Abstract
Effects of N-geranyl cyclopropylcarboxamide (NGCC) and four structurally related compounds (N-cyclopropyl E2,Z6-nonadienamide, N-geranyl isobutanamide, N-geranyl 2-methylbutanamide, and allyl N-geranyl carbamate) were evaluated on the chorda tympani (CT) nerve response to NaCl and monosodium glutamate (MSG) in rats and wild-type (WT) and TRPV1 knockout (KO) mice and on human salty and umami taste intensity. NGCC enhanced the rat CT response to 100 mM NaCl + 5 μM benzamil (Bz; an epithelial Na+ channel blocker) between 1 and 2.5 μM and inhibited it above 5 μM. N-(3-methoxyphenyl)-4-chlorocinnamid (SB-366791, a TRPV1t blocker) inhibited the NaCl+Bz CT response in the absence and presence of NGCC. Unlike the WT mice, no NaCl+Bz CT response was observed in TRPV1 KO mice in the absence or presence of NGCC. NGCC enhanced human salt taste intensity of fish soup stock containing 60 mM NaCl at 5 and 10 μM and decreased it at 25 μM. Rat CT responses to NaCl+Bz and human salt sensory perception were not affected by the above four structurally related compounds. Above 10 μM, NGCC increased the CT response to MSG+Bz+SB-366791 and maximally enhanced the response between 40 and 60 μM. Increasing taste cell Ca2+ inhibited the NGCC-induced increase but not the inosine monophosphate-induced increase in glutamate response. Addition of 45 μM NGCC to chicken broth containing 60 mM sodium enhanced the human umami taste intensity. Thus, depending upon its concentration, NGCC modulates salt taste by interacting with the putative TRPV1t-dependent salt taste receptor and umami taste by interacting with a Ca2+-dependent transduction pathway.
Keywords: chorda tympani, TRPV1t, benzamil, SB-366791, T1R1+T1R3
a subject's eating behavior is influenced by the sense of taste. Because salty taste is generally appetitive, people in developed countries ingest far more salt than is required to maintain a normal sodium balance (Weinberger 1996). Both clinical and experimental studies suggest a correlation between dietary salt intake and the prevalence and progression of hypertension (Haddy and Pamnani 1995; Karppanen and Mervaala 2006), a risk factor for stroke, coronary heart disease, and heart failure. Consistent with this, the clinical and public health recommendations in most developed countries and those of the Institute of Medicine of the National Academies in the US in 2010 are for reducing sodium intake currently to no more than 2,300 mg/day for persons 2 or more years of age. However, recent meta-analysis of the randomized control trials suggests that interventions to reduce dietary salt do not reduce mortality and cardiovascular morbidity in the long term (Taylor et al. 2011). Thus, to decrease the overall incidence of hypertension and to assist in its clinical management, it is imperative to find ways to decrease dietary Na+ intake without compromising on salty taste (Kahan 2012; Wenner 2008). One approach to lower dietary sodium would be to identify salt taste enhancers, compounds that can reversibly upregulate either the mammalian amiloride-sensitive or the amiloride-insensitive salt taste receptor (DeSimone and Lyall 2006, 2008; Katsumata et al. 2008).
The amiloride- and benzamil (Bz)-insensitive salt taste receptor(s) are the predominant transducers of salt taste in humans (Feldman et al. 2003; Katsumata et al. 2008; Ossebaard and Smith 1995). We have identified several modulators of the putative TRPV1t (a variant of the pain receptor TRPV1)-dependent amiloride- and Bz-insensitive salt taste receptor that might be useful as salt taste enhancers. Over a narrow range of concentrations, cetylpyridinium chloride (CPC) (DeSimone et al. 2001), resiniferatoxin (RTX), capsaicin (Lyall et al. 2004, 2005a), ethanol (Lyall et al. 2005b), nicotine (Lyall et al. 2007), and both natural (Rhyu et al. 2009) and synthetic (Coleman et al. 2011; Katsumata et al. 2008) Maillard reacted peptides (MRPs) upregulate TRPV1t reversibly and enhance chorda tympani (CT) taste nerve responses in rodents to NaCl+Bz and thus can, in principle, serve as salt taste enhancers. At higher concentrations, all of the above modulators inhibit TRPV1t and attenuate the CT response to NaCl+Bz and thus can be used as salt taste suppressors. Unfortunately, with the exception of MRPs, many of these compounds also produce either undesirable side tastes or thermal pain in behaving animals, properties that limit their practical use. Interestingly, at the concentrations above those that inhibit the neural response to NaCl+Bz, MRPs also modulate the CT response to glutamate and enhance umami taste (Katsumata et al. 2008) without introducing side tastes. MRPs also modulate human salty and umami taste in the distinct concentration ranges that alter CT responses respectively to NaCl+Bz and glutamate in rodents (Katsumata et al. 2008). These data suggest that in both rodents and humans MRPs induce changes in NaCl and glutamate taste by interacting with functionally similar salty and umami receptors.
In this report, we describe the effects of N-geranyl cyclopropylcarboxamide (NGCC), a compound synthesized by International Flavors & Fragrances (IFF), on salty and umami taste stimuli by monitoring the Bz-insensitive NaCl CT response and the glutamate CT response in Sprague-Dawley rats, wild-type (WT) C57BL/6J mice, and TRPV1 knockout (KO) mice and on the evaluation of salty and umami tastes in humans. Our results demonstrate that between 0.25 μM and 50 μM NGCC modulates the TRPV1t-dependent Bz-insensitive NaCl CT response in a biphasic manner. At low concentrations (1–2.5 μM) it enhances and at higher concentrations (>2.5 μM) it inhibits the Bz-insensitive NaCl CT response. At concentrations above 10 μM NGCC enhances the CT response to glutamate. At 100 μM NGCC enhanced the glutamate CT response by the same magnitude as 1 mM inosine 5′-monophosphate (IMP). In human sensory evaluation NGCC enhanced the perception of salty and umami taste at the same respective concentration range that produced the maximum enhancement in the Bz-insensitive NaCl CT response and the glutamate response, respectively, in rats and mice. We conclude that NGCC, depending upon its concentration, modulates both salty and umami taste (Dewis et al. 2006).
MATERIALS AND METHODS
CT taste nerve recordings.
Animals were housed in the Virginia Commonwealth University animal facility in accordance with institutional guidelines. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Virginia Commonwealth University. Female Sprague-Dawley rats (150–200 g) were anesthetized by intraperitoneal injection of pentobarbital (60 mg/kg), and supplemental pentobarbital (20 mg/kg) was administered as necessary to maintain surgical anesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anesthesia. Body temperatures were maintained at 37°C with a Deltaphase Isothermal Pad (model 39 DP; Braintree Scientific Braintree, MA). The left CT nerve was exposed laterally as it exited the tympanic bulla and placed onto a 32G platinum-iridium wire electrode. The stimulus solutions at room temperature were injected into a Lucite lingual perfusion chamber affixed by vacuum to a 28-mm2 patch of anterior dorsal lingual surface. For stimulation or rinsing, 3-ml aliquots were injected at a rate of 1 ml/s into the perfusion chamber (Lyall et al. 2004). The CT responses were recorded under zero lingual current clamp and analyzed as described previously (Katsumata et al. 2008).
The major part of the study involved recording CT nerve responses in rats so that the data from NGCC can be compared with the already published data in the rat from several other TRPV1t modulators (Katsumata et al. 2008; Lyall et al. 2004, 2005a, 2005b, 2005c, 2007, 2009, 2010a; Treesukosol et al. 2007). We also monitored CT responses in WT (C57BL/6J) and homozygous TRPV1 KO [B6.129S4-Trpv1tmijul, N10F19 (02-JAN-10); Jackson Laboratory, Bar Harbor, ME] mice to demonstrate that the effect of NGCC is species independent but is dependent upon the TRPV1 gene product (Lyall et al. 2004, 2005a). Mice (30–40 g) were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg), and supplemental pentobarbital (10 mg/kg) was administered as necessary to maintain surgical anesthesia. Our rat lingual perfusion chamber is too big for the mouse tongue. Therefore, in mice CT recordings were made without the lingual perfusion chamber. The mouse tongue was stimulated by superfusing 3 ml of rinse and stimulus solutions at the rate of 1 ml/s. The rest of the procedure was the same as described above for rats. At the end of each experiment animals were humanely killed by an intraperitoneal overdose of pentobarbital (∼195 mg/kg body wt for rats and 150 mg/kg body wt for mice).
When using a chamber, zero-current clamp mode is functionally equivalent to open-circuit mode, which is the electrical condition of the tongue without a chamber. Accordingly, one would expect the CT response in zero-current clamp mode (with a chamber) to closely resemble the CT response in open-circuit mode (without a chamber). In rats, where it can be tested, that is the case. In mice without a chamber we typically see the usual phasic-tonic characteristic without any unusual adaptation in the tonic CT response. This is also what other labs who have recorded from mice tend to see in the CT taste nerve and in the glossopharyngeal nerve (Horio et al. 2011).
The composition of rinse and NaCl stimulating solutions is shown in Table 1. In preliminary studies, a large number of in-house IFF compounds were tested in human sensory evaluation. Among the compounds tested, NGCC increased peripheral taste responsiveness. To examine the mechanism by which NGCC increases salt taste perception, CT responses in rats, WT mice, and TRPV1 KO mice were monitored while the anterior lingual surface was stimulated first with the rinse solution and then with salt solutions containing 0–100 μM NGCC and four additional compounds synthesized by IFF with a similar structural motif:
Table 1.
Taste stimuli used for CT experiments
| Rinse | Composition, mM | Stimuli | Composition, mM |
|---|---|---|---|
| R* | 10 KCl + 10 HEPES | N* | 10 KCl + 10 HEPES + 100 NaCl |
| R* | 10 KCl + 10 HEPES | N+Bz* | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz |
| R* | 10 KCl + 10 HEPES | N+Bz+IFF compound* | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz + IFF compound (0–0.1) |
| R* | 10 KCl + 10 HEPES | N+SB* | 10 KCl + 10 HEPES + 100 NaCl + 0.001 SB |
| R* | 10 KCl + 10 HEPES | N+SB+IFF compound* | 10 KCl + 10 HEPES + 100 NaCl + 0.001 SB + IFF compound (0.0025) |
| R* | 10 KCl + 10 HEPES | N+Bz+SB+IFF compound* | 10 KCl + 10 HEPES + 100 NaCl + 0.005 Bz + 0.001 SB + IFF compound (0–0.1) |
| R | 10 KCl | MSG+Bz+SB | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB |
| R | 10 KCl | MSG+Bz+SB+IMP | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB + 1 IMP |
| R | 10 KCl | MSG+Bz+SB+IFF compound | 10 KCl + 100 MSG + 0.005 Bz + 0.001 SB + IFF compound (0–0.1) |
| R† | 10 KCl + 10 CaCl2 | MSG+Bz+SB+CaCl2+IFF compound† | 10 KCl + 10 CaCl2+ 100 MSG + 0.005 Bz + 0.001 SB + IFF compound (0–0.1) |
| R | 10 KCl | Sweet | 10 KCl + 500 sucrose |
| R | 10 KCl | Bitter | 10 KCl + 10 mM quinine |
| R | 10 KCl | Sour | 10 KCl + 20 mM HCl |
| R | 10 KCl | Nonsodium salts | 10 KCl + 100 mM KCl |
| R | 10 KCl | Nonsodium salts | 10 KCl + 100 mM CaCl2 |
| R | 10 KCl | Control-1 | 300 NH4Cl |
| R | 10 KCl | Control-2 | 300 NaCl |
HEPES was used to buffer the pH of rinse and salt stimuli at pH 6.1.
These solutions were used after topical lingual application of 0.15 mM ionomycin for 30 min. Ionomycin was directly dissolved in dimethyl sulfoxide (DMSO). DMSO by itself does not alter CT responses to taste stimuli (Lyall et al. 1999). MSG, monosodium glutamate; IMP, inosine 5′-monophosphate; Bz, benzamil; SB-366791 (SB), N-(3-methoxyphenyl)-4-chlorocinnamide. International Flavors & Fragrances (IFF) compounds N-geranyl cyclopropylcarboxamide, N-geranyl isobutanamide, N-geranyl 2-methylbutanamide, allyl N-geranyl carbamate, and N-cyclopropyl E2,Z6-nonadienamide were used in the concentration range between 0 and 0.1 mM. All IFF compounds used were readily soluble in water.
The pH of the rinse solution and the salt solutions containing the IFF compounds was adjusted to 6.1. In previous studies, the TRPV1t agonist-induced increase in the N+Bz CT response varied with pH. The relationship between pH and the magnitude of the CT response was bell shaped. The maximum increase in the CT response was observed at a pH between 6.0 and 6.5 (Lyall et al. 2004). In some experiments Bz (5 μM) and N-(3-methoxyphenyl)-4-chlorocinnamide [SB-366791 (SB); 1 μM] were added to the salt solutions to block Na+ entry into taste receptor cells (TRCs) via the apical epithelial Na+ channels (ENaC) and putative nonselective TRPV1t cation channels (Lyall et al. 2004, 2005a, 2005c, 2007). CT responses were also recorded at varying temperatures (23–55.5°C). In some experiments we tested the effect of IFF compounds on the CT response to monosodium glutamate (MSG) and MSG+IMP (Table 1). IMP is a specific modulator of umami taste (Yamaguchi 1967). CT responses to MSG were monitored in the presence of Bz and SB (Table 1). This was done to eliminate the contribution of Na+ to the glutamate CT response (Lyall et al. 2004). Typically, stimulus solutions remained on the tongue for 1 min. Control stimuli consisting of 0.3 M NH4Cl and 0.3 M NaCl applied at the beginning and at the end of the experiment were used to assess preparation stability. As in our previous studies the control responses did not differ by more than 2–5% at the beginning and end of the experiment (Treesukosol et al. 2007). The following stimulus series were used in the CT experiments.
Series 1 was used to generate the relationship between IFF compound concentration and the magnitude of the NaCl+Bz (N+Bz) CT response:
The R→(N+Bz+IFF compound)→R step was repeated for each concentration of IFF compound between 0 and 100 μM. At the end of the IFF compound concentration series, the control stimuli were again applied (R→0.3 M NH4Cl→R→0.3 M NaCl→R).
Series 2 was used to generate the relationship between IFF compound concentration and the magnitude of the N+Bz CT response in the presence of TRPV1t blocker SB (1 μM):
The R→(N+Bz+SB+IFF compound)→R step was repeated for each concentration of IFF compound between 0 and 100 μM.
Series 3 was used to generate the relationship between the temperature of the N+Bz solution and the magnitude of the N+Bz CT response in the absence and presence of a fixed concentration of IFF compound:
The R→(N+Bz)→R→(N+Bz+IFF compound)→R step was repeated for (N+Bz) and (N+Bz+IFF compound) maintained between 23 and 55.5°C. In these experiments, the lingual surface was superfused (8 ml/min) with salt solutions by syringe pumps and heating coils maintained at temperatures between 23 and 55.5°C while the rinse (R) was maintained at 23°C (Lyall et al. 2004). At the end of the temperature series, the control stimuli were again applied (R→0.3 M NH4Cl→R→0.3 M NaCl→R).
Series 4 was used to generate the relationship between IFF compound concentration and the magnitude of the MSG+Bz+SB CT response:
The R→(MSG+Bz+SB+IFF compound)→R step was repeated for each concentration of IFF compound between 0 and 100 μM.
In CT experiments the tonic (steady state) part of the NaCl or MSG CT responses was quantified. To quantify the tonic part of a response, the area under the response vs. time curve was taken over the final 30 s of the response. To normalize this result, this area was divided by the area under the 0.3 M NH4Cl response curve over the final 30 s of the tonic response period. The normalized data are reported as means ± SE of the number of animals (n). Student's t-test was employed to analyze the differences between sets of data. Since we are comparing the normalized CT responses to N+Bz before and after IFF compounds in the same CT preparation, paired t-test was used to evaluate statistical significance.
For clarity the points on the graphs of the mean normalized tonic responses versus the logarithm of the IFF compound concentration were connected respectively by smooth curves. The curves were generated with a fitting function that models the characteristic biphasic property of the peptide agonists of TRPV1. The biphasic property has been observed with every agonist of TRPV1t examined thus far (Coleman et al. 2011; Katsumata et al. 2008; Lyall et al. 2004, 2005a, 2005c, 2007; Treesukosol et al. 2007). The fitting function used was
| (1) |
where
| (2) |
and
| (3) |
Here R is the response, x is the logarithm of the IFF compound concentration expressed in percentage, and a, b, d, m, n, and r are parameters chosen by least-squares criteria.
The plot of CT response versus temperature was fitted to Eq. 4 with least-squares criteria:
| (4) |
Here R is the CT response, t is the temperature, and a, k, b, c, n, and m are constants. The minimum CT response is c. The NaCl+Bz CT responses obtained as a function of varying temperature in the absence and presence of NGCC were analyzed by two-way analysis of variance (ANOVA), and statistically significant values were Bonferroni adjusted (Lyall et al. 2009).
The CT responses to MSG were also monitored under the conditions in which we altered TRC Ca2+. TRC Ca2+ levels were increased in vivo by topical lingual application of 150 μM ionomycin (a Ca2+ ionophore) for 30 min and including 10 mM CaCl2 in all rinse and stimulating solutions (Lyall et al. 2006, 2009).
The plot of the magnitude of the CT response versus the IFF compound concentration was fitted to Eq. 5 with least-squares criteria:
| (5) |
Here R is the CT response, x is the logarithm of the IFF compound concentration in moles per liter and a, b, c, and n are positive constants. The maximum CT response is a + c, the minimum CT response is c, and b is the value of x for which R is one-half its maximum value above c.
Human salt sensory evaluation.
The human sensory evaluations were performed in accordance with the standards established by IFF and the Korea Food Research Institute (KFRI) for taste testing and do not require Institutional Review Board approval. Previously trained panelists (men and women, ages between 25 and 37 yr) with no history of basic taste disorders were recruited and trained to recognize salt taste intensity with reference to 8.7, 14.4, 25.8, 60, 80, and 100 mM NaCl solutions, which were assigned scores of 0.5, 1.0, 2.0, 5.0, 6.8, and 8.5, respectively, with a 15-point intensity scale. Sensory evaluations were conducted at room temperature with a nose clip, and samples were served to panelists in a random order. The panelists washed their mouth after tasting each sample. To test the effect of NGCC on salt taste, NGCC (2.5, 5, 10, and 25 μM) dissolved in 80 mM NaCl solution was compared with the salt taste intensity of 80 mM NaCl solution, given an intensity of 6.8 (Meilgaard et al. 1987) with a 15-point intensity scale (n = 6–9). To evaluate the salt taste intensity of NGCC alone, NGCC (2.5, 5, 10, and 25 μM) dissolved in water was compared with NaCl solutions (8.2 mM, intensity = 0.5; 14 mM, intensity = 1; and 25.8 mM, intensity = 2; n = 6). The data were analyzed by one-way ANOVA to compare between-group differences.
According to the manufacturers' instructions, the Japanese fish soup base hondashi (2 g) was dissolved in 600 ml of water. Hondashi fish soup contains 20.8 mM NaCl. To this, 39.2 mM NaCl was added to achieve a final concentration of 60 mM NaCl. Hondashi solution containing 60 mM NaCl was used as a control and was given an intensity of 6 on a 15-point intensity scale. The effects of NGCC (2.5, 5, 10, and 25 μM) dissolved in hondashi solution containing 60 mM NaCl were evaluated for salty taste by the same trained panelists used in tests with NaCl solutions (n = 10–18).
The effect of IFF compounds on the umami taste was assessed with a 16-point intensity scale (0 = none and 16 = very strong). For umami taste, intensity rating of retorted IFF low-sodium chicken broth (control, 60 mM NaCl), control with added 6 mM MSG, and control with added 45 μM NGCC were evaluated by nine taste panelists. The broth without MSG was presented as a control and given an intensity of 8 on a 16-point intensity scale. The data were analyzed by one-way ANOVA to evaluate between-group differences.
RESULTS
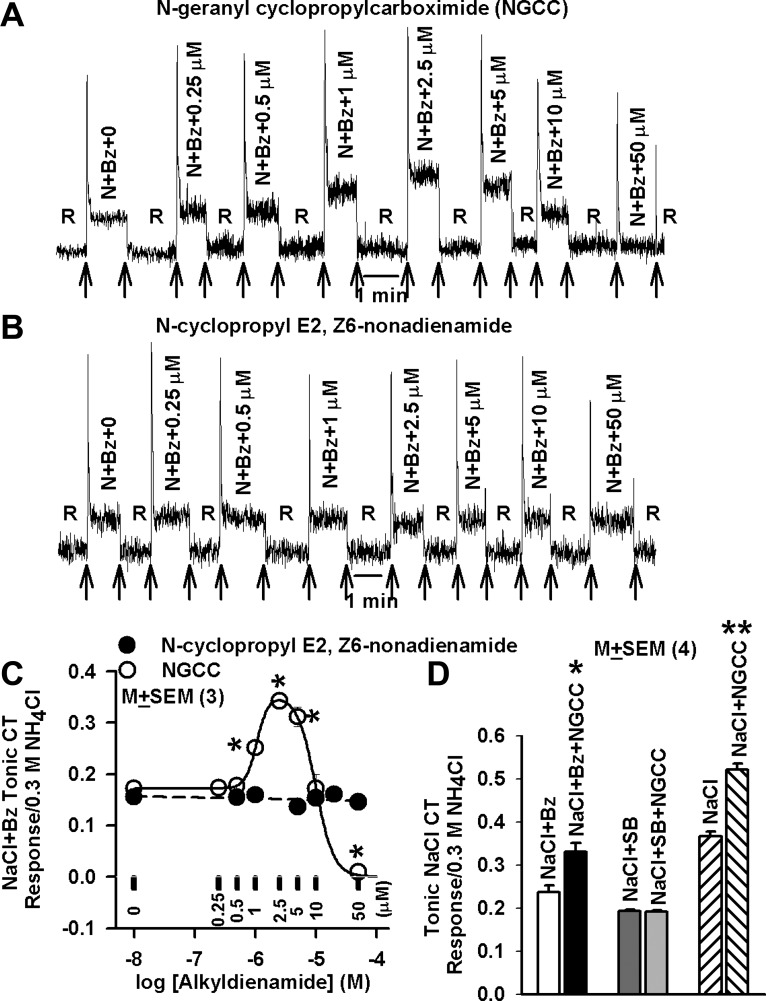
Adding increasing concentrations of NGCC to N+Bz solutions (Table 1) initially produced an increase in both phasic and tonic NaCl CT responses between 0.25 and 2.5 μM (Fig. 1A). Above 2.5 μM NGCC, the magnitudes of the phasic and tonic CT responses were less than their respective maximum values. At 50 μM NGCC, the tonic CT response decreased below the value of N+Bz alone (Fig. 1A). The normalized mean tonic N+Bz CT responses from three such experiments are summarized in Fig. 1C. NGCC produced a biphasic dose-response relationship for both phasic (data not shown) and tonic (Fig. 1C) N+Bz CT response. The maximum increase in the mean normalized tonic CT response occurred at 2.5 μM NGCC as estimated from the fitted curves, a 99.1% increase relative to N+Bz. At 10 μM NGCC, the tonic CT response was not statistically different from its value in N+Bz alone (P > 0.05). At 50 μM, NGCC the tonic CT response was not different from baseline rinse value (P > 0.05). Stimulating the tongue with the rinse solution (R)+NGCC (Table 1) elicited only transient CT responses that were concentration independent and were indistinguishable from the mechanical rinse artifact (data not shown). These results indicate that, at the concentrations used in these experiments, NGCC by itself is not a gustatory stimulus in the fungiform taste receptive field and modulates the CT response in the presence of salt (NaCl+Bz).
Fig. 1.
Effect of N-geranyl cyclopropylcarboximide (NGCC) and N-cyclopropyl E2,Z6-nonadienamide on the benzamil (Bz)-insensitive and Bz-sensitive NaCl chorda tympani (CT) response. A and B: representative traces of CT response obtained while the rat tongue was first stimulated with rinse solution (R) and then with NaCl (N)+Bz solutions containing 0.25 μM-50 μM NGCC (A) or N-cyclopropyl E2,Z6-nonadienamide (B) maintained at room temperature with the stimulus series 1 protocol. Arrows represent time periods when the rat tongue was superfused with R and the stimulating solutions. C: mean ± SE values of the normalized tonic CT responses from 3 animals plotted as a function of log[NGCC] concentration or log[N-cyclopropyl E2,Z6-nonadienamide] concentration expressed in moles/liter. The data with NGCC were fitted to Eq. 1. P values at 1 μM, 2.5 μM, 5 μM, and 50 μM NGCC for the mean tonic CT response were 0.0054, 0.0001, 0.0019 and 0.0002, respectively with reference to the tonic CT response at 0 NGCC. At 0.25 μM, 0.5 μM, and 10 μM NGCC the mean tonic CT responses were not significantly different (P > 0.05) from the mean CT response at 0 NGCC (paired; n = 3). D: mean normalized CT responses obtained in 4 rats while their tongues were first stimulated with R and then with N+Bz or N+ SB-366791(SB) solutions containing 0 or 2.5 μM NGCC maintained at room temperature with the stimulus series 1 protocol. Also shown are the mean normalized CT responses obtained in a separate set of 4 rats while their tongues were first stimulated with R and then with N and N+NGCC (2.5 μM). *P = 0.0107, **P = 0.0002 (unpaired; n = 4).
In contrast to NGCC, addition of N-cyclopropyl E2,Z6-nonadienamide, a chemically related compound, to the N+Bz solutions was ineffective in producing any changes in CT response between 0.25 and 100 μM (Fig. 1, B and C). In addition, three other chemically related IFF compounds, N-geranyl isobutanamide, N-geranyl 2-methylbutanamide, and allyl N-geranyl carbamate, had no effect on rat N+Bz CT responses between 0.25 and 100 μM (data not shown).
As shown in Fig. 1D, 2.5 μM NGCC enhanced the CT response when rat tongues were stimulated with solutions containing 100 mM NaCl (N) (P = 0.0002, n = 4; unpaired). In another set of four animals, NGCC produced the expected enhancement in the CT response when rat tongues were stimulated with solutions containing N+Bz (Fig. 1D; P = 0.0107, n = 4; unpaired) but not with solutions containing N+SB (P > 0.05, n = 4; unpaired). These results suggest that NGCC enhances the NaCl CT response by specifically modulating the activity of the SB-sensitive TRPV1t cation channel and does not affect the Bz-sensitive ENaC activity in salt-sensing TRCs.
Effect of TRPV1/TRPV1t inhibition on N+Bz CT response in the absence and presence of NGCC.
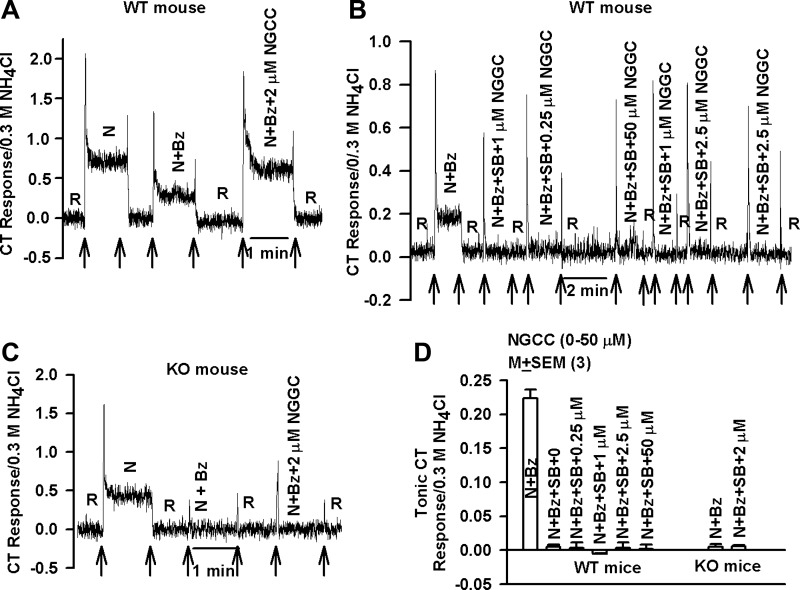
Similar to other putative TRPV1 agonists, NGCC (2 μM) enhanced the N+Bz CT response in both rats (Fig. 1) and WT mice (Fig. 2A). As shown previously (Katsumata et al. 2008; Lyall et al. 2004, 2005a, 2005c, 2007; Treesukosol et al. 2007), mixtures containing N+Bz+SB (1 μM), a specific blocker of TRPV1 (Gunthorpe et al. 2004), inhibited the constitutive CT response to N+Bz to baseline in WT mice (Fig. 2B). Subsequently stimulating the mouse tongue with solutions containing N+Bz+SB+NGCC (0.25–50 μM) elicited no increase in CT nerve response above the rinse baseline level (Fig. 2B). In WT mice, in mixtures containing N+Bz+SB the tonic CT responses in the presence of NGCC (0.25–50 μM) were not different from the rinse baseline value (Fig. 2D; P > 0.05; unpaired).
Fig. 2.
Effect of TRPV1/TRPV1t inhibition on the NGCC-induced changes in the Bz-insensitive NaCl CT response. A: representative CT trace obtained while the wild-type (WT) mouse tongue was first stimulated with R and then with N, N+Bz, and N+Bz+2 μM NGCC maintained at room temperature with the stimulus series 1 protocol. Arrows represent time periods when the mouse tongues were superfused with R and the stimulating solutions. B: representative CT trace obtained while the WT mouse tongue was first stimulated with R and then with N+Bz+SB solutions containing NGCC (0.25–50 μM) maintained at room temperature with the stimulus series 2 protocol. C: representative CT trace in a TRPV1 knockout (KO) mouse while the mouse tongue was first stimulated with R and then with N, N+Bz, and N+Bz+2 μM NGCC maintained at room temperature with the stimulus series 1 protocol. Arrows represent time periods when the mouse tongues were superfused with R and the stimulating solutions. D: summary of the normalized CT responses to N+Bz, N+Bz+SB, and N+Bz+SB+2 μM NGCC in 3 WT mice and N+Bz and N+Bz+2 μM NGCC in 3 TRPV1 KO mice.
In agreement with our earlier studies (Lyall et al. 2004), TRPV1 KO mice did not elicit CT responses to N+Bz (Fig. 2C) and demonstrated no increase in the CT response above baseline when the tongue was stimulated with N+Bz+2 μM NGCC (Fig. 2C), a concentration that induced a near-maximum increase in the N+Bz response in rats (Fig. 1, A and C). In three TRPV1 KO mice, the tonic CT response in the presence of N+Bz+2 μM NGCC was not different from the tonic response to N+Bz or the rinse baseline level (Fig. 2D). These results show that in WT and KO mice pharmacologically or genetically silencing TRPV1/TRPV1t activity, respectively, not only eliminates the constitutive CT response to N+Bz but also eliminates the modulation of the CT response by NGCC.
Effect of temperature on N+Bz CT response in absence and presence of NGCC.
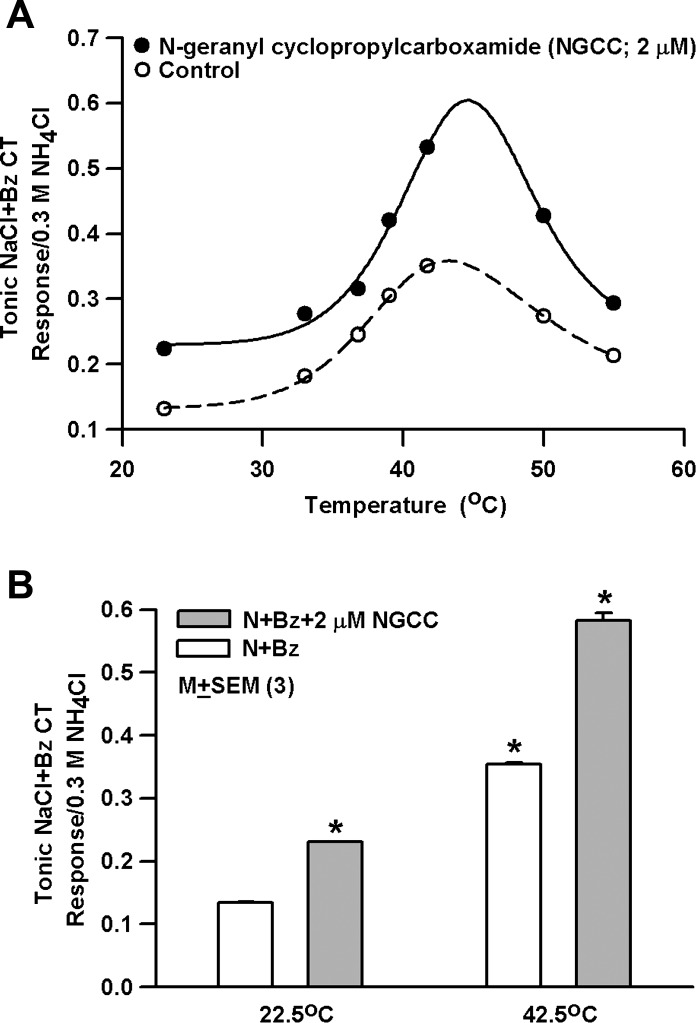
As shown in a representative rat CT recording (Fig. 3A), consistent with our earlier studies (Katsumata et al. 2008; Lyall et al. 2004, 2005a, 2005c, 2007), increasing the temperature from 23°C to 33, 36.8, 39, 41.7, 50, and 55.5°C (Table 1) increased the magnitude of the tonic N+Bz CT responses relative to 23°C (Fig. 3A). At 50°C and 55.5°C the magnitude of the CT response was less than its maximum value. At all temperatures studied, NGCC (2 μM) produced a significantly greater increase (P < 0.0001) in the tonic CT response (N+Bz+NGCC) relative to N+Bz (Fig. 3A). The plot of CT response versus temperature was fitted to Eq. 4 with least-squares criteria. For the control curve, c = 0.13, a = 1.0, k = 44.8, b = 0.21, n = 9.8, and m = 17.0. For the NGCC curve, c = 0.23, a = 1.0, k = 44.3, b = 0.12, n = 11.7, and m = 25.8. The maximum increase in the CT response in the presence of NGCC occurred at the same temperature as with N+Bz. In three rats N+Bz CT response at 22.5°C was enhanced by increasing the temperature to 42.5°C and by the addition of 2 μM NGCC (Fig. 3B; P = 0.0001; unpaired). These results indicate that elevated temperature and NGCC produce additive effects on the N+Bz CT response.
Fig. 3.
Modulatory effect of elevated temperature on the Bz-insensitive NaCl CT response in the absence and presence of NGCC. A: rat tongue was first stimulated with R and then with N+Bz solutions containing 0 (control) or 2 μM NGCC maintained at 23°C, 33°C, 36.8°C, 39°C, 41.7°C, 50°C, and 55°C with the stimulus series 3 protocol. The normalized CT response data at the various temperatures shown were fitted to Eq. 4. Tonic responses to increasing temperature were compared in the absence and presence of 2 μM NGCC. Significant differences were found for increasing temperature (P = 0.01 for both, 2-way ANOVA; Bonferroni corrected) and their interactions (P = 0.001). B: mean normalized CT response in 3 rats to N+Bz and N+Bz+2 μM NGCC at 22.5°C and 42.5°C. *P = 0.0001 with respect to CT response to N+Bz at 22.5°C (unpaired).
Effect of NGCC on CT response to MSG.
The CT response to glutamate alone can be recorded in WT mice or rats by stimulating the tongue with MSG+Bz+SB or in TRPV1 KO mice by stimulating with MSG+Bz without any contribution from Na+ (Katsumata et al. 2008). As shown in a representative trace (Fig. 4A), NGCC at 10, 20, and 40 μM enhanced the rat CT response to MSG+Bz+SB in a dose-dependent manner. Increasing the NGCC to 60 or 100 μM did not further increase the magnitude of the MSG+Bz+SB response relative to 40 μM NGCC. The normalized mean tonic MSG+Bz+SB CT responses as a function of NGCC concentration from three rats are summarized in Fig. 5A (Control). Similar to IMP, NGCC dissolved in rinse solution at 60 or 100 μM elicited only transient CT responses that were concentration independent and were indistinguishable from the mechanical rinse artifact (Fig. 6A). We also did not observe any tonic CT responses with rinse solution containing lower NGCC concentrations (0.25–50 μM) (data not shown). These results indicate that NGCC at 60 or 100 μM only modulates CT responses in the presence of glutamate.
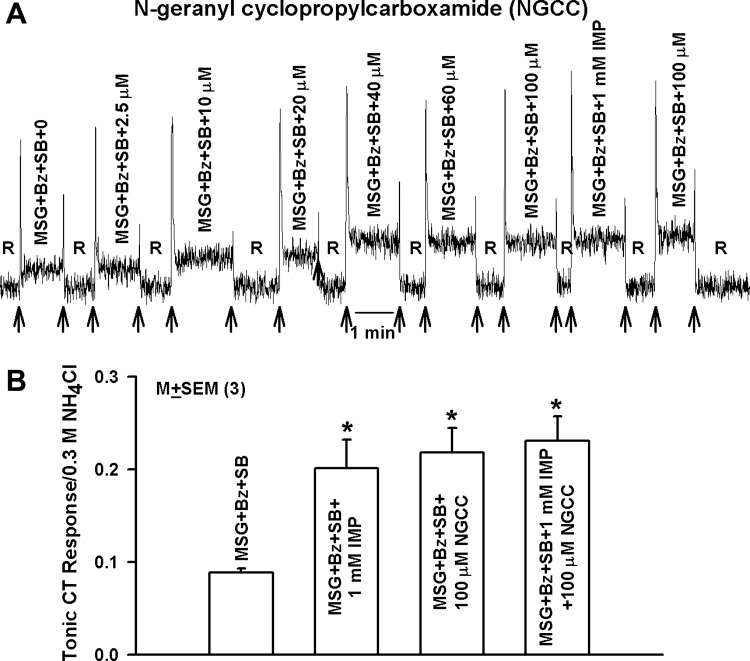
Fig. 4.
CT responses to monosodium glutamate (MSG), MSG+ inosine 5′-monophosphate (IMP), MSG+NGCC, and MSG+IMP+NGCC. A: representative CT trace in which the rat tongue was first stimulated with R and then with MSG+Bz+SB, MSG+Bz+SB+IMP (1 mM), and MSG+Bz+SB+NGCC (2.5–100 μM) maintained at room temperature with the stimulus series 4 protocol. Arrows show time period when the tongue was superfused with R and stimulating solutions. B: normalized mean ± SE tonic CT response from 3 animals for MSG+SB+Bz, MSG+SB+Bz+1 mM IMP, MSG+SB+Bz+100 μM NGCC, and MSG+SB+Bz+1 mM IMP+100 μM NGCC. *P values for mean MSG+Bz+SB CT response in the presence of 1 mM IMP, 100 μM NGCC, and 1 mM IMP+100 μM NGCC were 0.0226, 0.0085, and 0.0065, respectively, relative to MSG+Bz+SB (paired; n = 3).
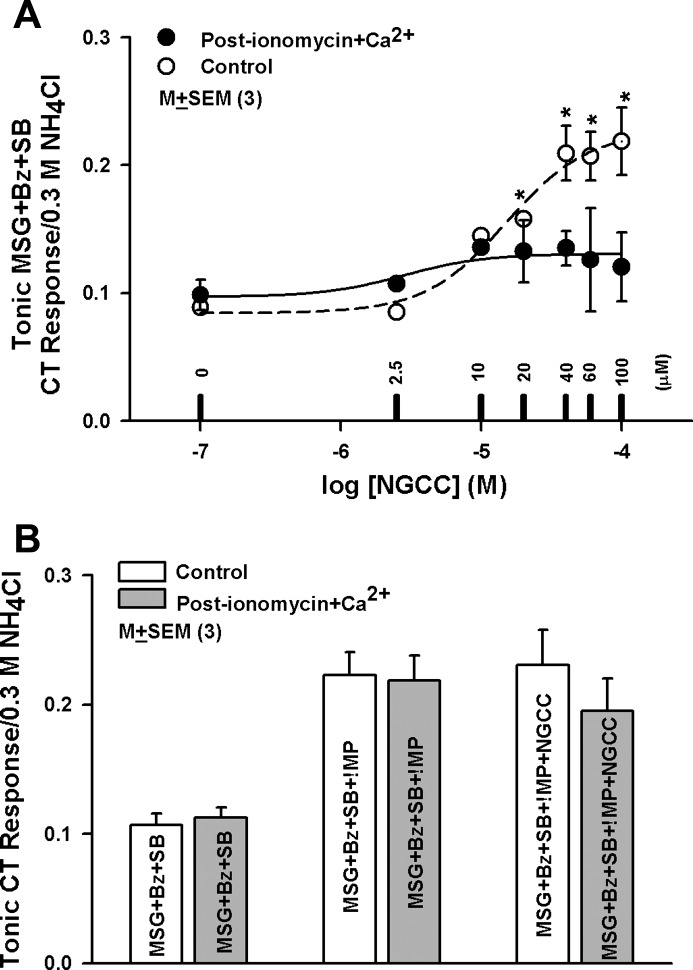
Fig. 5.
Effect of ionomycin+Ca2+ on the CT response to MSG, MSG+NGCC, MSG+IMP, and MSG+IMP+NGCC. A: relationship between the normalized mean ± SE tonic CT response to MSG+Bz+SB in 3 rats as a function of the log [NGCC] concentration between 0.25 and 100 μM (expressed as mol/l) under control conditions (Control). The CT data with varying NGCC concentration were fitted to Eq. 5. *P values for mean MSG+Bz+SB CT response in the presence of 20, 40, 60, and 100 μM NGCC were 0.0002, 0.0054, 0.0037, and 0.0085, respectively, relative to MSG+Bz+SB alone. Relationship between normalized mean ± SE tonic CT response to MSG+Bz+SB+10 mM Ca2+ in same 3 rats as a function of log [NGCC] concentration between 0.25 and 100 μM (in mol/l) after topical lingual application of 150 μM ionomycin for 30 min with the stimulus series 4 protocol is also shown (Post-ionomycin+Ca2+). The CT data with varying NGCC concentration were fitted to Eq. 5. P values at all NGCC concentrations were not significantly different (P > 0.05; paired; n = 3) relative to control. B: normalized mean ± SE tonic CT response from 3 animals for MSG+Bz+SB, MSG+Bz+SB+1 mM IMP, and MSG+Bz+SB+1 mM IMP+100 μM NGCC under control conditions and after topical lingual application of 150 μM ionomycin for 30 min with the stimulus series 4 protocol.
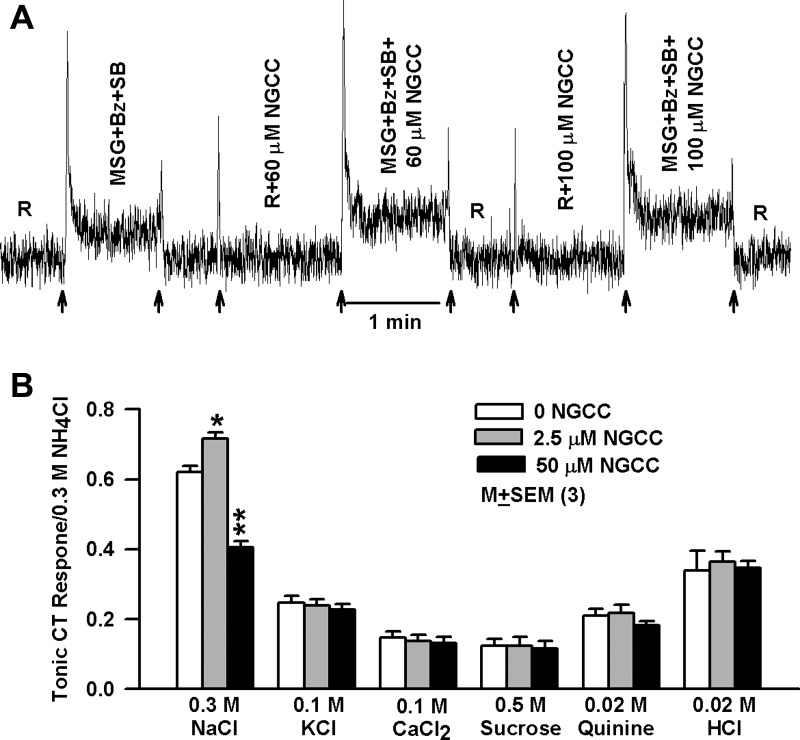
Fig. 6.
Effect of NGCC on the CT responses in rinse solution. A: representative CT trace obtained while the rat tongue was stimulated with R and then with MSG+Bz+SB solutions containing 60 μM or 100 μM NGCC maintained at room temperature. Arrows show time period when the tongue was superfused with R, R+NGCC, MSG+Bz+SB, or MSG+Bz+SB+NGCC. B: mean normalized tonic CT responses to 300 mM NaCl, 100 mM KCl, 100 mM CaCl2, 500 mM sucrose, 20 mM quinine, and 20 mM HCl in the absence and presence of 2.5 and 50 μM NGCC in 3 rats. *P = 0.0184, **P = 0.0002 (unpaired; n = 3).
As shown previously (Li et al. 2002), 1 mM IMP enhanced the CT response to MSG+Bz+SB (Fig. 4A). In three animals, the mean increase in the MSG+Bz+SB CT response in the presence of 1 mM IMP was not different from its increase in the presence of 100 μM NGCC (Fig. 4B). In the presence of 100 μM NGCC, adding 1 mM IMP did not produce any additional enhancement in the CT response. The effect of NGCC on the CT response to MSG+Bz+SB was not observed at concentrations ≤5 μM (Fig. 4A), i.e., at concentrations at which NGCC enhances the Bz-insensitive NaCl CT response (Fig. 1, A and C).
Stimulating the tongue with mixtures containing 500 mM sucrose, 20 mM quinine, or 100 mM KCl and 2.5 or 50 μM NGCC only elicited transient CT responses that were concentration independent and were indistinguishable from the mechanical rinse artifact (data not shown). Consistent with the data shown in Fig. 1, NGCC enhanced the tonic CT response to 300 mM NaCl at 2.5 μM (P = 0.0184) and inhibited the CT response at 50 μM (Fig. 6B; P = 0.001; unpaired; n = 3) relative to control. In contrast, the mean tonic CT responses to 100 mM KCl, 100 mM CaCl2, 500 mM sucrose, 20 mM quinine, and 20 mM HCl were not altered in the presence of 2.5 or 50 μM NGCC relative to control (Fig. 6B; P > 0.05; unpaired; n = 3). These results suggest that NGCC specifically modulates NaCl and glutamate responses without affecting responses to representative sweet, bitter, sour, and non-Na+ salt taste stimuli.
Effect of changes in TRC Ca2+ concentration on CT response to glutamate in absence and presence of NGCC and IMP.
To test whether changes in TRC Ca2+ concentration ([Ca2+]) modulate neural responses to glutamate, glutamate+IMP, or glutamate+NGCC, we increased TRC [Ca2+] in vivo by the topical lingual application of ionomycin and by adding 10 mM CaCl2 to the rinse and all stimulating solutions (DeSimone et al. 2012; Lyall et al. 2006, 2009). We have previously shown that 10 mM CaCl2 by itself does not elicit a CT response. In addition, SB inhibits the CT response to CaCl2 (Lyall et al. 2009). Thus, under these experimental conditions, there is no contribution of CaCl2 to the glutamate CT response. Increasing TRC Ca2+ with ionomycin did not alter the CT response to glutamate (Fig. 5A; post-ionomycin+Ca2+); however, it did inhibit the NGCC-induced enhancement in the glutamate CT response at NGCC concentrations between 20 and 100 μM relative to control (Fig. 5A). In contrast to its effects on glutamate+Bz+SB+NGCC, the CT responses to MSG+Bz+SB+1 mM IMP and MSG+Bz+SB+1 mM IMP+100 μM NGCC were not affected by ionomycin+Ca2+ treatment (Fig. 5B). These results suggest that an increase in TRC Ca2+ inhibits the enhancement in the glutamate CT response in the presence of NGCC but not in the presence of IMP. The plot of CT response versus the log[NGCC] concentration expressed in moles was fitted to Eq. 5 with least-squares criteria. For both the control and post-ionomycin+Ca2+ curves n equals 1.5, suggesting some positive cooperativity in the interaction between NGCC and the umami receptor. For the control curve, a = 0.034, b = −5.56, and c = 0.097. For the post-ionomycin+Ca2+ curve, a = 0.14, b = −4.82, and c = 0.084.
Effect of NGCC on human salt taste.
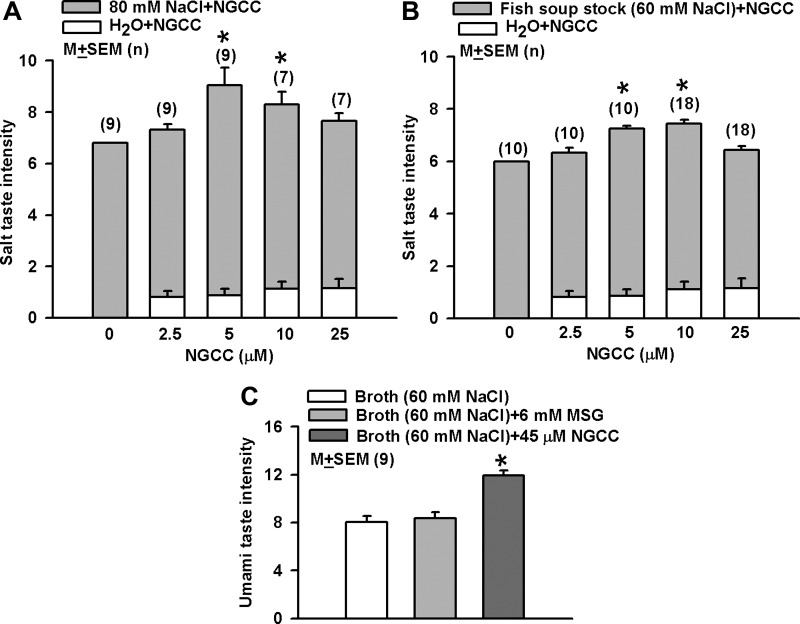
In human psychophysical tests 5 and 10 μM NGCC significantly increased the taste quality ratings of pure 80 mM NaCl solution (Fig. 7A; P < 0.5; n = 7–9; 1-way ANOVA) and of fish soup stock containing 60 mM NaCl (Fig. 7B; P < 0.5; n = 10–18; 1-way ANOVA) relative to control. At 25 μM NGCC the salt taste intensity was lower and was not different from control. NGCC dissolved in H2O did not elicit salty quality (Fig. 7). These results suggest that in humans NGCC produces a biphasic effect on salt taste. Salt taste intensities were calculated with a standard curve for NaCl. In solutions containing 80 mM NaCl plus 5 μM NGCC, the mean salt taste intensity was 8.45 (Fig. 7A). This value was greater than the sum (7.68) of individual mean intensities at 80 mM NaCl alone (6.8) and 5 μM NGCC alone (0.88). In solutions containing 80 mM NaCl plus 25 μM NGCC, the mean salt taste intensity was 7.5 (Fig. 7A). This value was smaller than the sum (7.96) of individual mean intensities at 80 mM NaCl alone (6.8) and 25 μM NGCC (1.16). These results suggest that the salt taste-enhancing effects of NGCC are synergistic. The results further suggest that at high concentrations NGCC inhibits the salt intensity by its interaction with the salt-sensing mechanism.
Fig. 7.
Effect of NGCC on human salt taste perception. A: NGCC solutions containing 2.5, 5, 10, and 25 μM NGCC were prepared in H2O or 80 mM NaCl and compared with reference solutions with a 15-point intensity scale. NaCl solutions containing 8.7, 14.4, 25.8, 60, 80, and 100 mM NaCl were assigned scores of 0.5, 1.0, 2.0, 5.0, 6.8, and 8.5, respectively. Values are means ± SE of relative salt taste intensity from 10–18 trained panelists at the Korea Food Research Institute (KFRI). The data were analyzed by 1-way ANOVA followed by Duncan's multiple-range test to evaluate between-group differences. *P < 0.05. B: effect of NGCC on salty taste was assessed with a 15-point intensity scale in a commercial fish soup stock in which the final concentration of Na+ was adjusted to 60 mM. The stock without NGCC was presented as a control and the stock plus 2.5, 5, 10, and 25 μM NGCC as test samples. Values are presented as means ± SE of relative salt taste intensity from 10–18 trained panelists at KFRI. The data were analyzed by 1-way ANOVA followed by Duncan's multiple-range test to evaluate between-group differences. *P < 0.05. C: effect of NGCC on umami taste was evaluated with a 16-point intensity scale in a retorted International Flavors & Fragrances (IFF) low-sodium chicken broth (control, 60 mM Na+) and control with added 6 mM MSG or 45 μM NGCC. Values are presented as means ± SE of relative umami taste intensity from 9 trained panelists at IFF. *P < 0.05.
The mean umami intensity of Control (retorted IFF low-sodium chicken broth)+6 mM MSG did not differ significantly from Control. In contrast, the mean umami intensity of Control+45 μM NGCC was significantly increased compared with Control (Fig. 7C; n = 9; P < 0.05; 1-way ANOVA). When presented with 25 or 45 μM NGCC dissolved in water the panelists mentioned unpleasant umami taste but no pungency. In contrast, N-cyclopropyl E2,Z6-nonadienamide, a compound chemically related to NGCC, did not affect human salty or umami taste (data not shown).
DISCUSSION
Modulation of salt responses by NGCC.
Our results show that NGCC modulates the Bz-insensitive NaCl CT response in a biphasic manner. It produced the maximum enhancement and inhibition of the N+Bz CT response at 2.5 μM and 50 μM, respectively (Fig. 1C). Compared with NGCC, RTX and capsaicin induced the maximum increase in the N+Bz CT response at 1 μM and 40 μM and the maximum inhibition at 10 μM and 200 μM, respectively (Lyall et al. 2004). Thus NGCC is a less potent agonist of the N+Bz CT response than RTX but is more potent than capsaicin.
The constitutive N+Bz CT response (Fig. 1, A and B, Fig. 2A) and its modulation by NGCC were not observed in TRPV1 KO mice (Fig. 2C) or in rats (Fig. 1D) and WT mice (Fig. 2B) in the presence of SB, a specific TRPV1 inhibitor. The TRPV1 cation channel does not conduct ions unless activated by elevated temperature, acidic pH, or TRPV1 agonists (Davis et al. 2002). The N+Bz CT response, however, appears to depend upon a constitutively conductive channel at neural pH, room temperature, and the absence of an agonist. CPC, which modulates the Bz-insensitive NaCl CT response, does not appear to act on the cloned TRPV1 (J. B. Davis, personal communication). These studies suggest that a putative variant of TRPV1 (TRPV1t) may be involved in the N+Bz CT response (Lyall et al. 2004).
NGCC increased the magnitude of the N+Bz CT response at all temperatures without a leftward shift in the relationship between temperature and the magnitude of the N+Bz CT response relative to control (Fig. 3). Similarly, mixtures of N+Bz+ethanol (Lyall et al. 2005c), N+Bz+nicotine (Lyall et al. 2007), or N+Bz+GalA-MRPs (Katsumata et al. 2008) also increased the magnitude of the N+Bz CT response at all temperatures without a leftward shift in the relationship between temperature and the magnitude of the N+Bz CT response. However, in mixtures containing nicotine or ethanol and a subthreshold RTX concentration (0.25 μM), the relationship between temperature and the magnitude of the tonic N+Bz CT response was shifted to the left to lower temperatures. Similarly, adenosine 5′-triphosphate (ATP) alone had no effect on the temperature threshold of the N+Bz CT response. However, in the presence of 0.25 μM RTX, ATP shifted the temperature threshold to lower temperatures (Lyall et al. 2004). Mixtures containing GalA-MRP and subthreshold concentrations of RTX or capsaicin enhanced the N+Bz CT response relative to the N+Bz CT response in the presence of RTX alone, capsaicin alone, or GalA-MRP alone (Katsumata et al. 2008). These studies suggest that binding of RTX either to the intracellular binding site for capsaicin (Lee et al. 2011) or to another ligand-independent allosteric site alters the conformation of the channel and sensitizes the channel to further stimulation with nicotine, ethanol, ATP, GalA-MRPs (Lyall et al. 2007), and NGCC (Lyall et al., unpublished data). The effect of NGCC on the N+Bz CT response was not mimicked by several structurally related compounds, N-cyclopropyl E2,Z6-nonadienamide (Fig. 1, B and C), N-geranyl isobutanamide, N-geranyl 2-methylbutanamide, and allyl N-geranyl carbamate (data not shown). These results suggest that NGCC specifically binds to a site on the putative TRPV1t cation channel that does not accommodate compounds with different structural motifs.
NGCC had no effect on the CT responses to representative sweet, bitter, and non-Na+ salt stimuli (Fig. 6), suggesting that it specifically modulates salty and glutamate CT responses. In contrast to NGCC, the other TRPV1/TRPV1t modulators such as CPC, RTX, and SB modulated CT responses to NaCl, KCl, NH4Cl, and CaCl2 (Lyall et al. 2004, 2009). We hypothesize that, unlike RTX and SB, which specifically modulate the activity of TRPV1/TRPV1t, NGCC may have additional effects on taste cells that prevent us from seeing the effects of NGCC on the CT response to KCl and CaCl2. These effects may include changes in transporters or other ion channels that regulate intracellular levels of K+ and/or Ca2+ in TRCs. In this study, the effect of NGCC was tested only at one concentration of KCl and CaCl2 (100 mM). It is likely that NGCC effects can be observed at lower or higher ionic strength of these salts. However, further studies are needed to resolve this issue.
In our earlier behavior studies with NaCl solutions containing amiloride, TRPV1 KO and WT mice demonstrated similar detection thresholds for NaCl (Treesukosol et al. 2007). This suggests that there may exist other amiloride-insensitive salt transduction mechanisms in taste receptor fields other than the anterior tongue that maintain normal salt detection performance in the TRPV1 KO mice. This hypothesis is supported by the observation that rats retain the preference for NaCl even when given a choice between H2O+Bz and NaCl+Bz (Coleman et al. 2011). In WT mice and Sprague-Dawley rats, the N+Bz CT response represents ∼30% of the total NaCl CT response. The N+Bz CT response can be enhanced by as much as 100% in the presence of some TRPV1t agonists (Katsumata et al. 2008; Lyall et al. 2004), including NGCC (Fig. 1C). In behavioral studies in P (alcohol preferring) rats, addition of 0.25% GalA-MRPs (a concentration that produced a maximum increase in the N+Bz CT response) to the solutions containing 150 mM NaCl+5 μM Bz produced a significant decrease in NaCl intake and NaCl preference (Coleman et al. 2011). In NP (alcohol nonpreferring) rats, GalA-MRPs produced a small but significant decrease in NaCl intake without a change in NaCl preference (Coleman et al. 2011). These results suggest that in P rats that already demonstrate a spontaneous upregulation of TRPV1/TRPV1t further enhancing the N+Bz CT response with GalA-MRPs alters NaCl behavior in a predictable manner.
The amiloride-insensitive salt taste receptors are the predominant transducers of salt taste in humans (Feldman et al. 2003; Ossebaard and Smith 1995). In human taste test studies, at low salt concentration 5 and 10 μM NGCC produced significantly higher salt taste quality ratings relative to control and the intensity decreased at 25 μM (Fig. 7, A and B). These results suggest that in humans NGCC also produces a biphasic effect on the salt taste. In humans the NGCC-salt taste intensity response curve (Fig. 7, A and B) is shifted slightly to the right on the NGCC concentration axis relative to the rat CT response curve (Fig. 1C). This difference may be due to a slight variation between human and rodent TRPV1t or in their regulation by intracellular effectors (Lyall et al. 2009, 2010a, 2010b). This suggests that a component of the amiloride-insensitive salt taste in humans may also be dependent upon the putative TRPV1t-salt taste receptor. Consistent with this notion, in a recent study (Hochheimer et al. 2012) the presence of TRPV1 mRNA was shown in human taste cell lines from lingual epithelium by RT-PCR, and TRPV1 expression has been observed immunohistochemically in primary afferents but also in oral epithelial cells in the rat tongue (Kido et al. 2003).
Similar to TRPV1, the putative TRPV1t-dependent salt taste receptor integrates the effect of multiple stimuli to produce additive effects on the N+Bz CT responses (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007) and is regulated by the same intracellular signaling intermediates that also regulate TRPV1 (Lyall et al. 2009, 2010a, 2010b). Decreasing TRC Ca2+ levels and cell membrane phosphatidylinositol 4,5-bisphosphate (PIP2) levels enhanced the effect of TRPV1t agonists on the CT response to NaCl+Bz and also decreased the inhibition in the CT response at high agonist concentrations (Lyall et al. 2009, 2010a, 2010b). Genetically induced alcohol preference in P rats or forced chronic alcohol exposure in NP rats elevated the expression of a putative TRPV1t-dependent Bz-insensitive salt taste receptor in the fungiform taste receptive field and increased the responsiveness of the CT nerve to salt taste stimuli in the absence and presence of TRPV1t modulators, with a leftward shift in the agonist concentration versus the magnitude of the N+Bz CT response (Coleman et al. 2011). It is likely that a combination of different strategies may be required to enhance the effectiveness of NGCC in human salt sensory evaluations.
In our human sensory evaluations 25 or 45 μM NGCC dissolved in water produced unpleasant umami taste but no pungency. However, at higher concentrations (>100 μM), NGCC is described by humans as pungent. NGCC modulates the putative TRPV1t-dependent N+Bz CT response and is, therefore, most likely also an agonist of TRPV1. However, glycoconjugated peptides modulate TRPV1 activity (Rhyu et al. 2011) but are not pungent (Katsumata et al. 2008). It is suggested that TRPV1 agonists that have high lipophilicity and are easily broken down in normal aqueous conditions have less accessibility to nociceptors (Iida et al. 2003). In addition, slow kinetics of TRPV1 agonists were previously found to be associated with stronger potencies as TRPV1-desensitizing agents, which in turn are usually associated with lower pungency and stronger antihyperalgesic activity (De Petrocellis et al. 2011).
We have recently tested the effect of NGCC on human embryonic kidney (HEK293) cells expressing cloned human TRPV1, using calcium imaging. NGCC enhanced the Ca2+ influx in HEK293 cells in a dose-dependent manner. Ca2+ influx in HEK293 cells was inhibited by ruthenium red and capsazepine (M. Rhyu, unpublished observations). We also tested the effect of NGCC on HEK293 cells expressing human α-, β-, and γ-ENaC subunits, using potential-sensitive dyes. NGCC did not induce changes in the cell membrane potential, suggesting that NGCC does not activate human ENaC (M. Rhyu, unpublished observations). These results suggest that in humans NGCC enhances salt taste intensity by specifically modulating TRPV1/TRPV1t activity.
Modulation of umami responses by NGCC.
Similar to MRPs conjugated with xylose (Xyl-MRPs) (Katsumata et al. 2008), at the concentrations (>5 μM) that inhibit the N+Bz CT response NGCC enhanced the CT responses to glutamate (Fig. 4A, Fig. 5A). NGCC increased the CT response to glutamate in a dose-dependent manner, giving a maximum enhancement in the glutamate CT response between 40 and 60 μM (Fig. 4A). At 100 μM, NGCC produced the same enhancement in the glutamate CT response as 1 mM IMP, a known umami taste enhancer. Thus NGCC is a more potent modulator of umami taste than IMP. Stimulation of the tongue with MSG+IMP+NGCC did not produce a further increase in the CT response relative to MSG+IMP (Fig. 4B). This indicates that in the presence of IMP (1 mM) and NGCC (100 μM) the umami receptor (T1R1+T1R3) is maximally activated, suggesting that IMP and NGCC bind to the same site on the receptor. In human sensory evaluations, NGCC enhanced umami taste (Fig. 7C) at the concentrations that produced a maximum enhancement in the glutamate CT response (Fig. 4A, Fig. 5A). These data strongly suggest that, like IMP (Shigemura et al. 2009; Yamaguchi 1967; Yoshii et al. 1986), NGCC also modulates umami taste in humans by interacting with the T1R1+T1R3 umami taste receptor.
To elucidate how NGCC may interact with the TRPV1 channel and umami taste receptor, we constructed homology models of TRPV1 channel and mouse T1R1/T1R3 (mT1R1/mT1R3) receptor based on the crystal structure templates Kv1.2_2.1 channel chimera (PDB: 2R9R) and metabotropic glutamate receptor (PDB: 1EWK), respectively. The refined TRPV1 channel and mT1R1/mT1R3 model structures were used for molecular docking of NGCC with the GOLD molecular docking program. The docking results suggest that NGCC shares the same binding regions with RTX in the TRPV1 channel and with IMP (umami enhancer) in the mT1R1 receptor (M. Cui, X. Meng, and V. Lyall, unpublished observations).
In our studies NGCC maximally enhanced the Bz-insensitive NaCl CT responses in rats between 2.5 μM and 5 μM and human salt taste perception between 5 μM and 10 μM. Similarly, NGCC maximally enhanced the rat CT response to glutamate between 40 μM and 60 μM and enhanced umami taste in humans at 45 μM. These data suggest that there is close correspondence between the effects of NGCC on the rat and human taste responses to NaCl and glutamate. However, we did not perform similar detailed experiments with NGCC in WT mice. Therefore, at present it is not clear whether there are species differences between WT mice, rats, and humans with respect to NGCC-induced changes in salty and umami taste responses.
It is suggested that glutamate is detected by multiple receptors and transduction pathways (Chaudhari et al. 1996, 2000; DeSimone et al. 2012; Yasumatsu et al. 2011; Yasuo et al. 2008). The candidate umami receptors include a variant of brain-expressed metabotropic glutamate receptor 4 (taste mGluR4), a heteromer T1R1+T1R3, and a variant of the type 1 metabotropic glutamate receptor that has a truncated NH2-terminal domain (truncated mGluR1). The mouse T1R1+T1R3, heterologously expressed in HEK cells, responds to many amino acids. In contrast, the human-type heteromer preferentially responds to glutamate and its response is synergistically enhanced by IMP (Li et al. 2002; Nelson et al. 2002). Downstream from the receptor, the signal transduction pathway seems to be common for the transduction of umami, sweet, and bitter taste and involves the G protein Gα-gustducin or Gαi, PLCβ2, type III inositol 1,4,5-trisphosphate (IP3R3), and TRPM5 (for a review see Yasuo et al. 2008).
The umami-responsive single CT fibers in WT mice were classified into sucrose-best responsive S-type and umami-best responsive M-type fibers. S-type fibers showed a large synergistic effect on responses to MSG or monopotassium glutamate (MPG) when IMP was present. More recent studies (Yasumatsu et al. 2011) suggest that the CT response to MPG is carried by single fibers of two major types, sucrose-best (S) and MPG-best (M) fibers. In addition, each fiber type has two subtypes: one type shows synergism between MPG and IMP (S1, M1), and the second type shows no synergism. T1R3 and TRPM5 KO mice demonstrate the absence of only S1-type fibers, while the other fiber types (S2, M1 and M2) are still present in these KO mice. Yasumatsu et al. (2011) further demonstrated that mGluR antagonists selectively suppressed MPG responses of M1 and M2 types. These data suggest that T1R3 KO mice lack the signals elicited by MSG in the anterior tongue that are not umami specific and may be similar to those elicited by sweet compounds (S-type cell and fibers).
In several studies, mice lacking intracellular signaling effectors such as Gα-gustducin, PLCβ2, IP3R3, and TRPM5 did not show large deficits in the behavioral and CT responses to umami taste stimuli (Damak et al. 2006; He et al. 2004; Hisatsune et al. 2007; Talavera et al. 2005). Taken together, the above results suggest that in the anterior tongue two pathways are involved in umami taste transduction. One pathway involves T1R3, Gα-gustducin, IP3R3, and TRPM5, and the second pathway is independent of the above intracellular signaling molecules (Yasuo et al. 2008). Increasing TRC Ca2+ inhibited the NGCC-induced enhancement of the CT response to glutamate (Fig. 5A) but had no effect on the CT response to glutamate alone or on the IMP- and IMP+NGCC-induced enhancement of the glutamate CT response (Fig. 5B). These results suggest that IMP and NGCC bind to different sites on the glutamate receptor. NGCC seems to bind to a site that also competes for Ca2+ binding. These results suggest that Ca2+-dependent and Ca2+-independent transduction mechanisms are involved in umami taste transduction (DeSimone et al. 2012). We speculate that in our study the Ca2+-independent CT response to glutamate may arise from its interactions with mGluRs.
In summary, the results presented in this report suggest that NGCC, depending upon its concentration, serves as a salt taste modifier or an umami taste enhancer. NGCC modulates the Bz-insensitive NaCl CT responses in rodents by interacting with the putative TRPV1t-dependent salt taste receptor. NGCC functions as a salt taste enhancer over a narrow concentration range as determined by neural responses in rodents and in human taste sensory evaluation. At higher concentrations NGCC behaves as a salt taste suppressor. However, at these concentrations it enhances CT responses to glutamate and increases umami taste intensity in humans. An increase in TRC Ca2+ inhibits the NGCC-induced increase in the glutamate CT response but not the synergistic effects of IMP on the glutamate response. These data suggest that NGCC modifies glutamate responses by presumably binding to the T1R1+T1R3 receptor. The Ca2+-independent glutamate neural responses in the absence of NGCC and IMP may occur via a PLCβ2- and TRPM5-independent mechanism, presumably by its interaction with mGluRs.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DC-005981 (V. Lyall), DC-000122 (V. Lyall), and DC-011569 (V. Lyall), NIH Grant S10RR-027411 (M. Cui), a Korea Food Research Institute (KFRI) grant (V. Lyall) and International Flavors & Fragrances (J. A. DeSimone). The computations were supported by the Center for High Performance Computing and Institute for Structural Biology and Drug Discovery at Virginia Commonwealth University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L.D., J.A.D., and V.L. conception and design of research; M.L.D., M.-R.R., J.A.D., and V.L. interpreted results of experiments; M.L.D., M.C., M.-R.R., J.A.D., and V.L. edited and revised manuscript; M.L.D., T.-H.T.P., X.M., M.C., S.M., M.-R.R., J.A.D., and V.L. approved final version of manuscript; T.-H.T.P., Z.R., X.M., M.C., S.M., M.-R.R., and V.L. performed experiments; T.-H.T.P., Z.R., X.M., M.C., S.M., M.-R.R., J.A.D., and V.L. analyzed data; M.C., M.-R.R., J.A.D., and V.L. prepared figures; J.A.D. and V.L. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gerard L. Heck for the technical help with the CT nerve recordings.
REFERENCES
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 16: 3817–3826, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113–119, 2000 [DOI] [PubMed] [Google Scholar]
- Coleman J, Williams A, Phan TH, Mummalaneni S, Melone P, Ren ZJ, Zhou H, Mahavadi S, Murthy KS, Katsumata T, DeSimone JA, Lyall V. Strain differences in the neural, behavioral, and molecular correlates of sweet and salty taste in naive, ethanol- and sucrose-exposed P and NP rats. J Neurophysiol 106: 2606–2621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006 [DOI] [PubMed] [Google Scholar]
- Davis JB, Smart D, Gunthorpe MJ. The vanilloid receptor and vanilloid receptor-like genes: a hot topic getting hotter. Celltransmissions 18: 3–9, 2002 [Google Scholar]
- De Petrocellis L, Guida F, Moriello AS, De Chiaro M, Piscitelli F, de Novellis V, Maione S, Di Marzo V. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the TRPV1 receptor and anti-hyperalgesic activity. Pharmacol Res 63: 294–299, 2011 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V, Heck GL, Phan TH, Alam RI, Feldman GM, Buch RM. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH4Cl chorda tympani responses. J Neurophysiol 86: 2638–2641, 2001 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol 291: G1005–G1010, 2006 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Amiloride-sensitive ion channels. In: The Senses: A Comprehensive Reference, Vol. 4, Olfaction and Taste, edited by Firestein S, Beauchamp G. San Diego, CA: Academic, 2008, p. 281–288 [Google Scholar]
- DeSimone JA, Phan TH, Ren ZJ, Mummalaneni S, Lyall V. Changes in taste receptor cell [Ca2+]i modulate chorda tympani responses to bitter, sweet, and umami taste stimuli. J Neurophysiol 108: 3221–3232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewis ML, DeSimone JA, Phan TH, Heck GL, Lyall V. Effect of N-geranyl cyclopropylcarboxamide (NGCC) on TRPV1 variant salt taste receptor (TRPV1t) (Abstract). Chem Senses 31: A105, 2006 [Google Scholar]
- Feldman GM, Mogyorósi A, Heck GL, DeSimone JA, Santos CR, Clary RA, Lyall V. Salt-evoked lingual surface potential in humans. J Neurophysiol 90: 2060–2064, 2003 [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 46: 133–149, 2004 [DOI] [PubMed] [Google Scholar]
- Haddy FJ, Pamnani MB. Role of dietary salt in hypertension. J Am Coll Nutr 14: 428–438, 1995 [DOI] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24: 7674–7680, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem 282: 37225–37231, 2007 [DOI] [PubMed] [Google Scholar]
- Hochheimer A, Krohn M, Zinke H. Endogenous gustatory response and properties of immortalized human taste cell lines from lingual epithelium (Abstract). 35th Annual AChemS Meeting Program P125, 2012 [Google Scholar]
- Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y. Sour taste responses in mice lacking PKD channels. PLoS One 6: e20007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Moriyama T, Kobata K, Morita A, Murayama N, Hashizume S, Fushiki T, Yazawa S, Watanabe T, Tominaga M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 44: 958–967, 2003 [DOI] [PubMed] [Google Scholar]
- Kahan S. Commentary. Am Coll Physicians Journal Club 156: 4–5, 2012 [Google Scholar]
- Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis 49: 59–75, 2006 [DOI] [PubMed] [Google Scholar]
- Katsumata T, Nakakuki H, Tokunaga C, Fujii N, Egi M, Phan TH, Mummalaneni S, DeSimone JA, Lyall V. Effect of Maillard reacted peptides on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t). Chem Senses 33: 665–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido MA, Muroya H, Yamaza T, Terada Y, Tanaka T. Vanilloid teceptor expression in the rat tongue and palate. J Dent Res 82: 393–397, 2003 [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S. Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J Comput Aided Mol Des 25: 317–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, DeSimone JA, Feldman GM. Effect of osmolarity on taste receptor cell size and function. Am J Physiol Cell Physiol 277: C800–C813, 1999 [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, DeSimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses 30, Suppl 1: i42–i43, 2005a [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+ flux. J Gen Physiol 125: 569–585, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol 125: 587–600, 2005c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Pasley H, Phan TH, Mummalaneni S, Heck GL, Vinnikova AK, DeSimone JA. Intracellular pH modulates rat taste receptor cell volume and the phasic part of the chorda tympani response to acid stimulation. J Gen Physiol 127: 15–34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol 98: 1662–1674, 2007 [DOI] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the benzamil-insensitive salt taste receptor by intracellular Ca2+, protein kinase C, and calcineurin. J Neurophysiol 102: 1591–1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Ren ZJ, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the amiloride-insensitive NaCl chorda tympani responses by phosphatidylinositol 4,5-bisphosphate. J Neurophysiol 103: 1337–1349, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Melone P, DeSimone JA. Differential regulation of chorda tympani (CT) taste nerve responses to sweet, salty, bitter and umami taste stimuli by phosphatidylinositol 4,5-bisphosphate (PIP2) (Abstract). Chem Senses 35: A63, 2010b [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Descriptive analysis techniques. In: Sensory Evaluation Techniques . Boca Raton, FL: CRC, 1987, p. 136–411 [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002 [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses 20: 37–46, 1995 [DOI] [PubMed] [Google Scholar]
- Rhyu MR, Song A, Abe K, Lyall V. Effect of Kokumi taste active peptides on amiloride-insensitive salt taste preference in C57BL/6J mice (Abstract). Chem Senses 34: A41–A42, 2009 [Google Scholar]
- Rhyu M, Lee BH, Kim YH, Song AY, Son HJ, Oh SB, Lyall V, Kim EY. Soy-derived glycopeptides induce inward current in TRPV1-expressing cells by whole-cell patch-clamp recording (Abstract). Chem Senses 31: A40, 2011 [Google Scholar]
- Shigemura N, Shirosaki S, Sanematsu K, Yoshida R, Ninomiya Y. Genetic and molecular basis of individual differences in human umami taste perception. PLoS One 4: e6717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review). Am J Hypertens 24: 843–853, 2011 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R1799–R1809, 2007 [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- Wenner M. Magnifying taste. Sci Am 299: 96–99, 2008 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J Food Sci 32: 473–478, 1967 [Google Scholar]
- Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol 590: 1155–1170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull 31: 1833–1837, 2008 [DOI] [PubMed] [Google Scholar]
- Yoshii K, Yokouchi C, Kurihara K. Synergistic effects of 5′-nucleotides on rat taste responses to various amino acids. Brain Res 367: 45–51, 1986 [DOI] [PubMed] [Google Scholar]
- Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA 105: 20930–20934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]