Abstract
Much is known about the neuronal cell types and circuitry of the mammalian respiratory brainstem and its role in normal, quiet breathing. Our understanding of the role of respiration in the context of vocal production, however, is very limited. Songbirds contain a well-defined neural circuit, known as the song system, which is necessary for song production and is strongly coupled to the respiratory system. A major target of this system is nucleus parambigualis (PAm) in the ventrolateral medulla, a structure that controls inspiration by way of its bulbospinal projections but is also an integral part of the song-pattern generation circuit by way of its “thalamocortical” projections to song-control nuclei in the telencephalon. We have mapped out PAm to characterize the cell types and its functional organization. Extracellular single units were obtained in anesthetized adult male zebra finches while measuring air sac pressure to monitor respiration. Single units were characterized by their discharge patterns and the phase of the activity in the respiratory cycle. Several classes of neurons were identified and were analogous to those reported for mammalian medullary respiratory neurons. The majority of the neurons in PAm was classified as inspiratory augmenting or preinspiratory, although other basic discharge patterns were observed as well. The well-characterized connectivity of PAm within the vocal motor circuit and the similarity of its neural firing patterns to the rostral ventral respiratory group and pre-Bötzinger complex of mammals make it an ideal system for investigating the integration of breathing and vocalization.
Keywords: song, brainstem, hemispheric coordination, motor patterns
normal breathing or eupnea is an automatic function controlled by brainstem respiratory circuits that can be altered during voluntary or involuntary motor behaviors. Respiratory patterns are suitably adjusted by integration of a variety of central (Guyenet et al. 2005; Smith et al. 2009) and peripheral inputs (Davies and Kubin 1986; Lipski et al. 1991; Wild 2004a, b) to the brainstem respiratory circuitry. Song production in songbirds is a particularly interesting example of such integration, because it requires coordination between respiratory circuits and the vocal motor “cortical nuclei” that control production of this learned vocal behavior (Schmidt and Ashmore 2008; Wild 2004b). Although vocal production is tightly linked to respiration in many taxa (Kelley and Bass 2010), and much is known about respiratory circuits, at least in mammals, the interaction between respiratory control and vocal production is poorly understood.
In mammals, the neural control of vocalization involves two pathways that converge on caudal brainstem vocalization centers. One pathway consists of projections from cortical and limbic areas through the midbrain periaqueductal gray (PAG), which when stimulated, can elicit changes in respiratory patterns associated with vocalizations (Katada et al. 1996; Subramanian et al. 2008) and when lesioned, produces mutism (Adametz and O'Leary 1959). The other pathway is thought to originate in the motor cortex and proceeds directly to areas in the reticular formation of the dorsocaudal medulla involved in vocalization (Jürgens and Ehrenreich 2007; Simonyan and Jürgens 2003). This latter pathway may be influenced by cerebellar and basal ganglia feedback loops through the thalamus [reviewed in Jürgens (2009)]. The motor cortex pathway along with its feedback loops appear to be involved with the production of learned vocal patterns, whereas the pathway through PAG, with vocal initiation.
The neural circuitry controlling vocal production in songbirds is referred to as the song system (Fig. 1) and contains a direct motor pathway that consists of the telencephalic structures HVC (used as a proper name) and robust nucleus of the arcopallium (RA). HVC is thought to encode many of the acoustic and temporal features of song (Hahnloser et al. 2002; Long and Fee 2008), and RA serves as an output structure analogous to layer V of the motor cortex (Dugas-Ford 2009; Jarvis et al. 2005). RA uniquely projects directly upon vocal motor neurons in the tracheosyringeal branch of the hypoglossal nucleus (nXIIts) (Nottebohm et al. 1976) and premotor neurons in the respiratory brainstem (Roberts et al. 2008; Vicario 1991; Wild 1993a). The dorsal portion of RA contains the projection neurons that innervate the nucleus parambigualis (PAm), nucleus retroambigualis (RAm), and the dorsomedial nucleus of the intercollicular complex (DM). These nuclei are commonly referred to as respiratory-vocal nuclei, because they control respiration and project to hypoglossal motor neurons controlling the vocal musculature of the syrinx—the avian vocal organ.
Fig. 1.
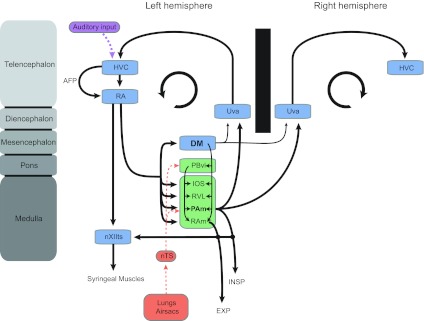
Schematic representation of the avian song system and its relationship to the respiratory system. Illustration of the avian song system (shown in blue) and its anatomical connections with ponto-medullary respiratory nuclei (shown in green), which receive sensory information from putative mechano- and chemosensitive receptors in the lungs and air sacs (red) via the nucleus tractus solitarius (nTS). The song system also receives auditory input (purple) by way of nucleus HVC. The inspiratory nucleus parambigualis (PAm) receives strong projections from robust nucleus of the arcopallium (RA) and projects bilaterally back to the thalamic nucleus, uvaeformis (Uva), which itself projects directly back to HVC. Dorsomedial nucleus of the intercollicular complex (DM) also receives projections from RA and projects bilaterally back to Uva, but its projections are much weaker than PAm. DM projections to nXIIts are not shown for purposes of clarity. The nucleus parabrachialis ventrolateralis (PBvl), which is likely the avian homologue of the Kölliker-Fuse nucleus, does not receive direct projections from RA but is intimately tied with other structures in the respiratory brainstem. The respiratory areas PAm and retroambigualis (RAm) project directly to neurons in the tracheosyringeal branch of the hypoglossal nucleus (nXIIts), which contain the motor neurons that innervate the syrinx. The circular arrow in each hemisphere symbolizes the recurrent nature of the song motor circuit. AFP, anterior forebrain pathway; IOS, infra-olivaris superior; RVL, ventrolateral nucleus of the rostral medulla; EXP, expiration.
Previous electrophysiological recordings in PAm have identified neurons that are active during the inspiratory phase of respiration (Ashmore et al. 2008; Reinke and Wild 1997, 1998). These premotor neurons project bilaterally to motor neurons in the spinal cord that innervate inspiratory muscles. Similarly, RAm neurons are reported to be active during the expiratory phase of respiration and project bilaterally to spinal motor neurons innervating expiratory muscles (Ashmore et al. 2008; Sturdy et al. 2003; Wild 1993b). In addition to downstream projections to spinal motor neurons controlling respiration, PAm projects directly to the nucleus uvaeformis (Uva) in the posterior thalamus. Uva (Reinke and Wild 1998; Striedter and Vu 1998), a nucleus involved in sensory integration and necessary for song production, sends a projection back up to the HVC, thereby completing a recurrent loop in the song system (Coleman and Vu 2005; Striedter and Vu 1998; Williams and Vicario 1993). Ashmore et al. (2005) have proposed that PAm, along with the telencephalic nuclei HVC and RA in this recurrent loop, all contribute to the song motor pattern and that PAm provides essential timing signals for the generation of vocal motor sequences. In addition to its vocal motor inputs from RA, PAm receives afferent projections from subnucleus parasolitarius, a small subnucleus of the nucleus tractus solitarius (nTS) that receives syringeal and pulmonary afferents over the vagus nerve (Reinke and Wild 1998; Wild 2004a).
PAm has been referred to as the avian homolog of the mammalian rostral ventral respiratory group (rVRG), because it contains bulbospinal inspiratory premotor neurons and is adjacent to the nucleus ambiguus (Reinke and Wild 1997, 1998). In mammals, the rVRG is one component of the ventral respiratory column (VRC), which extends from the caudal end of the facial nucleus to the rostral cervical spinal cord. The VRC consists of a rostrocaudally oriented column of cells that includes, in descending order, the Bötzinger Complex (BötC), the pre-BötC, the rVRG, and the caudal VRG (cVRG). The VRC is controlled by afferents from higher medullary and pontine centers and generates the motor pattern of breathing [reviewed in Smith et al. (2009)].
Normal eupneic breathing in mammals consists of three phases: inspiration, postinspiration, and a late stage of expiration. In the mammalian literature, neurons located in the VRC have been classified according to their firing patterns and phase relationship to the respiratory cycle (Bianchi et al. 1995; Richter 1982; Smith et al. 2009). In birds, respiratory nuclei, having a similar rostrocaudal distribution throughout the ventrolateral medulla as that found in mammals, have been documented in pigeons and zebra finches (Reinke and Wild 1997, 1998), but little is known about the detailed respiratory-modulated discharge patterns of PAm neurons. Given that PAm likely plays a critical role in vocal motor production and hemispheric coordination, we aimed to characterize the different cell types in PAm and describe their organization within the ventrolateral medulla. We report here that the majority of PAm neurons have discharge profiles similar to those found in the mammalian rVRG. Consistent with a previous study (Ashmore et al. 2008), we also recorded from neurons that fired in bursts that were unrelated to the respiratory rhythm, yet they were recorded at sites where we could also record inspiratory activity.
MATERIALS AND METHODS
Animals.
Adult male zebra finches (Taeniopygia guttata), at least 120 days of age, were obtained from a local supplier (Canary Bird Farm, Old Bridge, NJ). Birds were given food and water ad libitum and kept on a 12:12 light:dark cycle in a colony room. All procedures described here were approved by an Institutional Animal Care and Use Committee at the University of Pennsylvania.
Recording.
Adult male zebra finches were anesthetized with an intramuscular injection of ketamine (25 mg/kg)/xylazine (5 mg/kg; Sigma, St. Louis, MO), supplemented with diazepam (7.5 mg/kg; Hospira, Lake Forest, IL). PAm was identified using stereotaxic coordinates combined with electrophysiological recordings of a respiratory rhythm in phase with inspiration, as determined by simultaneous recordings of air sac pressure. Birds were placed in the stereotaxic apparatus at a head angle of 45°, and coordinates for PAm were relative to the bifurcation of the midsagittal sinus. Initial coordinates for finding PAm were anteroposterior, 0; lateral, 1.4 mm. Extracellular activity was recorded with thin tungsten electrodes (1 MΩ; FHC, Bowdoin, ME). Neural signals were amplified using a Bioamp headstage connected to a DB4 module (Tucker-Davis Technologies, Alachua, FL). Signals were filtered between 300 Hz and 3 kHz using a four-channel amplifier (model 440; Brownlee Precision, San Jose, CA) and digitized at a sampling rate of 20 kHz using a four-channel data acquisition device (Micro1401 mk II; Cambridge Electronic Design, Cambridge, UK) in conjunction with Spike2 software (Cambridge Electronic Design). Small electrolytic lesions (4 μAmp negative current; 10 s on, 10 s off; given twice; isolated pulse stimulator model 2100; A-M Systems, Carlsborg, WA) were made through the recording electrode at the bottom of a penetration and several hundred microns above the first recorded cell to mark the electrode track and determine cell locations. Tissue shrinkage was accounted for by measuring the distance from the midline to the lateral position of the lesion and comparing that with the known lateral position of the electrode.
Respiratory monitoring.
Air sac pressure recording was performed as described previously (Ashmore et al. 2008). Briefly, a thin piece of silastic tubing was inserted into the thoracic air sac just below the lowest rib and sutured in place. This cannula was connected to a pressure transducer (FHM-02PGR-02; Fujikura, Tokyo, Japan), powered by a custom-built power source. In some experiments, respiratory monitoring was accomplished by placing a small plastic pillow between the bird's chest and a cloth jacket that held the pillow in place. The pillow was attached to the pressure transducer by a short length of tubing. Signals from the transducer in both cases were amplified and filtered between 0.75 Hz and 30 Hz using a four-channel amplifier (model 440; Brownlee Precision) and digitized at a sampling rate of 20 kHz using a four-channel data acquisition device (Micro1401 mk II; Cambridge Electronic Design) in conjunction with Spike2 software (Cambridge Electronic Design).
Analysis
Spike sorting.
Clustering-based spike sorting was carried out using Spike2 software (version 6; Cambridge Electronic Design). Cluster values were extracted using the principal components analysis (PCA). The principal components were generated from spike waveforms, and clusters were visually inspected in PCA space to ensure that spikes were sorted correctly. We were frequently able to isolate more than one unit in a given recording with the use of this technique.
Respiratory phase plots and respiratory rhythm markers.
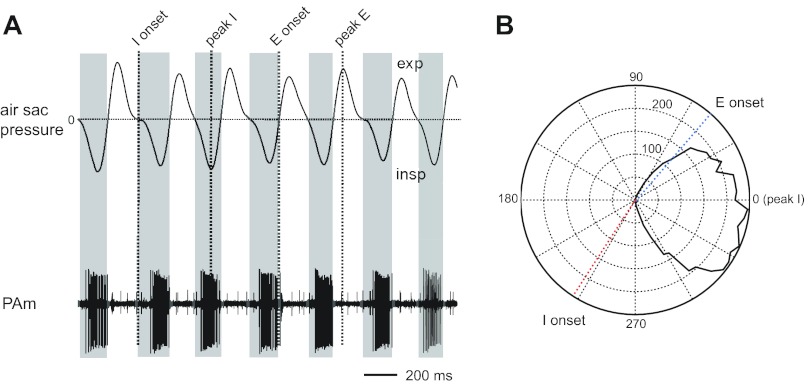
For each recording location, neural activity and air sac pressure measures were obtained for ∼2 min. Respiratory measures were analyzed using Spike2 software. The trace from the respiratory air sac pressure cannula was smoothed and the direct current level removed to account for drifts in the average signal. The most negative trough in this rhythmic trace corresponds with the peak inspiratory air sac pressure. For each isolated unit, a phase histogram was generated, where all spikes in each respiratory cycle were binned into histograms relative to the occurrence of the peak inspiratory air sac pressure, i.e., the trough in the cannula trace. Each cycle was divided into 75 bins, regardless of the cycle length. Other markers obtained from the air sac pressure waveform include the peak, which corresponds to the peak expiratory air sac pressure; the point where the trace falls through zero volts, which corresponds to the onset of inspiration; and the point where the trace rises through zero volts, which corresponds to the switch from inspiration to expiration (Cohen 1968). Phase histograms were also created for each of the respiratory markers relative to the peak inspiratory pressure. These phase plots were fit with a Gaussian using Spike2 software, and the center of the Gaussian was used as the value demarcating peak expiratory air sac pressure, onset of inspiration, and onset of expiration in phase plots for each cell. Figure 2 illustrates how we divided the respiratory cycle. In the polar plots (see Figs. 2–6), 0° corresponds to the peak of the inspiratory phase. For illustrative purposes, the data in the phase histograms (see Figs. 3–7) have been shifted so that the onset of inspiration occurs at 0°.
Fig. 2.
Air sac pressure recording and PAm unit activity. A: expiration leads to a positive deflection in the air sac pressure trace, and inspiration is indicated by a negative deflection. Air sac pressure markers: I onset, onset of inspiration; peak I, inspiratory peak; E onset, onset of expiration; peak E, peak expiration. B: polar plot of PAm unit activity counted in a 120-s trace at different phases of respiration. Respiratory phase plots were generated by binning spikes with respect to the cyclical occurrence of the peak in the inspiratory phase, which occurs at 0° in the polar plot.
Fig. 3.
Phase histograms, polar plots, air sac pressure traces, and PAm unit activity for 2 inspiratory-related PAm units. Dotted lines represent markers in the air sac pressure trace; red, onset of inspiration, followed by peak inspiration; blue, onset of expiration, followed by peak expiration. A: early I. Cell fires robustly early in inspiration, and firing rate slows as inspiration progresses. B: I augment. Firing rate increases throughout inspiration. In the polar plots, 0° is the peak of the inspiratory phase. For illustrative purposes, in the phase histograms, these data have been shifted so that the onset of inspiration occurs at 0°.
Fig. 4.
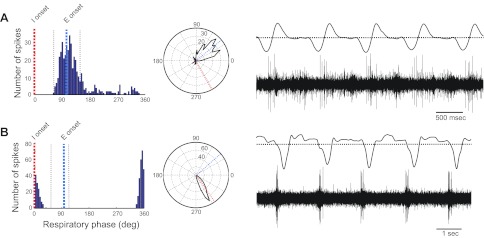
Phase histograms, polar plots, air sac pressure traces, and PAm unit activity for 2 PAm units that fired at phase transitions. A: late I. Cell fires at the transition from inspiration to expiration. B: pre-I. Cell fires at the transition from expiration to inspiration.
Fig. 5.
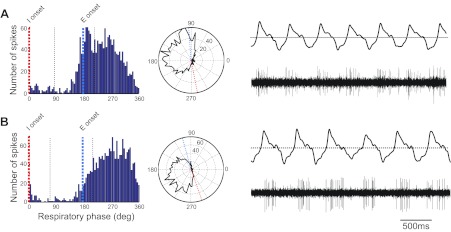
Phase histograms, polar plots, air sac pressure traces, and PAm unit activity for 2 expiratory-related PAm units. A: post-I. Cell fires robustly early in expiration, and firing rate slows as expiration progresses. B: E augment. Firing rate increases throughout expiration.
Fig. 6.
Several neurons exhibited firing patterns that were phase locked to the respiratory rhythm; however, discharge patterns did not match any of the 6 patterns described in Figs. 3–5. The discharge patterns in B and C were obtained from 2 neurons in the same electrode track, 85 μm apart.
Fig. 7.
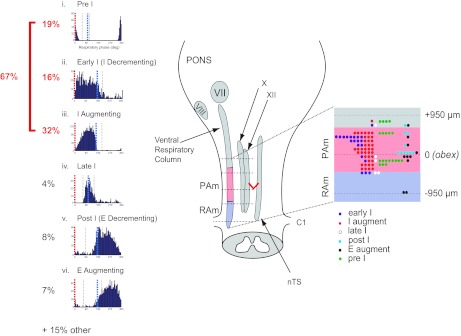
Summary of cell types and their spatial localization in PAm. Left: phase histograms of PAm respiratory-related neurons aligned vertically. Respiratory cycles are aligned to the onset of the inspiratory phase (red; 0°) to show the respective contribution of different cell types throughout the respiratory cycle. The time of occurrence of the IE transition (blue) could vary within the respiratory cycle. i, pre-I phase histogram; ii, early I phase histogram; iii, I-augment phase histogram; iv, late I phase histogram; v, post-I phase histogram; vi, E-augment phase histogram. Right: the rostrocaudal distribution of recorded respiratory-related cells with respect to the obex. The ventral respiratory column (VRC) and other brainstem nuclei are illustrated schematically in the brainstem drawing on the left. The rostrocaudal location and cell type are schematically represented in an enlargement of the portion of the VRC spanning the obex (red V) on the right. VII, facial nucleus; VIII, cochlear nucleus; X, dorsal motor nucleus of the vagus nerve; XII, hypoglossal nucleus; C1, spinal cord cervical section 1.
Data in the phase plots were exported to MATLAB (MathWorks, Natick, MA) for further analysis. From these data, we used circular statistics to calculate a mean vector that determined a mean direction or angle for each phase plot, given in degrees (Batschelet 1981). A variety of other measurements were obtained from the data using Spike2 software and MATLAB, including a plot of the interspike interval (ISI), peak firing rate, and spike width, which was defined as the width at 25% maximum spike height. All values are given as means ± SE and are reported in results and in Table 1.
Table 1.
Properties of PAm cell types
| Cell Type | n | Mean Angle (deg) | Mean Spike Width (ms) | Mean Firing Rate (Hz) | ISI Peak (s) |
|---|---|---|---|---|---|
| Early I | 21 | 286.0 ± 19.7 | 0.31 ± 0.03 | 55.1 ± 9.3 | 0.02 ± 0.007 |
| I Augmenting | 41 | 237.0 ± 22.6 | 0.29 ± 0.01 | 42.4 ± 5.0 | 0.04 ± 0.02 |
| Post-I | 10 | 153.7 ± 5.4 | 0.34 ± 0.03 | 33.3 ± 9.7 | 0.04 ± 0.009 |
| E Augmenting | 10 | 204.6 ± 9.1 | 0.32 ± 0.05 | 28.0 ± 5.7 | 0.01 ± 0.003 |
| Late I | 5 | 30.4 ± 17.5 | 0.28 ± 0.05 | 23.5 ± 8.4 | 0.03 ± 0.01 |
| Pre-I | 24 | 292.9 ± 5.6 | 0.43 ± 0.02 | 22.2 ± 4.1 | 0.06 ± 0.03 |
PAm, nucleus parambigualis; ISI, interspike interval; I, inspiratory; E, expiration.
Histology.
After 4–5 days of recovery to permit glial scarring to occur at lesion sites, animals were deeply anesthetized with 0.1 ml of 50 mg/kg Nembutal (Abbott Laboratories, Abbott Park, IL) and perfused with 0.9% saline and 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose and 4% paraformaldehyde and sectioned coronally at 40 μm on a freezing microtome. All sections were then stained with cresyl violet.
Anatomical reconstruction of functional maps.
For each recorded cell, we located the recording site on histological sections and determined the rostrocaudal distance of that site from the obex. Nissl-stained tissue sections were traced using a Bausch and Lomb microprojector, and cell locations were plotted in the traced section. Relative positions for all cells were then plotted on a common “anatomical map” (see Fig. 7). We used two nonparametric statistical tests to determine if the locations of the cells relative to the obex were segregated significantly. Neither the Kruskal-Wallis ANOVA by Ranks nor the Median Test revealed any significant segregation.
RESULTS
We recorded extracellular neural activity from 130 neurons in the rostral ventrolateral medulla of anesthetized adult male zebra finches. Recordings were performed in regions extending from ∼1 mm caudal to 1 mm rostral to the obex and from 1.2 mm to 1.6 mm lateral to the midsagittal sinus (see Fig. 7). We combined neural recordings with air sac pressure recording using a cannula inserted into the thoracic air sac. Respiratory-related activity was encountered at electrode depths ranging between 6 mm and 8 mm from the surface of the telencephalon. We assigned neurons into categories based on their discharge patterns (augmenting or decrementing) and the phase of the activity in the respiratory cycle. We identified a total of six broad categories of cell types in the songbird rostral ventrolateral medulla. The naming scheme applied to the various categories was guided by the mammalian literature (Bianchi et al. 1995; Smith et al. 2009). Phase plots for cells characteristic of each class are illustrated in Figs. 3–6.
Characterization of discharge patterns
Inspiratory neurons: early I and I-augmenting cells.
Inspiratory (I) neurons made up nearly one-half of all neurons recorded in the ventrolateral medulla (62/130 neurons) and were generally characterized by firing in the interval between the onset of inspiration and the onset of expiration. These neurons fired robustly during this interval and exhibited the highest firing rates in our population.
Early I cells, also known as I-decrementing cells, fired robustly, early in inspiration, and then, the firing rate slowed as inspiration progressed (n = 21). These cells represented 16.2% of all of the cells in our study (Fig. 3A). Most of these cells fired at the onset of inspiration, and firing slowed throughout the entire inspiratory phase; however, two cells fired only at the onset of inspiration, and firing rate slowed rapidly, ending before the peak of inspiration. For one cell, it was not clear that the activity was phase locked to the respiratory cycle until generating the phase plot. Variations in discharge patterns of neurons in a given class may be dependent on the level of respiratory drive and respiratory-related feedback from the periphery (Ballantyne and Richter 1986). The average mean angle for early I neurons, calculated from phase plots, was 286.0 ± 19.7°, i.e., with peak inspiration at 0° or 360°. Mean spike width for these cells was 0.31 ± 0.03 ms, mean firing rate was 55.1 ± 9.3 Hz, and mean ISI peak was 0.02 ± 0.007 s.
I-augmenting neurons also fired during inspiration, but their firing rate increased throughout inspiration, peaking near the transition to expiration (n = 41). These were the most common cell type encountered in our population, representing 31.5% of all cells (Fig. 3B). All of these cells fired briskly and rhythmically like the majority of the early I cells. The average mean angle for I-augmenting neurons calculated from phase plots was 237.0 ± 22.6°. Mean spike width for these cells was 0.29 ± 0.01 ms, mean firing rate was 42.4 ± 5.0 Hz, and mean ISI peak was 0.04 ± 0.02 s.
Phase-spanning cells: late I and pre-I cells.
Phase-spanning neurons made up approximately one-fourth of all neurons recorded in the ventrolateral medulla (31/130 neurons) and were generally characterized by firing within a narrow time window during the transitions between the inspiratory and expiratory cycles. These neurons had the lowest firing rates in our population.
Late I cells fired late in the inspiratory phase and into the very early part of the postinspiratory phase (n = 5; 3.8%). These cells tended to fire only a few spikes throughout a rather narrow range in the respiratory cycle surrounding the transition from inspiration to expiration (Fig. 4A). The average mean angle for these cells was 30.4 ± 17.5°. Mean spike width for these cells was 0.28 ± 0.05 ms, mean firing rate was 23.5 ± 8.4 Hz, and mean ISI peak was 0.03 ± 0.01 s.
Pre-I neurons fired at the transition from expiration to inspiration (EI; n = 24). Like the late I neurons, these cells typically fired a short burst of action potentials over a very narrow window in the respiratory cycle (see Fig. 4B) compared with the other cell types. Five of these cells fired spikes in doublets or triplets. This group was the second-most commonly encountered cell type in our population, representing 18.5% of all cells. The average mean angle for these cells was 292.9 ± 5.6°. Mean spike width for these cells was 0.43 ± 0.02 ms, mean firing rate was 22.2 ± 4.1 Hz, and mean ISI peak was 0.06 ± 0.03 s.
Expiratory neurons: postinspiratory and E-augmenting cells.
Expiratory neurons made up ∼15% of all neurons recorded in the ventrolateral medulla (20/130 neurons) and were generally characterized by firing in the interval between the onset of expiration and the onset of inspiration. These neurons fired robustly during this interval and exhibited intermediate firing rates.
Postinspiratory, also known as E-decrementing neurons, fired early in expiration, and then the firing rate slowed (n = 10; 7.7%). According to mammalian literature, these neurons fired during the second phase of the respiratory cycle—E1 or post-I (Fig. 5A). Most of these cells fired robustly at the onset of expiration; however, for three cells, the firing pattern was not clear until the phase plot was inspected. The average mean angle for these cells was 153.7 ± 5.4°. Mean spike width for these cells was 0.34 ± 0.03 ms, mean firing rate was 33.3 ± 9.7 Hz, and mean ISI peak was 0.04 ± 0.009 s.
E-augmenting neurons fired late in the second expiratory phase, E2, and peak firing rates occurred just prior to the transition from expiration to inspiration (n = 10). These cells comprised 7.7% of our cells, and we observed that all cells fired a strong burst of spikes within each cycle (Fig. 5B). The average mean angle for these cells was 204.6 ± 9.1°. Mean spike width for these cells was 0.32 ± 0.05 ms, mean firing rate was 28.0 ± 5.7 Hz, and mean ISI peak was 0.01 ± 0.003 s.
Other.
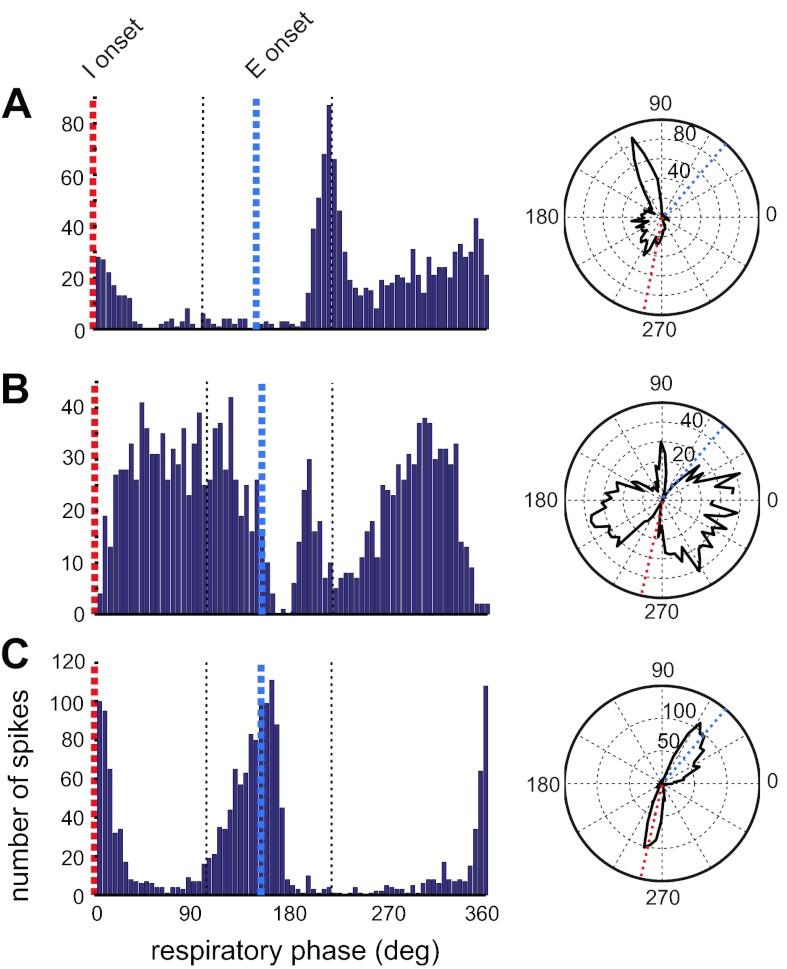
We recorded several neurons whose firing rate was modulated with the respiratory rhythm; however, the firing patterns did not match any of the categories described above (n = 13; 10%). Two of these neurons fired robustly, shortly after the onset of expiration, followed by a rapid reduction in firing rate and finally, an augmenting pattern of firing peaking nearing the transition to inspiration (Fig. 6A). Two other neurons displayed a high rate of firing throughout the respiratory cycle, and this rate was suppressed near the transition point from expiration to inspiration as well as from inspiration to expiration (Fig. 6B). Finally, three other neurons exhibited peak firing during the IE transition and also just prior to the EI transition points in the respiratory rhythm (Fig. 6C).
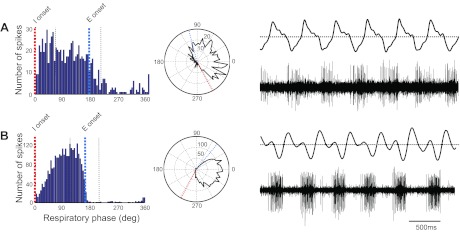
Another group of neurons (n = 6; 4.6%) was recorded at sites where we could also record inspiratory activity; however, these neurons did not show any phase locking to the respiratory rhythm. Most of these neurons fired in bursts that seemed to be unrelated to the respiratory rhythm. These phasic patterns of bursting were identical to those described in our previous study (Ashmore et al. 2008).
Cell class and firing rate.
On average, the lowest firing rates were observed for the two classes of cells that fired maximally at the transitions: the late I and the pre-I cells. The highest firing rates were observed in those cell classes that fired during the inspiratory phase: the early I and the I-augment cells. The two classes of cells that fired during the expiratory phases—the postinspiratory and the E-augmenting cells—exhibited intermediate firing rates. Overall, we found that peak firing rates were significantly correlated with the respiratory rate (r = 0.33; P < 0.001); i.e., when respiratory rates were low, firing rates were correspondingly lower as well. However, when considering the relationship between peak firing rates and respiratory rate for each individual cell class, only the I-augment cells were found to be correlated significantly (r = 0.32; P < 0.05). The E-augmenting cells had the largest correlation coefficient (r = 0.59), but the correlation was not significant (P = 0.07). The phase histograms for these six classes of cells are illustrated in Fig. 7.
Anatomical localization of identified respiratory cell types.
Two small electrolytic lesions were made through the recording electrode at depths just ventral and dorsal to the PAm recording sites. Histological analysis verified that recordings were obtained in the ventrolateral medulla in regions previously identified as PAm (Reinke and Wild 1998). We determined the precise location of recording sites in the rostrocaudal axis relative to the obex. The six classes of respiratory neurons that we encountered did not appear to be localized to any specific region in PAm, because all classes of neurons were encountered throughout the area we probed. The location of all cells relative to the obex is plotted in Fig. 7. Neurons in this plot were recorded 1.2–1.6 mm lateral to the midsagittal sinus, between 920 μm posterior and 920 μm anterior to the obex, and roughly 6–8 mm from the surface of the telencephalon.
Whereas all types of neurons could be found in PAm, the majority of early I cells appeared to be localized in more rostral regions of the structure. We also found that E-augment cells, which have been identified in mammals as premotor bulbospinal cells innervating muscles associated with expiration (Ezure 1990; Monteau and Hilaire 1991), were more likely to be encountered caudal to the obex with the two most caudal E-augment cells likely being recorded in RAm.
DISCUSSION
In this study, we sought to characterize the firing patterns and functional organization of neurons recorded in the respiratory-vocal nucleus PAm. Here, we provide a physiological characterization of PAm as a first step in understanding the interaction between the respiratory system and vocal production.
Respiratory neuron types in PAm.
In mammals, the rVRG contains a variety of neuron types but chiefly, inspiratory-related bulbospinal neurons that project to spinal phrenic (innervating the diaphragm) and intercostal inspiratory motor neurons (Bianchi et al. 1995). Whereas birds have neither a diaphragm nor a phrenic nucleus, PAm is thought to be homologous to the mammalian rVRG, based on the similar distributions of bulbospinal and inspiratory-related neurons within the ventrolateral medulla at levels spanning the obex (Reinke and Wild 1997, 1998). Respiratory discharge patterns found in PAm were similar to those reported in mammalian respiratory brainstem. In mammalian studies, respiratory neurons are classified according to their firing patterns and the phase of their activity within the respiratory cycle. We found that the most frequently encountered cell types recorded in PAm (47.7%) were inspiratory related, as reported previously (Ashmore et al. 2008; Reinke and Wild 1997, 1998). Most of our inspiratory-related neurons were classified as I augment (31.5%) and early I (16.2%). In mammals, I-augment neurons tend to be the inspiratory premotor neurons that project to the spinal cord (Bianchi et al. 1995; Feldman et al. 1985; Guyenet et al. 2002). In songbirds, retrograde tracer injections into the upper thoracic spinal cord at levels where motor neurons to inspiratory muscles are located will label neurons in PAm (Reinke and Wild 1997, 1998). The distribution of these retrogradely labeled neurons overlaps with levels of the ventrolateral medulla, where we observed inspiratory-related neurons, suggesting that I-augment neurons in PAm are well placed to play the same role as they do in mammals.
In this same region where I-augment neurons were observed, we also recorded from late I (3.8%) and pre-I (18.5%) neurons, often referred to as phase-spanning neurons, as well as from expiratory-related neurons (15.4%). Connelly et al. (1992) also reported a similar heterogeneity of cell types in the rVRG of the adult cat, including the six classes that we report here. The majority of cells in the cat rVRG were also I augment, but early I, expiratory, and phase-spanning neurons were recorded as well.
In addition to these neuron classes, several neurons were recorded with discharge patterns that were phase locked to the respiratory cycle but did not fit into any of the six categories (Fig. 6). Three of these neurons fired during the IE transition and just prior to the EI transition (Fig. 6C). This discharge pattern has been referred to as pre-I (Bianchi et al. 1995), although the pre-I classification is more typically associated with EI phase-spanning neurons. Smith et al. (2009) refer to neurons that start firing before the onset of inspiration and continue throughout the inspiratory phase as pre-I or also pre-I/I neurons.
Is rostral PAm equivalent to the mammalian pre-BötC?.
Whereas the various cell classes were distributed throughout PAm, there was a tendency for early I neurons to be found in more rostral regions. In mammals, the pre-BötC is situated just rostral to the rVRG in the ventrolateral medulla. In cat, inspiratory, expiratory, and phase-spanning neurons are observed in the pre-BötC, but most of the neurons encountered are of the Early I (I-decrement) class. Several investigators have found that whereas all classes of respiratory neurons are seen in the pre-BötC, the complex is characterized by having pre-I neurons localized specifically to that area and having a high density of early I cells (Connelly et al. 1992; Schwarzacher et al. 1995). We hypothesize that neurons localized to the more rostral regions represent the avian homologue of the pre-BötC. In mammals, the pre-BötC projects to the hypoglossal nucleus, but the rVRG does not (Borgmann et al. 2011). In songbirds, tracer injections into the nXIIts retogradely labeled neurons in PAm at sites corresponding to those where inspiratory-related activity was recorded (Reinke and Wild 1998). It is possible that these sites may correspond to an avian homolog of the pre-BötC. We are unaware of any nomenclature associated with an avian homolog for the pre-BötC.
The mammalian hypoglossal nucleus innervates the intrinsic tongue muscles and has a respiratory rhythm that is modulated by pre-BötC (Smith et al. 2009). In birds, PAm neurons also project to the rostral part of the hypoglossal nucleus that innervates intrinsic tongue muscles (nXII l; nucleus hypoglossus, pars lingualis), as well as to the more caudal tracheosyringeal component (XIIts) that innervates muscles of the vocal organ (Reinke and Wild 1998). XIIts has a respiratory rhythm in phase with expiration (Sturdy et al. 2003), but whether nXII l also has a respiratory rhythm is not known. Nevertheless, the tongue, larynx, and associated hyoid apparatus can all be seen to move rhythmically in concert during breathing (Wild JM, unpublished observations).
PAm is both a target and a driver of vocal motor control circuits.
An emerging view of the respiratory system in mammals is that different respiratory neuronal types are distributed throughout the VRC but that each compartment within the VRC has dominant populations that may define its functional role with each compartment under the control of more rostral compartments (Smith et al. 2009). In mammals, the pre-BötC is believed to be a part of the core respiratory circuitry and the source of rhythmic inspiratory input to premotor neurons. The rVRG is therefore driven by excitatory inputs from the pre-BötC during inspiration and inhibitory inputs from the BötC during expiration. Whereas PAm has been proposed to act similarly to the rVRG in driving inspiratory motor neurons, in songbirds (Reinke and Wild 1998), PAm is also known to project rostrally to Uva in the diencephalon, which in turn, projects to HVC. It has been hypothesized that these projections relay song and respiratory-related activity back to higher centers for timing and interhemispheric synchronization. Whether Uva-projecting neurons make up a distinct physiological class of cells within PAm is not known, but the nonrespiratory bursting (NRB) neurons recorded in PAm (∼5% of the population) [Ashmore et al. (2008) and the present study] might represent such a population, given that their activity is correlated with activity in HVC and RA in anesthetized birds (Ashmore et al. 2008). Further studies using antidromic identification will be necessary to fully characterize the properties of Uva-projecting neurons in PAm.
The recurrent projection to Uva and eventually to HVC places PAm as a key structure for providing critical song-timing information (Andalman et al. 2011; Schmidt and Ashmore 2008) as well as for playing a pivotal role in integrating information relating to the motor program for song and the respiratory system. PAm receives inputs from telencephalon regarding the motor program, sends outputs to the spinal cord to control inspiratory muscles, and is reciprocally connected to other brainstem respiratory centers, including nucleus parabrachialis ventrolateralis, nucleus infra-olivaris superior, ventrolateral nucleus of the rostral medulla, and RAm (Reinke and Wild 1998). In addition, PAm is likely to receive chemoreceptor and perhaps mechanoreceptor information from the lung, air sacs, and syrinx via vagal projections to nTS (Wild 2004a). Such information could then be routed to the telencephalon via the projections of PAm to Uva (Mendez et al. 2010).
Respiratory neurons and vocal production.
In mammals, there are likely two pathways that control vocalization: one originating in cortex and limbic structures that operate through the PAG (Holstege 1989) and the other, a more direct pathway from motor cortex to areas in the reticular formation of the dorsocaudal medulla involved in vocalization (Jürgens 2009; Jürgens and Ehrenreich 2007; Simonyan and Jürgens 2003). The PAG is likely to be critical in vocal-respiratory coupling, given that lesions result in mutism in animals, including humans [Adametz and O'Leary (1959); reviewed in Jürgens (2009)], and stimulation causes increased activity in the diaphragm, which is often followed by alternate inspiration and natural-sounding vocalization (Katada et al. 1996; Subramanian et al. 2008).
The effects of PAG stimulation on respiration are consistent with projections from the PAG to respiratory control centers in the caudal brainstem (Holstege 1989), which when stimulated, produce vocalization (Subramanian and Holstege 2009). The notion that the PAG might act as a general motor organizer for respiratory-vocal coupling is strengthened by recent work showing that neurons in the ventrolateral medulla, such as E-augment neurons in the cVRG, show significantly increased activity during PAG stimulation and vocalization. Under these conditions, many more neurons appear to be recruited compared with normal, quiet breathing, and approximately two-thirds of these neurons shows altered firing patterns (Subramanian and Holstege 2010).
In birds, there might also exist at least two separate vocal-control pathways. There clearly exists a “cortical” pathway, in which RA—proposed to be analogous to layer 5 of the motor cortex—innervates vocal motor neurons in the nXIIts and premotor neurons in the respiratory brainstem nuclei. It also innervates nucleus DM, which is thought to be the avian equivalent to the lateral PAG (Gerrits and Holstege 1996; Holstege 1989; Jürgens 1994; Kingsbury et al. 2011; Wild et al. 1997). This cortical pathway exists in all oscine songbirds who learn their song and has therefore been implicated in the production of learned vocalizations. In birds that do not learn their vocalizations, this pathway is lacking, and it is thought that vocalizations are mediated primarily through the midbrain nucleus DM, which when stimulated, causes the production of innate vocalizations (Potash 1970; Vicario and Simpson 1995; Wild et al. 1997). In songbirds, DM receives a strong input from RA and is connected reciprocally with all of the characterized brainstem respiratory nuclei (Wild et al. 1997). In the canary, lesions of DM do not appear to alter learned vocalizations (Nottebohm et al. 1976), which are driven by RA. Similar to the situation in mammals, both the cortical pathway (RA) and midbrain pathway (DM) in songbirds send direct projections to expiratory (RAm; cVRG)- and inspiratory (PAm; rVRG)-related medullary nuclei, as well as to vocal motor neurons (hypoglossal nucleus).
In the current study, we characterized neurons in PAm in anesthetized birds; however, given its prominent role in vocal production and hemispheric coordination during singing (Schmidt and Ashmore 2008), it will be important to record from the different neuronal subtypes in PAm in singing birds. While this has not been achieved, owing to technical challenges, it has nevertheless been possible to record from PAm during electrically evoked vocalizations. Ashmore et al. (2008) found that stimulation of DM evoked call-like vocalizations in sedated birds, which caused a significant elevation in the activity levels in PAm NRB neurons. The effect of such electrically evoked calls was not measured for other classes of PAm neurons.
Our work provides the first physiological description of the various respiratory-related cell types found in PAm. The majority of cells encountered in PAm was identical to those described in mammalian systems. This similarity provides a basis for using the birdsong system as a model for understanding how respiratory centers interact with motor systems during vocalization.
GRANTS
Support for this research was provided by the National Institute on Deafness and Other Communication Disorders (R01DC6102) to M. F. Schmidt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M. and M.F.S. conception and design of research; J.M. and S.B. performed experiments; J.M. and S.B. analyzed data; J.M and M.F.S. interpreted results of experiments; J.M and M.F.S. prepared figures; J.M. and M.F.S. drafted manuscript; J.M. and M.F.S. edited and revised manuscript; J.M., S.B., and M.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Martin Wild for his continuous encouragement and advice throughout this project. We also thank Drs. Wild and Lindsey for their careful reading of the manuscript.
REFERENCES
- Adametz J, O'Leary JL. Experimental mutism resulting from periaqueductal lesions in cats. Neurology 9: 636–642, 1959 [DOI] [PubMed] [Google Scholar]
- Andalman AS, Foerster JN, Fee MS. Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. Plos One 6: e25461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J Neurosci 28: 2613–2623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci 25: 8543–8554, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurons of the cat. J Physiol 370: 433–456, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics. New York: Academic, 1981 [Google Scholar]
- Bianchi AL, Denavitsaubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995 [DOI] [PubMed] [Google Scholar]
- Borgmann A, Abdala APL, Zhang R, Rybak IA, Paton JFR, Smith JC. Spiking behavior and membrane potential trajectories of pre-BotC and hypoglossal neurons recorded from the rat in situ (Abstract). Experimental Biology 2011. Bethesda, MD: FASEB, 1074.11 [Google Scholar]
- Cohen MI. Discharge patterns of brain-stem respiratory neurons in relation to carbon-dioxide tension. J Neurophysiol 31: 142–165, 1968 [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Vu ET. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J Neurobiol 63: 70–89, 2005 [DOI] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Botzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992 [DOI] [PubMed] [Google Scholar]
- Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol 373: 63–86, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas-Ford J. A Comparative Molecular Study of the Amniote Dorsal Telencephalon (PhD dissertation) Chicago: University of Chicago, 2009 [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol 35: 429–450, 1990 [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol 373: 173–185, 1996 [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806–3816, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65–70, 2002 [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol 284: 242–252, 1989 [DOI] [PubMed] [Google Scholar]
- Jarvis E, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB, Avian Brain Nomenclature C Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6: 151–159, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. The neural control of vocalization in mammals: a review. J Voice 23: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behavior. Behav Brain Res 62: 107–117, 1994 [DOI] [PubMed] [Google Scholar]
- Jürgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motorneurons in the squirrel monkey. Brain Res 1148: 90–95, 2007 [DOI] [PubMed] [Google Scholar]
- Katada A, Sugimoto T, Utsumi K, Nonaka S, Sakamoto T. Functional role of ventral respiratory group expiratory neurons during vocalization. Neurosci Res 26: 225–233, 1996 [DOI] [PubMed] [Google Scholar]
- Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol 20: 748–753, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One 6: e20720, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JM, Dall'Asen AG, Goller F. Disrupting vagal feedback affects birdsong motor control. J Exp Biol 213: 4193–4204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol 37: 83–144, 1991 [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457–486, 1976 [DOI] [PubMed] [Google Scholar]
- Potash LM. Vocalizations elicited by brain stimulation in Coturnix coturnix Japonica. Behaviour 36: 149–167, 1970 [DOI] [PubMed] [Google Scholar]
- Reinke H, Wild JM. Distribution and connections of inspiratory premotor neurons in the brainstem of the pigeon (Columba livia). J Comp Neurol 379: 347–362, 1997 [PubMed] [Google Scholar]
- Reinke H, Wild JM. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J Comp Neurol 391: 147–163, 1998 [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982 [DOI] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci 28: 3479–3489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Ashmore RC. Integrating breathing and singing: forebrain and brainstem mechanisms. In: Neuroscience of Birdsong, edited by Zeigler HP, Marler P. New York: Cambridge University Press, 2008, p. 115–135 [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Botzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995 [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res 974: 43–59, 2003 [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Rybak IA, Paton JFR. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF, Vu ET. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. J Neurobiol 34: 27–40, 1998 [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J Neurosci 23: 1072–1086, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci 28: 12274–12283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Periaqueductal gray control of breathing. Adv Exp Med Biol 669: 353–358, 2010 [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. The nucleus retroambiguus control of respiration. J Neurosci 29: 3824–3832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario DS. Organization of the zebra finch song control system: II. functional organization of outputs from nucleus robustus archistriatalis. J Comp Neurol 309: 486–494, 1991 [DOI] [PubMed] [Google Scholar]
- Vicario DS, Simpson HB. Electrical stimulation in forebrain nuclei elicits learned vocal patterns in songbirds. J Neurophysiol 73: 2602–2607, 1995 [DOI] [PubMed] [Google Scholar]
- Wild J. Pulmonary and tracheosyringeal afferent inputs to the avian song system. In: Seventh Congress of the International Society for Neuroethology, Nyborg, Denmark, 2004a, p. 070 [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338: 225–241, 1993a [DOI] [PubMed] [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. In: Behavioral Neurobiology of Birdsong, 2004b, p. 438–462 [DOI] [PubMed] [Google Scholar]
- Wild JM. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Res 606: 319–324, 1993b [DOI] [PubMed] [Google Scholar]
- Wild JM, Li DF, Eagleton C. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columba livia) and zebra finch (Taeniopygia guttata). J Comp Neurol 377: 392–413, 1997 [DOI] [PubMed] [Google Scholar]
- Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J Neurobiol 24: 903–912, 1993 [DOI] [PubMed] [Google Scholar]