seen in sensory cortical plasticity. As described previously in studies conducted with the same behavioral sessions as the present work (Spingath et al. 2011), if responses to the task distractor are evaluated in the No Task condition monkey each day, sensory discrimination learning causes suppression at locations that respond to the task distractor. The suppression observed in the present study matches the selectivity of the plasticity effect in the prior study, which suggests these suppressive effects may be inherited from a common cause. The second possible role for the suppression is in task performance. The suppression creates a cue for the brain that can aid in task performance by creating a discrepancy between the strength and time course of the task distractor and task target responses. Because the earliest phase of the distractor response is similar to the target responses, the suppression may be cued from an area outside primary somatosensory cortex, like area SII, which plausibly could receive inputs from SI in a network setup for template matching, and cause selective feedback suppression, within 30–35 ms.
The task distractor-dependent suppression observed is likely caused by spatial attention effects. Whereas in principle the cognitive state of the No Task condition animal is uncontrolled, this cognitive state, and the cognitive state during the behavior, are matched between the task target and task distractor effects. Thus the cause of the differences between task distractor and task target effects cannot be caused by changes in arousal or by changes in sustained attention. Instead, they are likely caused by selective spatial attention. The responses to the task target during behavior resemble amplified versions of the responses in the No Task condition animal to the same stimuli, but the responses to the task distractor fall off roughly 30 ms after their initial, fairly unperturbed, response. Spatial attention in somatosensory cortex is already documented in macaques (Burton and Sinclair 2000) and humans (Jones et al. 2010), but in those studies attention was diverted cross-hemisphere or between the hand and foot ipsilaterally. In the present study, the spatial effects occurred within the same hand representation. Our use of near impulse stimuli in the experimental design also provides an advantage in comparing early and late components of the responses that cannot be compared as cleanly when temporally longer stimuli are used. Similar phenomena have been observed in spatial attention studies in the visual system. Suppressive effects for nontarget stimuli have been observed more strongly at longer latencies in area MT (Seidemann and Newsome 1999) or with greater attentional modulation at longer latencies in V4 (Hayden and Gallant 2005).
Caveats about sampling from implants.
The sampling in the present study is not commonly used. Cortical implants are positioned in somatosensory cortex and allowed to stabilize for >3 mo (deCharms et al. 1998). Once reasonable stability is achieved, sampling continued from sites that enabled clear audio-tactile receptive field assessments on a daily basis. Accordingly, all sampling in this study were performed on 25 implanted microelectodes that had responses to either the task target, or the task distractor. Multiunit samples were defined on 12–20 days from each electrode. Each of those samples were positioned in a fixed spatial grid relative to the cranium and lowered to a depth subjectively judged to have a maximally vigorous driven action potential filtered response. The choice of locations for recording is not biased with respect to size of electrophysiological waveform, but only for areas with strong driven responses. Typically, multiunit sampling was available with mean peak-to-peak amplitudes equal to 3.75 times the noise standard deviation or on the order of 75 μV. Such signals are not out of range for the middle cortical lamina of area 3b, a brain area well-known for its small uniform cell body size (Powell and Mountcastle 1959). Because the same sites were resampled for data collection, it is also possible that the samples possess some redundancy. Of the three basic discoveries in this work, two are not likely impacted by the form of sampling. The overall increased responsiveness during task performance and the μ-rhythm suppression during task performance were statistically robust. The task distractor response findings of less enhancement than target responses, and of changes in time course, were a statistical finding but could have been the result of a few electrodes finding cortical locations with these types of responses. Whereas it is a certainty that these types of responses exist in somatosensory cortex, it would be of further value to replicate these effects in a large statistically independent group of single units that were each used as a target, as a distractor, and sampled under No Task conditions. Such a replication would give a more robust picture of the prevalence of such response types across the population.
Translating from μ-suppression to spiking in SI.
These data also provide some more invasive translation into the field potential, EEG, and MEG work on the μ-rhythm. Suppression of this rhythm has been reported at 10, 20, and 30 Hz (Pfurtscheller et al. 2006). More simply, the EEG energy in those specific frequency bands decreases in a spatial region localized to the somatosensory hand region if input is delivered to the hand or if input is anticipated to be delivered to the hand. Human studies have found prestimulus magnetic field potential suppression across the μ-range of frequencies, even in studies that control arousal, control mode of attention, but direct attention spatially between the hand and the foot (Jones et al. 2010). The suppression observed in the present study closely matches the frequency range of suppression observed in interhemispheric somatosensory attention work in humans (Bauer et al. 2006). Monkey studies have similarly studied interhemispheric attention effects, or attention effects across sensory modalities, to achieve neural effects similar to the broad enhancement of responses we observed during active behaviors (Hsiao et al. 1993; Burton and Sinclair 2000; Meftah et al. 2002). Our recordings find a nonstimulus-dependent suppression from 12 to 40 Hz that occurred when animals were required to attend to the somatosensory inputs to receive reward and that this suppression of the field potential occurs concurrently with an amplification of ongoing and evoked somatosensory responses.
Conclusions and future directions.
These studies also suggest that mechanisms related to selective attention are related to plasticity. Prior studies have looked at effects of selective attention in primary and secondary somatosensory cortex (Hsiao et al. 1993; Burton and Sinclair 2000; Meftah et al. 2002). Whereas cross-modal attention effects in secondary sensory cortex exhibited changes in form and appear dependent on potential for reward, the changes in primary sensory cortex are mostly changes in responsiveness. Our finding that distractor tap responses exhibit late phase suppression suggests that a common neural cause may instruct both within trial suppression and longer term, stable, suppression of the responses to task distractors that is observable in studies of plasticity. The suppression may be derived from biasing responses from higher cortical areas, like SII.
GRANTS
This work supported by National Institute of Neurological Disorders and Stroke Grant 5R01-NS-055173 (to D. T. Blake).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.S. led all studies; E.S. collected all data from all animals and processed all data; H.-S.K. aided in data collection; D.T.B. and E.S. designed all studies; D.T.B. wrote all analysis software; E.S. and D.T.B. wrote the manuscript.
ACKNOWLEDGMENTS
We acknowledge the assistance of A. Khan, L. Mundo, S. McConnell, and J. Crawford in data collection, G. White and S. Patterson in machining, and J. Cisco in electronic work. Useful commentary on this manuscript was provided by C. I. Moore and C. Constantinidis.
REFERENCES
- Barbour D, Wang X. Contrast tuning in auditory cortex. Science 299: 1073–1075, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26: 490–501, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel R, Schreiner C, Cheung S, Wang X, Merzenich M. Reward dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA 100: 11070–11075, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA 99: 10114–10119, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Heiser M, Caywood M, Merzenich M. Experience-dependent adult cortical plasticity requires cognitive association between sensation, and reward. Neuron 52: 371–381, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Strata F, Kempter R, Merzenich M. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol 94: 2239–2250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair R. Tactile-spatial, and cross-modal attention effects in the primary somatosensory cortical areas 3b and 1–2 of rhesus monkeys. Somatosens Mot Res 17: 213–228, 2000 [DOI] [PubMed] [Google Scholar]
- Chapman C, Meftah EM. Independent controls of attentional influences in primary, and secondary somatosensory cortex. J Neurophysiol 94: 4094–4107, 2005 [DOI] [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. Optimizing sound features for cortical neurons. Science 280: 1439–1443, 1998 [DOI] [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. A multielectrode implant device for the cerebral cortex. J Neurosci Meth 93: 27–35, 1999 [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222, 1995 [DOI] [PubMed] [Google Scholar]
- Ghose G, Yang T, Maunsell J. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol 87: 1867–1888, 2002 [DOI] [PubMed] [Google Scholar]
- Hayden BY, Gallant JL. Time course of attention reveals different mechanisms for spatial and feature-based attention in area V4. Neuron 47: 637–643, 2005 [DOI] [PubMed] [Google Scholar]
- Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 70: 444–447, 1993 [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104, 1990 [DOI] [PubMed] [Google Scholar]
- Jones S, Kerr C, Wan Q, Pritchett D, Häamäläinen M, Moore C. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci 30: 13760–13765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Pritchett D, Stufflebeam S, Häamäläinen M, Moore C. Neural correlates of tactile detection: a combined magnetoencephalography, and biophysically based computational modeling study. J Neurosci 27: 10751–10764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulics A. Cortical neural evoked correlates of somatosensory stimulus detection in the rhesus monkey. Electroencephalogr Clin Neurophysiol 53: 78–93, 1982 [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci 4: 1131–1138, 2001 [DOI] [PubMed] [Google Scholar]
- Meftah EM, Shenasa J, Chapman C. Effects of a cross-modal manipulation of attention on somatosensory cortical neuronal responses to tactile stimuli in the monkey. J Neurophysiol 88: 3133–3149, 2002 [DOI] [PubMed] [Google Scholar]
- Miller J, Sutton D, Pfingst B, Ryan A, Beaton R, Gourevitch G. Single cell activity in the auditory cortex of rhesus monkeys: behavioral dependency. Science 177: 449–451, 1972 [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784, 1985 [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R. Cortical neuronal periodicities, and frequency discrimination in the sense of flutter. Cold Spring Harbor Symp Quant Biol 55: 861–872, 1990 [DOI] [PubMed] [Google Scholar]
- Pei Y, Hsiao S, Craig J, Bensmaia S. Neural mechanisms of tactile motion integration in somatosensory cortex. Neuron 69: 536–547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva F. Mu rhythm (de) synchronization and eeg single-trial classification of different motor imagery tasks. Neuroimage 31: 153–159, 2006. . [DOI] [PubMed] [Google Scholar]
- Polley D, Heiser M, Blake D, Schreiner C, Merzenich M. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci USA 101: 16351–16356, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley D, Steinberg E, Merzenich M. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci 26: 4970–4982, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T, Mountcastle V. The cytoarchitecture of the postcentral gyrus of the monkey macaca mulatta. Bull Johns Hopkins Hosp 105: 108, 1959 [PubMed] [Google Scholar]
- Raiguel S, Vogels R, Mysore S, Orban G. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci 26: 6589–6602, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol 67: 1031–1056, 1992a [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol 67: 1071–1091, 1992b [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13:87–103, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Miller J, Pfingst B, Martin G. Effects of reaction time performance on single-unit activity in the central auditory pathway of the rhesus macaque. J Neurosci 4:298–308, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature 412: 549–553, 2001 [DOI] [PubMed] [Google Scholar]
- Seidemann E, Newsome W. Effect of spatial attention on the responses of area MT neurons. J Neurophysiol 81: 1783–1794, 1999 [DOI] [PubMed] [Google Scholar]
- Spingath E, Kang H, Plummer T, Blake D. Different neuroplasticity for task targets and distractors. PLos One 6: e15342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968 [DOI] [PubMed] [Google Scholar]
- Thakur PH, Fitzgerald PJ, Hsiao SS. Second-order receptive fields reveal multidigit interactions in area 3b of the macaque monkey. J Neurophysiol 108: 243–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382: 539–541, 1996 [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75, 1995 [DOI] [PubMed] [Google Scholar]
- Weinberger N. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5: 279–290, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Jenkins W, Santucci S. Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex 9: 264–276, 1999 [DOI] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci 14: 1710–1721, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell J. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci 24: 1617–1626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ding M. Detection of a weak somatosensory stimulus: Role of the prestimulus mu rhythm, and its top-down modulation. J Cogn Neurosci 22: 3071617–322, 20112 [DOI] [PubMed] [Google Scholar]