Abstract
Deep brain stimulation (DBS) in the internal segment of the globus pallidus (GPi) relieves the motor symptoms of Parkinson's disease, yet the mechanism of action remains uncertain. To address the question of how therapeutic stimulation changes neuronal firing in the human brain, we studied the effects of GPi stimulation on local neurons in unanesthetized patients. Eleven patients with idiopathic Parkinson's disease consented to participate in neuronal recordings during stimulator implantation surgery. A recording microelectrode and a DBS macroelectrode were advanced through the GPi in parallel until a single neuron was isolated. After a baseline period, stimulation was initiated with varying voltages and different stimulation sites. The intra-operative stimulation parameters (1–8 V, 88–180 Hz, 0.1-ms pulses) were comparable with the postoperative DBS settings. Stimulation in the GPi did not silence local neuronal activity uniformly, but instead loosely entrained firing and decreased net activity in a voltage-dependent fashion. Most neurons had decreased activity during stimulation, although some increased or did not change firing rate. Thirty-three of 45 neurons displayed complex patterns of entrainment during stimulation, and burst-firing was decreased consistently after stimulation. Recorded spike trains from patients were used as input into a model of a thalamocortical relay neuron. Only spike trains that occurred during therapeutically relevant voltages significantly reduced transmission error, an effect attributable to changes in firing patterns. These data indicate that DBS in the human GPi does not silence neuronal activity, but instead disrupts the pathological firing patterns through loose entrainment of neuronal activity.
Keywords: basal ganglia, entrainment, Parkinson's disease, neuronal inhibition
deep brain stimulation (DBS) is a powerful therapeutic tool in the treatment of intractable neurological diseases and movement disorders (Aouizerate et al. 2004; Laxton et al. 2010; Mayberg et al. 2005). For patients suffering from Parkinson's disease, DBS improves quality of life and motor function after medications lose efficacy or side-effects become intolerable. The most common stimulation targets for Parkinson's disease are motor-related nuclei within the thalamus and basal ganglia, including the subthalamic nucleus (STN) and globus pallidus internus (GPi) (Ghika et al. 1998; Siegfried and Lippitz 1994). Although the locations of efficacious clinical targets are now well established, the therapeutic mechanisms of action are still not fully understood (Benazzouz and Hallett 2000; Hammond et al. 2008; McIntyre et al. 2004; Montgomery and Baker 2000). The expanding range of uses of DBS and increasing number of patients with implants emphasize the need to understand how stimulation produces its therapeutic effects to improve existing therapies and develop new treatments.
In the treatment of Parkinson's disease, the symptomatic relief from stimulation of the GPi is similar to that of an ablative lesion, and so, inhibition of local neuronal activity was proposed as the mechanism of action for DBS (Bergman et al. 1990; Limousin et al. 1995; Siegfried and Lippitz 1994). This idea was supported by observations that firing rates for GPi neurons are pathologically increased in parkinsonian states and that high-frequency stimulation using microelectrodes suppresses somatic activation of local neurons (Boraud et al. 1996; Dostrovsky et al. 2000; Filali et al. 2004; Lafreniere-Roula et al. 2010). However, the idea of complete inhibition has been challenged by recent observations of sustained or even increased neuronal firing of local GPi neurons during therapeutic stimulation. When using DBS macroelectrodes, stimulation in the GPi of a parkinsonian nonhuman primate produces complex patterns of neuronal entrainment relative to the stimulus pulse (Bar-Gad et al. 2004; McCairn and Turner 2009). Additionally, therapeutically effective stimulation in the STN, an area that sends excitatory projections to the GPi, produces similar firing patterns of GPi neurons (Hashimoto et al. 2003; Moran et al. 2011). Thus rather than simply silencing GPi activity, the therapeutic action of DBS in basal ganglia targets may involve entrainment or regularization of neuronal firing (Garcia et al. 2005a; Guo et al. 2008; Hahn et al. 2008; Meissner et al. 2005; Montgomery and Baker 2000; Rubin and Terman 2004). The idea of blocking pathological patterns through increased regularity is supported by observations that irregular high-frequency stimulation is less therapeutically efficacious than temporally regular stimulation in reducing bradykinesia and tremor (Birdno et al. 2012; Dorval et al. 2010).
These results from primate models suggest that therapeutic DBS in humans likely does not simply silence GPi neurons, although significant physiological differences often exist between animal models and true human disease states. To complement work in animal models, we evaluated how DBS in the human GPi changed firing of local neurons in unanesthetized patients with Parkinson's disease. Stimulation with DBS macroelectrodes decreased somatic firing and entrained activity of local neurons, and these changes in firing were sufficient to reduce error in a model of thalamocortical (TC) firing.
METHODS
Patient consent.
Eleven patients receiving a bilateral DBS implant in the GPi as treatment for Parkinson's disease consented to participate in this study, which was approved by the local Institutional Review Board (Oregon Health & Science University Institutional Review Board #6169). The standard surgical procedure uses two neuronal recordings during implantation to verify correct stimulator placement within the boundaries of the GPi. Participation in the research study added ∼15–30 min to the normal duration of the DBS implantation surgery.
Intraoperative recordings.
The standard stimulator implantation surgery is done with only local anesthesia and consists of placing the stimulating electrode (Activa Therapy model 3387 lead; Medtronic, Minneapolis, MN) into the GPi using predetermined stereotaxic coordinates, based on the Schaltenbrand and Wahren (1977) stereotactic atlas. Coordinates within the GPi were chosen using MRI with FrameLink Stereotaxic Linking System 4.1.9 (Medtronic), and patients were placed in a Leksell Frame G stereotaxic frame (Elekta AB, Stockholm, Sweden) for electrode guidance. Bilateral burr holes were drilled under local anesthesia; no study patients received intraoperative narcotics or sedation prior to the recordings. After the dura was opened, two microelectrodes (320–700 kΩ, 2 mm separation; FHC, Bowdoin, ME) were advanced through the introducer cannula until a clear transition from the external globus pallidus (GPe) to the GPi was identified. This transition generally occurs >5 mm from the ventral border of the GPi. The center (target) microelectrode was then replaced with the low-impedance DBS macroelectrode (∼4 kΩ), with contact 0 at the same depth as the microelectrode (Fig. 1). Based on the DBS electrode radius of 0.635 mm, the surface of DBS electrode contact 0 was 1.365-mm away from the tip of the remaining recording microelectrode (Carlson et al. 2009).
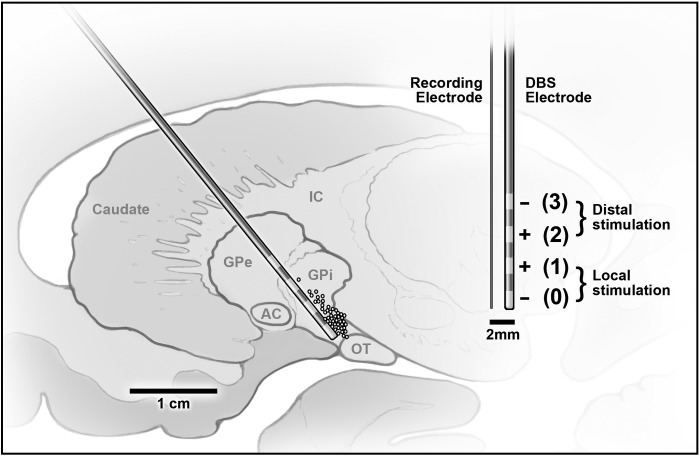
Fig. 1.
Left: map of the globus pallidus internus (GPi) and surrounding regions, with the estimated locations of recorded neurons (circles) and the deep brain stimulation (DBS) electrode trajectory into the GPi. Approximate locations of cells are plotted as circles, with cell positions estimated by the depth of the electrode pair relative to the stereotaxically determined ventral edge of GPi. Since recording sessions were not started until the electrode approached the target (the base of GPi), most of the recordings are clustered at the ventral edge of GPi. AC, anterior commissure; GPe, globus pallidus externus; IC, internal capsule; OT, optic tract. Right: The DBS and the recording electrodes are pictured to scale, showing the distance between the tip of the recording electrode and the DBS contacts.
Standard microelectrode recording/mapping of the GPi was carried out using a MicroGuide Pro microrecording system (Alpha Omega, Nazareth Illit, Israel). The DBS/microelectrode pair was advanced in micron increments, until the stable waveform of a single GPi neuron was distinguished. Baseline firing was recorded for at least 60 s, and then stimulation (88–180 Hz, 0.1-ms pulse width, 1–8 V) was applied through the DBS electrode using a handheld portable stimulator device (Medtronic Navigation, Louisville, CO). The majority of trials was centered on 100 Hz as the stimulation frequency, although the stimulation trials for one patient were conducted at 180 Hz (Table 1). Bipolar stimulation (5–25 s) was applied either locally, with the pair of contacts closest to the recording electrode (contact 0 as cathode, contact 1 as anode; referred to as “local stimulation”), or distally, with the pair farthest from the tip of the recording electrode (contact 2 as anode, contact 3 as cathode; referred to as “distal stimulation”; Fig. 1). For each cell, a number of trials at different voltages for either local or distal stimulation were performed, with at least 30 s between trials. Because of limitations and variability in human neuronal recordings and the artifact removal process, usable data were not available for all stimulation conditions for all neurons. In the occasional case where the same stimulation condition was applied more than once to the same neuron, the averaged response for the two trials was incorporated into the analysis. The electrode pair was advanced until encountering the ventral border of the GPi, which was identified from stereotaxic coordinates and characteristics of the microelectrode recording.
Table 1.
Patient intra- and postoperative stimulation parameters
| Intraoperative Stimulation Settings |
Postoperative Stimulation Settings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Gender | Recording Site | Number of Cells | Voltage | Frequency | Pulse Width | Contacts | Voltage | Frequency | Pulse Width | Contacts |
| 1 | F | Left GPi | 2 | 2–8 V | 90 Hz | 100 μs | 0−, 1+ or 2+, 3− | Follow-up unavailable | |||
| 2 | M | Left GPi | 2 | 1–8 V | 90 Hz | 100 μs | 0−, 1+ or 2+, 3− | 2.5 V | 180 Hz | 60 μs | 3−, Case+ |
| 3 | M | Left GPi | 3 | 1–8 V | 90 Hz | 100 μs | 0−, 1+ or 2+, 3− | Follow-up unavailable | |||
| 4 | M | Left GPi | 1 | 4–8 V | 90 Hz | 100 μs | 0−, 1+ or 2+, 3− | Follow-up unavailable | |||
| 5 | F | Left GPi | 2 | 1–8 V | 180 Hz | 100 μs | 0−, 1+ or 2+, 3− | 3.5 V | 180 Hz | 90 μs | 3−, Case+ |
| Right GPi | 3 | 1–8 V | 180 Hz | 100 μs | 0−, 1+ or 2+, 3− | 5.0 V | 180 Hz | 90 μs | 2−, Case+ | ||
| 6 | M | Left GPi | 6 | 1–8 V | 112 Hz | 100 μs | 0−, 1+ or 2+, 3− | 4.5 V | 185 Hz | 90 μs | 2−, 3+ |
| 7 | M | Left GPi | 5 | 1–8 V | 100 Hz | 100 μs | 0−, 1+ or 2+, 3− | 4 V | 185 Hz | 90 μs | 3−, Case+ |
| 8 | F | Left GPi | 5 | 2–8 V | 100 Hz | 100 μs | 0−, 1+ or 2+, 3− | 2.5 V | 140 Hz | 90 μs | 3−, Case+ |
| 9 | M | Left GPi, Right GPi | 8 | 2–8 V | 100 Hz | 100 μs | 0−, 1+ or 2+, 3− | Follow-up unavailable | |||
| 10 | M | Left GPi | 6 | 2–8 V | 88 Hz | 100 μs | 0−, 1+ or 2+, 3− | 2.5 V | 185 Hz | 60 μs | 1−, 0+ |
| 11 | F | Left GPi | 2 | 2–8 V | 100 Hz | 100 μs | 0−, 1+ or 2+, 3− | 4.6 V | 185 Hz | 60 μs | 3−, 2+ |
GPi, globus pallidus internus.
After neuronal recording and placement of the DBS electrode, the locations of the recording and DBS electrodes were verified as being on target using intraoperative fluoroscopy. For each cell, the relative distance of the DBS electrode from the ventral border of the GPi (the stereotaxic target) was recorded at the end of each procedure.
Spike sorting and artifact removal.
Neuronal activity was displayed in real time and simultaneously recorded for offline analysis. Recordings were digitized at 15 kHz and stored as Alpha Omega systems files. Spike sorting and subsequent analyses were performed using Spike2 (CED, Cambridge, UK) and MATLAB (MathWorks, Natick, MA). Stimulation artifacts were apparent in most recordings, and these were detected and removed (Erez et al. 2010). Briefly, designated periods of the stimulation artifact were used to design an artifact template that was then subtracted from the original recording at the start of each stimulation impulse (Fig. 2A). The resulting signal often included a period at the beginning of the stimulation artifact, where complete amplifier saturation prevented recovery of the original signal; this unusable period was recognized and measured by the software. The mean duration of the unusable period was noted for each stimulation trial, and calculations were adjusted accordingly.
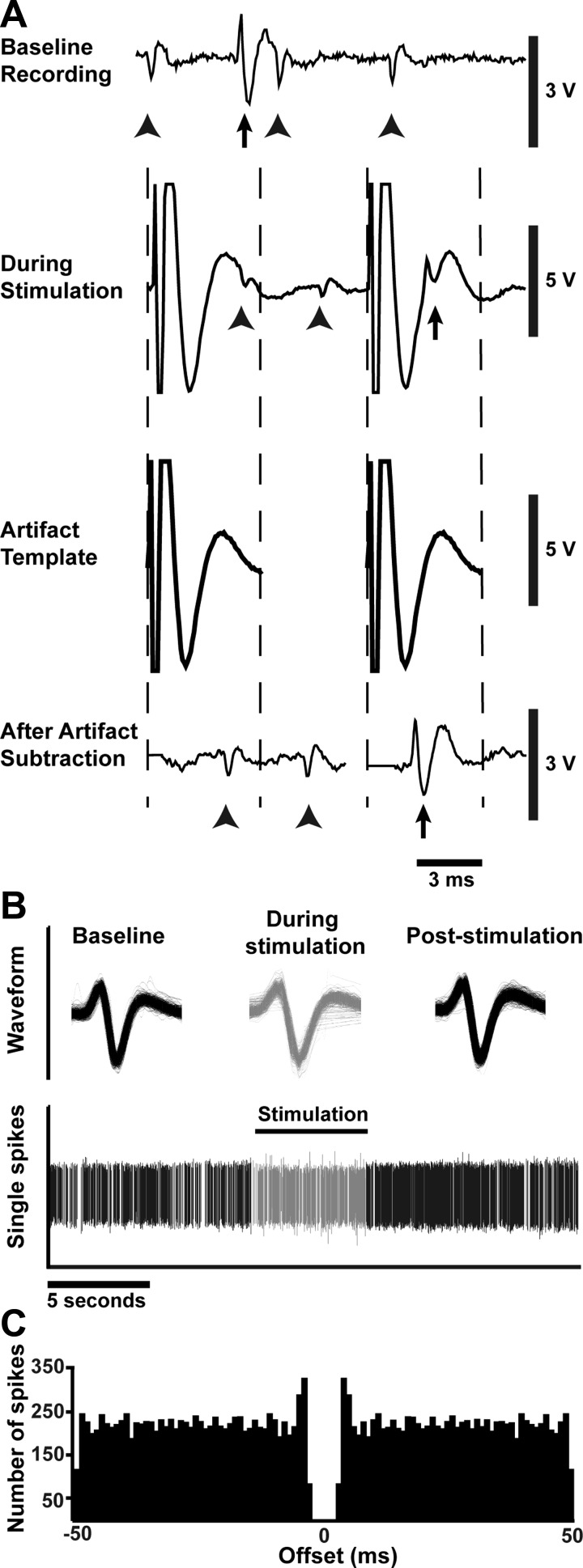
Fig. 2.
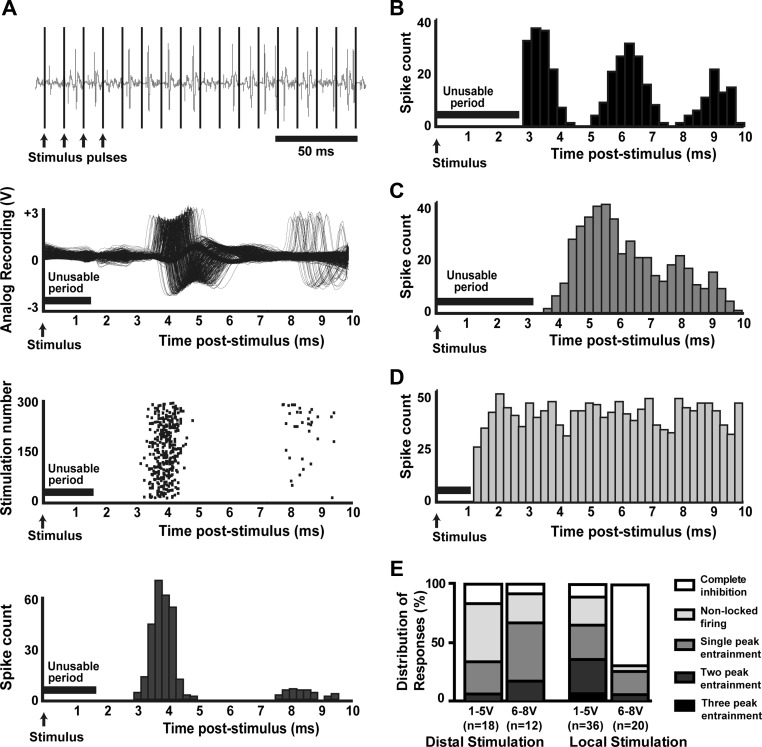
Template subtraction, spike sorting, and waveform analyses allow individual action potentials to be discerned during stimulation with a high degree of confidence. A: in an example of artifact removal, at baseline, 2 distinct action potential waveforms are distinguishable (arrows and arrowheads indicate the 2 waveforms). During stimulation, a large artifact prevents distinguishing neuronal activity or matching action potentials to previously identified waveforms, but after subtraction, waveforms are again distinguishable. B: action potential waveforms are overlaid to show continuity of recording from a single neuron after artifact removal throughout the entire stimulation trial. Spikes recorded before and after stimulus train are shown in black; those during the stimulus train in gray. C: in this example autocorrelogram, the refractory period around time 0 and surrounding short-term peaks indicate that only a single neuron was under consideration during the recording (Bar-Gad et al. 2001; Montgomery 2006).
Following artifact removal, individual action potentials were sorted using waveform template-matching to confirm the continuity of a single cell recording before, during, and after stimulation (Fig. 2B). After satisfactory spike sorting, an autocorrelogram was generated for every neuronal recording using MATLAB to verify the presence of only a single neuron (Fig. 2C). The presence of the refractory period and the accompanying short-term peak indicates that only a single neuron was under consideration for a given period (Bar-Gad et al. 2001; Montgomery 2006). Forty-five neurons and 186 stimulation trials from 11 patients were of sufficiently high quality to ensure that a single neuron was recorded throughout. Only these data were used in subsequent analyses.
Analysis of DBS-driven changes in firing rate.
Analysis of firing focused on changes in firing rate and firing pattern in response to stimulation. Mean pre- and poststimulation firing rates were calculated using the number of action potentials in the 10 s before and after the stimulation epoch, respectively. The firing rate during stimulation was measured as the cumulative number of action potentials divided by the total stimulation time and corrected for the amount of unusable time resulting from stimulation artifact removal for that neuron, which was, on average, 3.2 ± 0.16 ms or roughly 32% of the duty cycle for 100 Hz stimulation. Comparisons of firing rates before, during, and after stimulation were made using a one-way, repeated-measures ANOVA, followed by a Newman-Keuls post hoc test, with P < 0.05 considered statistically significant. Voltage-dependent decreases in firing rate for local and distal stimulation were analyzed using linear regression. The confidence interval (CI) of the slope was analyzed using an F-test to determine if the slope was significantly different than zero.
The neuronal response to stimulation during each trial was classified as no change, increased firing, decreased firing, or complete silence. This classification was determined using a t-test to compare mean firing rates in 1-s bins for 10 s at baseline of the trial with mean corrected firing rates in 1-s bins during stimulation. A value of P < 0.05 was considered a significant change in firing rate.
The characterized neuronal responses to stimulation were then separated into trials with low (1–5 V) or high (6–8 V) stimulus voltages. This division was based on therapeutic voltages reported previously (Garcia et al. 2005a) and on the effective voltages selected postoperatively for this cohort of patients, in which 1–5 V was found to be clinically relevant (Table 1). When a single neuron had multiple stimulation trials in the low-voltage range, only the lowest-voltage trial was included in the distribution analysis to avoid repeated, nonindependent values. Similarly, only the highest-voltage trial was included in the distribution analysis when multiple trials were performed in the higher-voltage range. A χ2 test was used to check for a difference in the distribution of responses between high- and low-voltage cases.
Analysis of DBS-driven changes in firing pattern.
A peristimulus time histogram (PSTH) for each trial was made by aligning action potentials relative to stimulus pulses. Entrained firing was evident in the PSTH of a subset of trials for almost all neurons; therefore, we examined the distribution of these effects based on lower (1–5 V) and higher (6–8 V) voltages.
To quantify the effect of DBS on burst-firing, a Poisson distribution of interspike intervals classified activity as random or burst-firing for pre- and poststimulus periods (Carlson et al. 2009; Kaneoke and Vitek 1996). With the use of this method, most trials showed no gross change from “bursting” to “nonbursting”, and so a more specific quantification of the time in burst-firing was made using the Poisson surprise method (Legendy and Salcman 1985). With the use of this method, the period of time that the neuron spent in burst mode was measured for the pre- and poststimulation periods in each trial, for a minimum S-value of three (S = −log probability of Poisson distribution, a measure of the likelihood of a given segment of firing being a burst). The total time in burst-firing was analyzed using a two-way, mixed-design ANOVA, with stimulus voltage (low vs. high) and time (pre- vs. poststimulation; repeated measure) as the factors. Separate analyses were undertaken for local and distal stimulation.
Simulation of TC relay-cell responses to input from GPi.
To understand the underlying therapeutic mechanism of DBS in the GPi, we modeled the effects of the changes in GPi firing on TC relay cells. This model of a TC cell receiving synaptic input from GPi neurons has been validated and described in detail elsewhere (Guo et al. 2008; Rubin and Terman 2004). Briefly, the TC cell model consists of a system of differential equations that use membrane capacitance and ionic currents to determine changes in membrane voltage and action potential initiation. GPi neuronal activity is integrated into the model as an inhibitory synaptic input current to the TC cell. The model also includes a periodic train of excitatory synaptic inputs to the TC cell.
For each recording of a GPi neuron, the prestimulation, during, and poststimulation firing times of each trial were entered separately into the simulation. Trials with no GPi spikes were excluded, as these trivially give perfect relay, and thus the model is not informative for such cases. The output variable from this analysis was the error index, computed as the fraction of excitatory inputs to which the TC cell failed to generate an action potential or responded with excessive spiking. Lower error indices are associated with greater relay fidelity and have been shown to correspond to an enhanced therapeutic benefit (Guo et al. 2008; Rubin and Terman 2004). The mean effect of stimulation on the error index was compared for each set of stimulation voltages and contact pairs using repeated-measures ANOVA followed by a Dunnett's post hoc test of the index during stimulation vs. its baseline value.
RESULTS
For each patient, correct stereotaxic placement of the DBS electrode was confirmed using microelectrode recordings and intraoperative X-ray. With final placement of the DBS electrode, the approximate position of each cell was recorded relative to the stereotaxic target, which was the ventral base of the GPi. The majority of cells was clustered along the ventral end of the GPi, with no cells likely recorded from the GPe (Fig. 1).
DBS depressed but did not uniformly silence GPi neuronal firing.
Of the cumulative recordings, 45 neuronal recordings and 186 stimulus trials were of sufficient quality to ensure that a single neuron was monitored throughout an entire recording session. Of these trials, 137 were in the local configuration (contact 0 as cathode, contact 1 as anode) and 49 were in the distal configuration (contact 3 as cathode, contact 2 as anode).
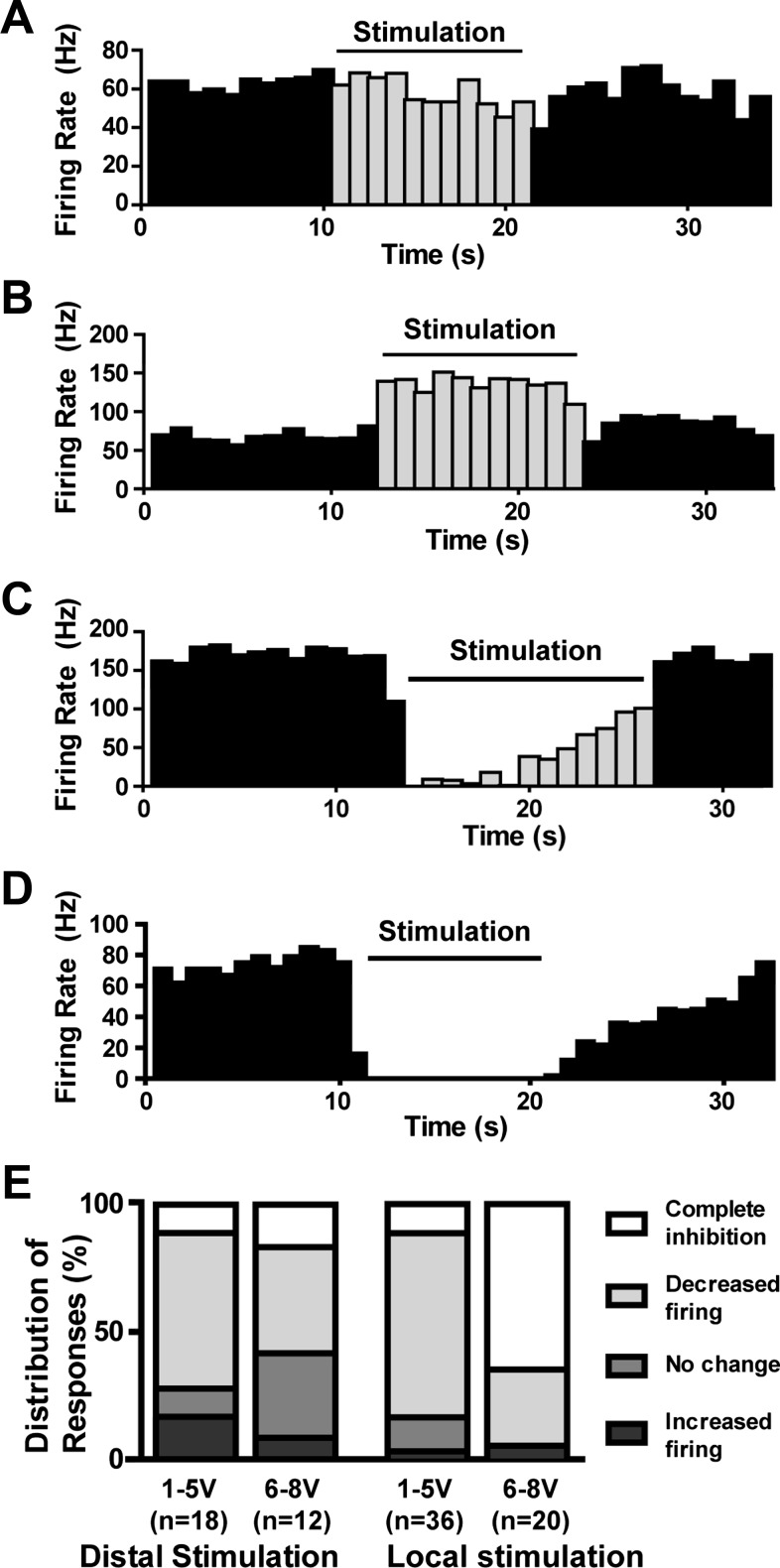
The effects of DBS on firing rate in individual trials ranged from an increase in firing rate to complete silencing (Fig. 3, A–D). The effect on each trial was classified as increased firing, partially decreased firing, complete silence, or no change in firing rate, and the distribution of effects categorized for local and distal stimulation between high and low voltages (examples in Fig. 3E). For distal stimulation, no difference was found in the distribution of responses between low- and high-voltage stimulation [χ2(3 df) = 2.7; P > 0.1]. Conversely, for local stimulation, a significant difference exists between the distribution of responses between high and low voltages [χ2(3 df) = 20.5; P < 0.001], with a greater number of cells silenced completely at high voltages.
Fig. 3.
Examples from separate neurons showing responses to local GPi stimulation. Responses ranged from (A) no change in firing rate during stimulation, (B) a significant increase in firing, (C) a significant decrease in firing, and (D) complete silence. E: the relative distributions of responses to distal and local stimulation are shown for low (1–5 V) and high (6–8 V) voltages. With distal stimulation, the most common neuronal response was decreased firing, although some cells showed no change in firing rate, and a small number of cells had a well-defined increase in firing. With low-voltage (1–5 V) local stimulation, most neurons had decreased firing but were not silenced completely. With local stimulation at higher voltages (6–8 V), most cells were silenced.
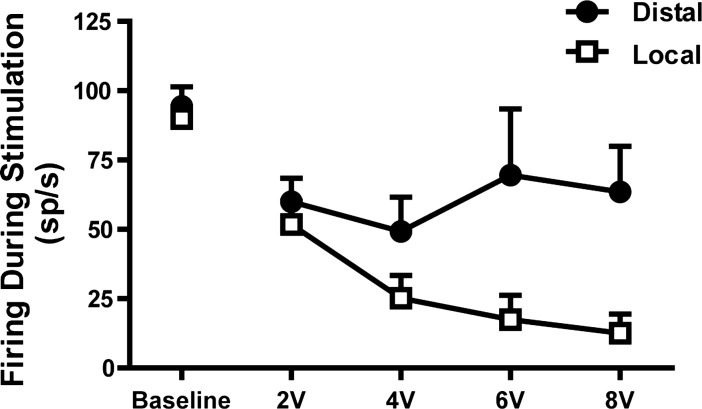
Stimulation at all voltages, both locally and distally, significantly decreased the mean firing rate (Fig. 4), compared with pre- and poststimulation rates [repeated-measures ANOVA followed by a Newman-Keuls post hoc: local stimulation, 2 V F(2,29) = 32.66, P < 0.001; 4 V F(2,30) = 87.95, P < 0.0001; 6 V F(2,17) = 36.75, P < 0.001; 8 V F(2,22) = 64.93, P < 0.001; distal stimulation: 2 V F(2,8) = 8.35, P < 0.01; 4 V F(2,15) = 15.93, P < 0.001; 6 V F(2,6) = 8.13, P < 0.01; 8 V F(2,8) = 14.58, P < 0.001]. No significant differences existed between pre- and poststimulus mean firing rates, either for local or distal stimulation.
Fig. 4.
Mean firing rate of GPi neurons at baseline and during stimulation with different voltages. Both local and distal stimulation produced significant decreases in mean firing rate compared with baseline, but only the effect of local stimulation was voltage dependent. sp/s, spikes/s. Values are means + SE.
These changes in mean firing rates with local stimulation were voltage dependent, with greater decreases at higher voltages (slope of linear regression: −9.4 Hz/V; 95% CI: −16.2 to −2.5 Hz/V; P < 0.05; Fig. 4). Distal stimulation did not show a similar voltage-dependent decrease in firing (slope of linear regression: −5.5 Hz/V; 95% CI: −10.5 to 0.4 Hz/V; P > 0.05).
Loose entrainment to stimulation occurred in the majority of human GPi neurons.
The effect of stimulation on firing pattern differed across voltages, stimulation parameters, and neurons. Changes in firing pattern were examined through PSTHs. Individual neurons and trials showed complex responses relative to the stimulus impulse, with alternating periods of increased and decreased likelihood of firing relative to each pulse (Fig. 5A). Cells often showed two distinct time periods of increased likelihood of firing separated by 1- to 2-ms periods when the cell was less likely to fire an action potential (Fig. 5A). Some cells showed other mixed responses, including fewer or more periods of increased likelihood of firing (Fig. 5, B and C), and a portion of cells did not change firing pattern (Fig. 5D). Figure 5E shows the distribution of response types with distal and local stimulation at low- and high-stimulus voltages. With distal stimulation, most cells were not entrained at lower voltages, but loose entrainment of firing was prevalent at higher voltages. With local stimulation, at lower voltages, the dominant change in pattern was entrainment, and at higher voltages, complete cessation of firing was most common.
Fig. 5.
Loosely entrained firing during local stimulation. A: when aligned along stimulus pulses (100 Hz, local stimulation), raw spike shapes from a single neuron show 2 periods of increased likelihood of firing, surrounded by periods of decreased likelihood of firing. The raster plot (3rd graph from top) and histogram (bottom graph) of these data reveal this pattern more concisely. Peristimulus time histograms demonstrated additional patterns of entrainment, including (B) 3 periods of increased likelihood of firing or (C) a single period. D: some neurons had no discernible modulation of firing pattern relative to the stimulus. E: distributions of entrainment responses to distal and local stimulation are shown for low (1–5 V) and high (6–8 V) voltages. For distal stimulation, the majority of cell responses at low voltages was not entrained to the stimulus, although at higher voltages, the proportion of cells showing entrainment increased. With local stimulation at low voltages, most cells showed some form of complex entrainment. At higher voltages with local stimulation, most cells were silent, although those that did fire showed some entrainment.
Neurons with loose entrainment showed decreased firing at the onset of the stimulus train.
In the seconds after the start of stimulation, some neurons showed a period of silence, followed by a slow increase in firing rate over the course of stimulation (example in Fig. 3C). This effect was observed predominantly in cells that also showed entrainment of firing. Without regard to voltage or contacts, cells that showed any form of entrained firing had a significantly lower average firing rate in the 1st s of stimulation compared with the last second of stimulation (respectively, 40.9 ± 5.4 Hz vs. 69.2 ± 5.4 Hz; P < 0.001, paired t-test). Cells with a flat PSTH (no entrained firing) did not have decreased firing at the start of stimulation and did not show a significant difference between rates at the start and end of stimulation (respectively, 75.7 ± 11.0 Hz vs. 82.3 ± 11.5 Hz; P = 0.62, paired t-test). Notably, by the end of the stimulus period, the firing rates of cells with entrainment were not significantly different from those of nonlocked cells (P = 0.24, unpaired t-test). Thus neurons exhibiting entrainment consistently fired fewer action potentials at the start of stimulation but resumed firing at a rate comparable with cells without entrainment by the end of the stimulus train. This effect was maintained even when the responses were subdivided into local and distal responses (data not shown).
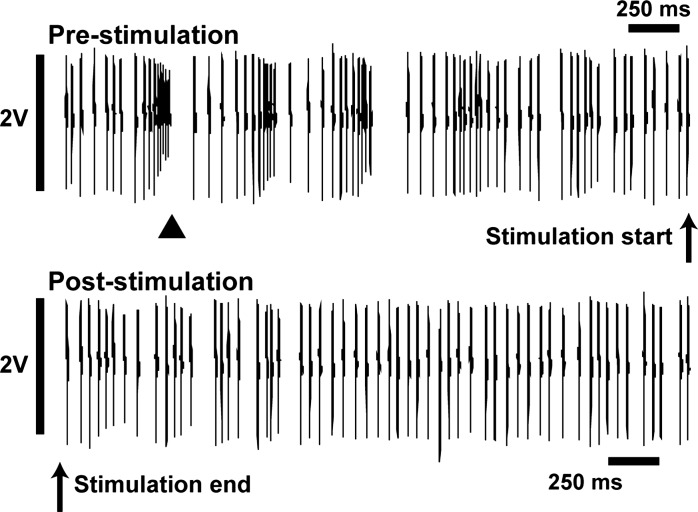
Poststimulus-bursting was decreased.
Neurons spent less time in burst-firing in the period immediately after the cessation of stimulation compared with the period of time just prior to stimulation (Fig. 6). Specific time in burst activity was quantified before and after stimulation, and these values were compared using a two-way ANOVA with time (pre- vs. poststimulation) and voltage (low vs. high) as factors. Stimulation at both local and distal contacts significantly decreased the percent of time bursting after stimulation compared with immediately before, independent of voltage. Local stimulation significantly reduced burst activity from 41.2 ± 2.2% before the onset of stimulation to 33.7 ± 1.9% immediately after the end of stimulation [F(1,119) = 15.4; P = 0.0001]. In the distal configuration, stimulation also significantly reduced the time in burst activity, from 42.6 ± 6.8% to 28.6 ± 3.7% [F(1,33) = 6.66; P = 0.015].
Fig. 6.
Raw recording showing an example of decreased burst-firing after stimulation. Prior to the onset of stimulation, this neuron showed a rapid burst of action potentials with decreasing amplitudes, followed by a short period of quiescence (arrowhead marks bursting, as detected by the Poisson surprise method). Immediately after a 14-s period of stimulation, the same cell displayed more regular firing without burst activity. These example segments were taken from 10-s epochs before and after stimulation, with mean firing rates of 67.1 Hz and 66.1 Hz, respectively.
Low-voltage DBS in GPi reduced the TC cell error index.
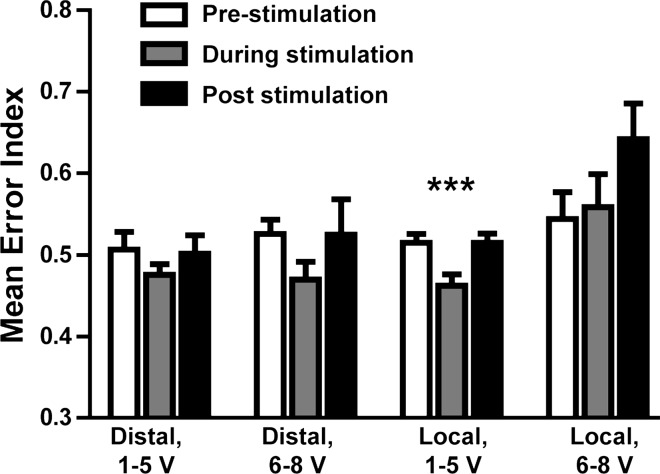
For each stimulation trial with GPi spiking, the prestimulation, during, and poststimulation spike times were used to generate an inhibitory synaptic input into a conductance-based model of a TC relay cell (Guo et al. 2008), which was also subject to a computationally generated excitatory synaptic signal. An error index was computed as a measure of the fidelity of the resulting signal transmission. When using the distal contacts, both low- and high-voltage stimulation produced a slight but not statistically significant decrease in the error index during stimulation compared with baseline values (Fig. 7). With local stimulation, lower voltages (1–5 V) produced a statistically significant decrease in the error index [F(2,48) = 3.71; P < 0.001]. Local stimulation with higher voltages (6–8 V) did not produce a significant change in the error index during stimulation but did mildly increase the error index after cessation of stimulation.
Fig. 7.
Low-voltage local stimulation significantly improves model thalamocortical (TC) cell relay. Spiking data from human recordings prior to, during, and after DBS were used as input for a model TC relay-cell subject to a periodic excitatory signal, and an error index was computed based on TC relay performance. Only local stimulation at therapeutic voltages produced a statistically significant decrease in the error index during stimulation relative to nonstimulation periods (***P < 0.001, repeated-measures ANOVA, Dunnett's post hoc test). Values are means + SE.
DISCUSSION
DBS in the GPi is thought to relieve movement-related symptoms of Parkinson's disease through effects on the local neuronal somata, fibers of passage, and other nearby neuronal elements (Benazzouz and Hallett 2000; Garcia et al. 2005a; Hammond et al. 2008; Holsheimer et al. 2000; McIntyre and Hahn 2009; Montgomery and Baker 2000; Ranck 1975). The present experiments showed that DBS in the GPi of awake, unanesthetized patients with Parkinson's disease induces changes in both firing rate and firing patterns of nearby neurons. Stimulation in the therapeutic voltage range reduced the mean neuronal firing rate, although not all neurons displayed decreased firing during stimulation. DBS also loosely entrained neuronal firing in complex patterns and reduced the time spent in burst firing immediately after the cessation of stimulation. To understand how these changes in firing could improve symptoms, the recorded spike times from human GPi were applied to a model TC cell with an excitatory input. The changes in firing associated with therapeutically relevant stimulation improved the reliability of TC relay, an effect likely related to changes in firing pattern and increased regularity.
DBS decreases the mean firing rate but does not uniformly silence GPi neurons.
In Parkinson's disease and animal models of Parkinson's disease, GPi neurons have increased firing rates, and decreasing this pathologically increased firing has been proposed as a therapeutic mechanism of action of DBS (Bergman et al. 1994; Filion and Tremblay 1991; Starr et al. 2005). Indeed, decreasing the inhibitory output from GPi correlates with therapeutic effect (Boraud et al. 1996). However, in other settings, therapeutically effective DBS can paradoxically increase the firing of GPi neurons, so decreased GPi activity may not be the only, or even primary, therapeutic mechanism of DBS (Garcia et al. 2005a; Hahn et al. 2008; Hashimoto et al. 2003; Montgomery and Baker 2000; Obeso et al. 2000; Rubin and Terman 2004). In the present experiments, local stimulation decreased the mean neuronal firing rate in a voltage-dependent manner. Although some neurons exhibited no significant change in firing or even increased firing in response to stimulation, during local stimulation, the majority of neurons had moderately decreased firing at lower voltages and complete silencing at higher voltages. These decreases in firing are similar to predicted changes in firing rates from a computational model (Johnson and McIntyre 2008), although the interpretation of these decreases in firing should take into consideration the presence of an irremovable portion of the stimulation artifact. This remaining artifact after template subtraction could still obscure some tightly entrained action potentials occurring immediately after the pulse. Therefore, the changes reported here represent lower bounds on firing and may, in fact, underestimate the firing rate during stimulation.
These observations of voltage-dependent decreases in firing compare well with previous recordings from human GPi, where increasing stimulation intensity produced greater decreases in firing of local neurons (Dostrovsky et al. 2000; Lafreniere-Roula et al. 2010). However, in prior observations, the decreased activity was in the period immediately after each stimulation pulse, and increasing the stimulation frequency completely silenced neuronal activity by connecting these short periods of inhibition. In the present work, we observed cessation of neuronal activity primarily at higher voltages, and during therapeutically relevant voltages we found modest decreases in firing in conjunction with loose entrainment. These differences may be explained by the use of recording microelectrodes for stimulation, which have a higher impedance and lower surface area (500 kΩ impedance, approximate point stimulation) than DBS macroelectrodes (1 kΩ impedance, 6 mm2 of stimulation surface area). Electrode choice could thus result in a difference in current density by as much as two orders of magnitude (Carlson et al. 2009; Garcia et al. 2005b). Also, the greater distance from the recording electrode to the stimulating electrode in the present work could contribute to the differences in observed effects (Holsheimer et al. 2000). That is, cathodic stimulation can produce a core of complete neuronal inhibition near the electrode and then progressively decrease neuronal inhibition farther from the source (Kiss et al. 2002; Perlmutter and Mink 2006; Ranck 1975). Here, complete silencing of neuronal activity occurred primarily during local stimulation with higher voltages when the stronger stimulation source was closer to the neuron. During distal stimulation, the neurons did not show the same voltage-response relationship, although they still frequently showed entrainment.
Entrained firing and increased neuronal regularity occur during DBS.
Reduction of burst activity and alteration of pathological firing patterns have also been proposed as therapeutic mechanisms in the treatment of Parkinson's disease (Benabid et al. 1991; Fogelson et al. 2005; Grill et al. 2004; Rubin and Terman 2004). Neurons recorded here had both decreased burst-firing after stimulation and increased regularity during stimulation. This regularity manifests as a loose entrainment of firing relative to each stimulation impulse. The entrainment included complex patterns of alternating increases and decreases in firing probability, although the activity was not sufficiently time locked to be antidromic. These complex patterns were observed more frequently in stimulation with therapeutically relevant parameters than with higher voltages or distal stimulation. Such loose entrainment is not unique to GPi stimulation, as similar patterns of entrainment have been observed in other therapeutic contexts. GPi neurons in the nonhuman primate show entrainment with either local GPi stimulation or stimulation in the STN (Bar-Gad et al. 2004; Hashimoto et al. 2003; McCairn and Turner 2009). Stimulation in either the STN or GPi likewise entrains firing of thalamic neurons (Anderson et al. 2003; Montgomery 2006; Xu et al. 2008), and DBS in the STN produces similar complex firing patterns in local neurons and in neurons of the contralateral STN (Carlson et al. 2009; Walker et al. 2011). Since DBS in either GPi or STN is sufficient to entrain firing in both GPi and thalamic neurons, high-frequency stimulation in different sites could engage a common therapeutic mechanism. Loose entrainment has been proposed to replace pathological rhythms with more ordered, regular firing, and indeed, regular stimulation is more therapeutically efficacious than irregular stimulation (Anderson et al. 2003; Dorval et al. 2010; Garcia et al. 2005a; Montgomery 2005).
A computational model of the basal ganglia-thalamic pathway predicts that regular, high-frequency stimulation produces therapeutic effects by entraining or otherwise regularizing activity downstream from the stimulation site (Rubin and Terman 2004). In this model, tonic or highly regular firing produces the lowest error index in simulated TC neurons, whereas irregular firing and bursting produce the highest error indices (Guo et al. 2008). A decrease in the thalamic relay error index has previously been observed when driving model neurons using spike times from GPi neurons recorded during application of STN–DBS at therapeutic voltages (Guo et al. 2008). Here, we used activity recorded from human GPi during stimulation, and we tested whether the observed stimulation effects on activity recorded from the somata of local neurons were sufficient to improve thalamic relay fidelity. A significant decrease in error index was only seen during local stimulation in the therapeutic voltage range, and these stimulation parameters also most consistently produced loose entrainment of neuronal activity. From these observations, we conclude that the increased regularity of somatic activity in human GPi neurons is sufficient to improve TC cell relay fidelity and that this mechanism could contribute to the therapeutic action of DBS.
The observed changes in somatic activity of GPi neurons are sufficient to improve TC relay fidelity, but the definitive neuronal mechanism underlying symptomatic relief remains unclear. The changes in somatic activity seen here, including variable latency responses with stereotyped patterns of activation, are consistent with presynaptic axonal stimulation with subsequent synaptic effects—a mechanism that has been implicated previously in the therapeutic effects of DBS (Birdno et al. 2012; Gradinaru et al. 2009). However, DBS can also drive axonal firing, independent of somatic activity (McIntyre and Grill 1999; Miocinovic et al. 2006), and fibers of passage and axons are now accepted as important therapeutic targets for DBS (Gradinaru et al. 2009; Johnson and McIntyre 2008; Maks et al. 2009). Axonal activation, independent of somatic activity, would explain how increased neuronal output could occur during stimulation of either STN or GPi (Anderson et al. 2003; Hashimoto et al. 2003; Xu et al. 2008), despite decreased or unchanged somatic firing rates (Benazzouz et al. 1995; Beurrier et al. 2001; Carlson et al. 2009; Dostrovsky et al. 2000; Lafreniere-Roula et al. 2010). Regardless, a significant physiological effect from axonal activation does not rule out a separate contribution from alterations in somatic activity, as pharmacological inhibition of GPi neuronal activity is alone sufficient to relieve parkinsonian motor symptoms in primate models (Baron et al. 2002). The symptomatic benefits of DBS in GPi, therefore, may arise from combined effects on multiple neuronal elements, as suggested by Johnson et al. (2012). They proposed that DBS decreases rigidity through the activation of fibers of passage in the adjacent section of the internal capsule, and the improvements in bradykinesia come from activation of neurons in GPi and GPe (Johnson et al. 2012). Just as Parkinson's disease manifests with complex symptoms involving multiple brain sites, symptomatic relief with DBS may involve multiple neuronal mechanisms.
Conclusion.
These results show that DBS, using implanted macroelectrodes in the GPi of the unanesthetized human, does not uniformly silence GPi activity but instead modestly decreases population activity while entraining somatic firing. Modeling of TC relay-cell activity shows that these changes in GPi firing patterns, if synaptically transmitted to downstream targets, would have the capacity to restore relay fidelity. These results illustrate one possible therapeutic mechanism of DBS, although other effects of DBS likely contribute in parallel, including axonal activation and entrainment of neurons in other regions.
GRANTS
Funding for this work was provided by National Institute of Neurological Disorders and Stroke (NS070374 to D. R. Cleary; NS070865 to J. E. Rubin; and NS066159 to M. M. Heinricher); the National Science Foundation (DMS 1021701 to J. E. Rubin); and the Oregon Health & Science University Brain Institute (to D. R. Cleary).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.R.C., A.V., M.M.H., and K.J.B. conception and design of research; D.R.C., A.M.R., J.E.R., D.B., A.V., and K.J.B. performed experiments; D.R.C., A.M.R., J.E.R., A.V., and M.M.H. analyzed data; D.R.C. interpreted results of experiments; D.R.C. prepared figures; D.R.C. drafted manuscript; D.R.C., J.E.R., and M.M.H. edited and revised manuscript; D.R.C. and M.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Valerie Anderson (Oregon Health & Science University, Department of Neurological Surgery), Jonathan Carlson (Inland Neurosurgery and Spine), and Justin Cetas (Oregon Health & Science University, Department of Neurological Surgery) for helpful discussion, feedback, and comments. Andy Rekito (Oregon Health & Science University, Department of Neurological Surgery) provided the illustration.
REFERENCES
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150–1160, 2003 [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, Tignol J, Burbaud P. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg 101: 682–686, 2004 [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci 24: 7410–7419, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. J Neurosci Methods 104: 155–163, 2001 [DOI] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22: 592–599, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: 403–406, 1991 [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology 55: S13–S16, 2000 [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189: 77–80, 1995 [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249: 1436–1438, 1990 [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520, 1994 [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356, 2001 [DOI] [PubMed] [Google Scholar]
- Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Gross RE, Grill WM. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol 107: 364–383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal globus pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215: 17–20, 1996 [DOI] [PubMed] [Google Scholar]
- Carlson JD, Cleary DR, Cetas JS, Heinricher MM, Burchiel KJ. Deep brain stimulation does not silence neurons in subthalamic nucleus in Parkinson's patients. J Neurophysiol 103: 962–967, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol 104: 911–921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84: 570–574, 2000 [DOI] [PubMed] [Google Scholar]
- Erez Y, Tischler H, Moran A, Bar-Gad I. Generalized framework for stimulus artifact removal. J Neurosci Methods 191: 45–59, 2010 [DOI] [PubMed] [Google Scholar]
- Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156: 274–281, 2004 [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547: 142–151, 1991 [PubMed] [Google Scholar]
- Fogelson N, Kuhn AA, Silberstein P, Limousin PD, Hariz M, Trottenberg T, Kupsch A, Brown P. Frequency dependent effects of subthalamic nucleus stimulation in Parkinson's disease. Neurosci Lett 382: 5–9, 2005 [DOI] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci 28: 209–216, 2005a [DOI] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Fernagut PO, Bioulac B, Hammond C. Impact of high-frequency stimulation parameters on the pattern of discharge of subthalamic neurons. J Neurophysiol 94: 3662–3669, 2005b [DOI] [PubMed] [Google Scholar]
- Ghika J, Villemure JG, Fankhauser H, Favre J, Assal G, Ghika-Schmid F. Efficiency and safety of bilateral contemporaneous pallidal stimulation (deep brain stimulation) in levodopa-responsive patients with Parkinson's disease with severe motor fluctuations: a 2-year follow-up review. J Neurosurg 89: 713–718, 1998 [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15: 1137–1140, 2004 [DOI] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol 99: 1477–1492, 2008 [DOI] [PubMed] [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, Vitek JL. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol 211: 243–251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Ammari R, Bioulac B, Garcia L. Latest view on the mechanism of action of deep brain stimulation. Mov Disord 23: 2111–2121, 2008 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsheimer J, Demeulemeester H, Nuttin B, de Sutter P. Identification of the target neuronal elements in electrical deep brain stimulation. Eur J Neurosci 12: 4573–4577, 2000 [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 100: 2549–2563, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Zhang J, Ghosh D, McIntyre CC, Vitek JL. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J Neurophysiol 108: 567–577, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods 68: 211–223, 1996 [DOI] [PubMed] [Google Scholar]
- Kiss ZH, Mooney DM, Renaud L, Hu B. Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanisms of deep brain stimulation. Neuroscience 113: 137–143, 2002 [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, Kim E, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. High-frequency microstimulation in human globus pallidus and substantia nigra. Exp Brain Res 205: 251–261, 2010 [DOI] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol 68: 521–534, 2010 [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345: 91–95, 1995 [DOI] [PubMed] [Google Scholar]
- Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry 80: 659–666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660, 2005 [DOI] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol 101: 1941–1960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 76: 878–888, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 38: 329–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248, 2004 [DOI] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128: 2372–2382, 2005 [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 96: 1569–1580, 2006 [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Effect of subthalamic nucleus stimulation patterns on motor performance in Parkinson's disease. Parkinsonism Relat Disord 11: 167–171, 2005 [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 117: 2691–2702, 2006 [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr, Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res 22: 259–266, 2000 [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Belelovsky K, Bar-Gad I. Dynamic stereotypic responses of basal ganglia neurons to subthalamic nucleus high-frequency stimulation in the parkinsonian primate. Front Syst Neurosci 5: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci 23: S8–S19, 2000 [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci 29: 229–257, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975 [DOI] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci 16: 211–235, 2004 [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. Chicago: Thieme, 1977 [Google Scholar]
- Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35: 1126–1129; discussion 1129–1130, 1994 [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol 93: 3165–3176, 2005 [DOI] [PubMed] [Google Scholar]
- Walker HC, Watts RL, Schrandt CJ, Huang H, Guthrie SL, Guthrie BL, Montgomery EB., Jr Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. J Neurophysiol 105: 1112–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci 28: 11916–11924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]