Abstract
The cortico-striato (Str)-globus pallidus external segment (GPe) projection plays major roles in the control of neuronal activity in the basal ganglia under both normal and pathological conditions. The present study used rat brain-slice preparations to address our hypothesis that the gain of this disynaptic projection is dynamically controlled by activations of short-term plasticity mechanisms of Str-GPe synapses. The Str-GPe projection neurons fire with very different frequency and firing patterns in vivo depending on the condition of the animal. The results show that the Str-GPe synapses have very strong short-term enhancement mechanisms and that repetitive burst activation of the Str-GPe synapses, which mimic oscillatory burst firing of Str neurons, can sustain enhanced states of synaptic transmission for tens of seconds. The results reveal that the short-term enhancement of Str-GPe synapses contributes to the generation of pauses in the firing of GPe neurons and that signal transfer function in the Str-GPe projection is highly dependent on the firing pattern of Str neurons.
Keywords: striatum, globus pallidus, IPSC, short-term plasticity
the cortico-striato (Str)-globus pallidus external segment (GPe) projection plays major roles in the control of neuronal activity in the basal ganglia under both normal and pathological conditions (Kita and Kita 2011a; Surmeier et al. 2010; Wu et al. 2011). We believe that the gain of this disynaptic projection is dynamically controlled by activations of short-term plasticity mechanisms of Str-GPe synapses.
Striatal projection neurons fire with very different frequency and patterns depending on the condition of the animal. Unit recordings in rodents and monkeys showed that most Str projection neurons were quiescent or only fired occasionally when animals were at rest. In task-performing animals, most behavioral-related unit activities occur as a train of firing lasting from 100 ms to over 1 s, with a maximum frequency of 100 Hz (Chen et al. 2001; Kimura 1990; Kubota et al. 2009). After dopamine (DA) depletion, a small population of Str neurons projecting to GPe fires oscillatory bursts under both anesthetized and wakeful animals due to altered glutamatergic and GABAergic inputs and altered membrane properties of the neurons (Chen et al. 2001; Kita and Kita 2011b; Mallet et al. 2006; Pang et al. 2001; Tseng et al. 2001; West and Grace 2002). These diverse activity patterns should alter the efficacy of the Str-GPe synapses. However, studies on the plasticity of Str-GPe synapses are scarce, and the results are inconsistent. Some studies reported that postsynaptic GABAergic responses recorded from GPe neurons to repetitive stimulation of Str exhibited a progressive facilitation during stimulation that developed in hundreds of milliseconds (Sims et al. 2008; Zucker and Regehr 2002). A number of studies also observed a moderate paired-pulse facilitation of the Str-GPe inputs (Cooper and Stanford 2001; Hashimoto and Kita 2008; Ogura and Kita 2000; Rueda-Orozco et al. 2009; Sims et al. 2008). Others reported progressive depression during stimulation (Rav-Acha et al. 2005, 2008) or slowly developing strong, long-term depression after the termination of repetitive stimulation (Rueda-Orozco et al. 2009). One of the aims of this study was to examine systematically the short-term plasticity of Str-GPe synapses.
Another aim was to gain insight about the induction of pauses in the most abundant type of high-frequency firing neurons in GPe (DeLong 1971). We have suspected that Str inputs generate the pauses and that the increased pause and burst activity of GPe neurons observed after DA depletion is due, in part, to the increased gain of the cortico-Str-GPe projection (Elias et al. 2007; Kita and Kita 2011a, b). Str-GPe synapses are known to generate weak (<10 pA), unitary inhibitory postsynaptic currents/inhibitory postsynaptic potentials (IPSCs/IPSPs) in GPe (Kita 2007; Tecuapetla et al. 2009), implying that a synchronous activation of a large number of Str projection neurons is required to induce pauses in GPe. However, since the population of spontaneously active Str projection neurons in resting animals appeared to be small, even after DA depletion (Kita and Kita 2011b), it was puzzling how such a small number of Str neurons could generate pauses in GPe. The present study addressed these questions using rat brain-slice preparations.
METHODS
Slice preparations.
Animal handling and all procedures were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee. Sprague-Dawley juvenile rats (16–21 days old) were used. Previous studies suggested that the physiological features of Str-GPe and Str-substantia nigra pars reticulata (SNr) synapses in rodents become adult like by approximately postnatal day 16 (Connelly et al. 2010; Ogura and Kita 2000). Rats were anesthetized with intraperitoneal injection of a mixture of ketamine (85 mg/kg) and xylazine (15 mg/kg) and perfused through the heart with cold, oxygenated (95% O2–5% CO2 gas mixture) solution containing (in mM): 252 sucrose, 3 KCl, 1.24 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 6.3 MgSO4, 0.2 thiourea, 0.2 ascorbic acid, and 20 glucose. After decapitation, the brains were removed rapidly, and blocks containing Str and GPe were obtained. Oblique sagittal slices (350 μm thick) were cut from the blocks on a vibrating blade microtome (Leica VT1000S; Leica Microsystems, Nussloch, Germany). The slices were incubated in oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 3 KCl, 1.24 NaH2PO4, 26 NaHCO3, 2.4 CaCl2, 1.3 MgSO4, 0.2 thiourea, 0.2 ascorbic acid, and 10 glucose at 34°C for 30 min and then at room temperature until the recordings were started.
Electrophysiological recordings.
The slices were transferred to a recording chamber with continuous perfusion of oxygenated ACSF at a flow rate of 1 ml/min. After placing the slice in the chamber, the temperature was increased gradually from room temperature to 32°C. Whole cell patch-clamp recording pipettes with a tip diameter of ∼1.5 μm were pulled from 1.5 mm, thin-wall, borosilicate glass capillaries on a horizontal electrode puller (P-97; Sutter Instrument, Novato, CA). The whole cell recording pipettes contained (in mM): 135 K-gluconate, 5 KCl, 10 HEPES, 0.1 CaCl2, 1 EGTA, 2 Mg-ATP, 0.2 Na-GTP, 10 phosphocreatine, and 0.2% Neurobiotin, with pH adjusted to 7.3 with KOH. The pipette resistance was 3–6 MΩ. Whole cell liquid junction potentials were calculated to be −11.6 mV for the internal solution, and membrane potentials were not corrected. Neurons and recording pipettes were visualized using an infrared differential interference contrast microscope (BX50WI; Olympus, Tokyo, Japan) with a ×40 water-immersion objective and charge-coupled device camera (4990 series; Cohu Electronics, San Diego, CA). The neurons were voltage clamped at −50 mV to eliminate the generation of action potentials. Our goal was to demonstrate the short-term enhancement of Str-GPe inputs in a setting with minimal deviations from normal physiological conditions. Thus the treatments to decrease membrane conductance, such as intracellular K+ and Na+ channel blockers, were not used. We believe that the conditions are adequate for acquiring the necessary data, even though the voltage clamp of distal dendrites is imperfect. In the current clamp recording of GPe neurons, the firing rates were maintained at ∼20 Hz by current injection through recording pipettes. Data were corrected and digitized with a sampling rate of 20 kHz using a MultiClamp 700B amplifier and an analog-to-digital converter board, Digidata 1322A (Molecular Devices, Sunnyvale, CA). Signals were filtered at 3 kHz and recorded on a hard disk using data acquisition and analysis software, pCLAMP 10 (Molecular Devices).
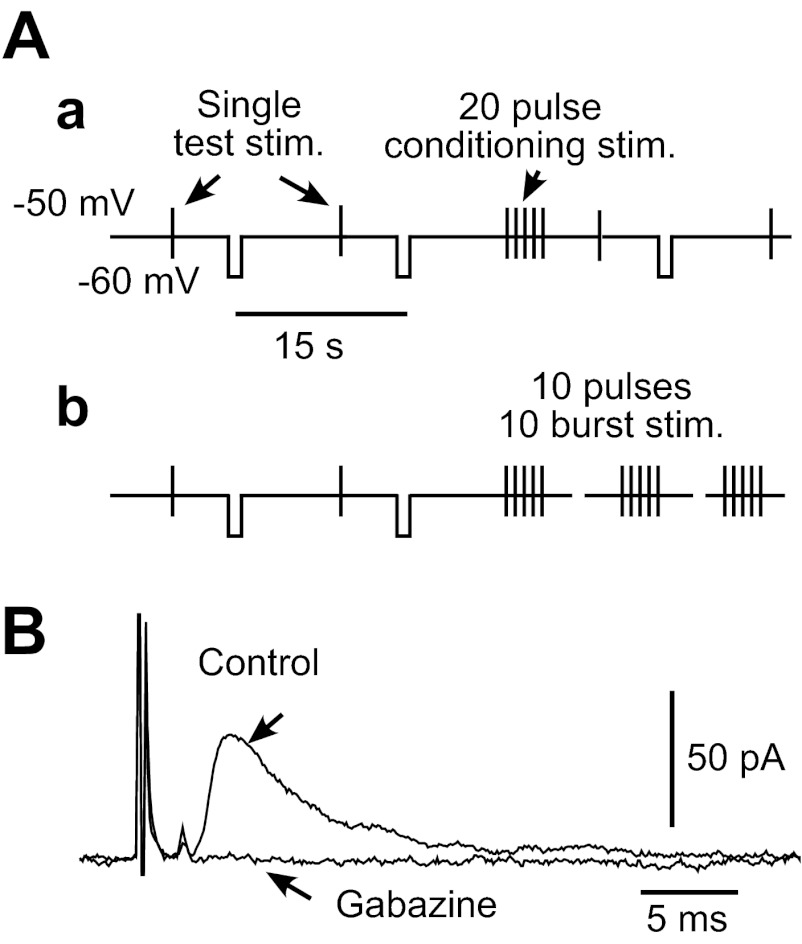
To activate Str-GPe fibers, electrical stimuli (each pulse 200 μs in duration) were applied through a bipolar-stimulating electrode (tip distance of 0.2–0.5 mm), placed in the Str. To block glutamatergic currents and isolate IPSCs and IPSPs, ACSF contained AMPA/kainate receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (10 μM; Tocris Cookson, Ellisville, MO) and N-methyl-d-aspartate antagonist 3-(2-carboxypiperzin-4-yl)-pro-pyl-1-phosponic acid (30 μM; Sigma-Aldrich, St. Louis, MO). The isolated, fast IPSCs were blocked completely by 10 μM gabazine (SR-95531; Sigma-Aldrich; Fig. 1B).
Fig. 1.
A: diagrams show stimulus (stim.) and the membrane-clamp schedules used in this study. Globus pallidus external segment (GPe) neurons were voltage clamped at −50 mV to block autonomous firing. Single test stimuli were given every 15 s, and −10 mV pulses were applied 5 s following each test stimulation to monitor the input impedance of the neuron. We used 2 repetitive stimulation schedules: single burst of 20 repetitive pulses with frequencies of 2–100 Hz (a) or 10 bursts of 10 repetitive pulses with 20 or 50 Hz and various interburst intervals (IBIs; b). B: stimulus intensity was adjusted to evoke 30–50 pA inhibitory postsynaptic currents (IPSCs) in test stimulation unless noted otherwise. For all recordings, artificial cerebrospinal fluid contained 10 μM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide and 30 μM 3-(2-carboxypiperzin-4-yl)-pro-pyl-1-phosponic acid to block AMPA/kainate and N-methyl-d-aspartate responses. Application of 10 μM gabazine completely abolished the fast IPSCs.
RESULTS
Type of neurons recorded.
The recordings were obtained from GPe neurons having spontaneous firing of 0–20 Hz and spike amplitudes >60 mV. The neurons generated repetitive firing without prominent spike accommodation upon depolarizing current injection, and hyperpolarizing currents induced either prominent or moderate sags due to an inwardly rectifying hyperpolarization-cyclic, nucleotide-activated current (Chan et al. 2004; Cooper and Stanford 2000; Günay et al. 2008). Intracellular staining with Neurobiotin revealed that these were also the most prominent rodent GPe neurons having medium-sized fusiform- or multipolar-shaped somata and long, slowly tapering, smooth dendrites (data not shown) (Kaneda et al. 2007; Kita and Kitai 1991).
Frequency-dependent, short-term plasticity.
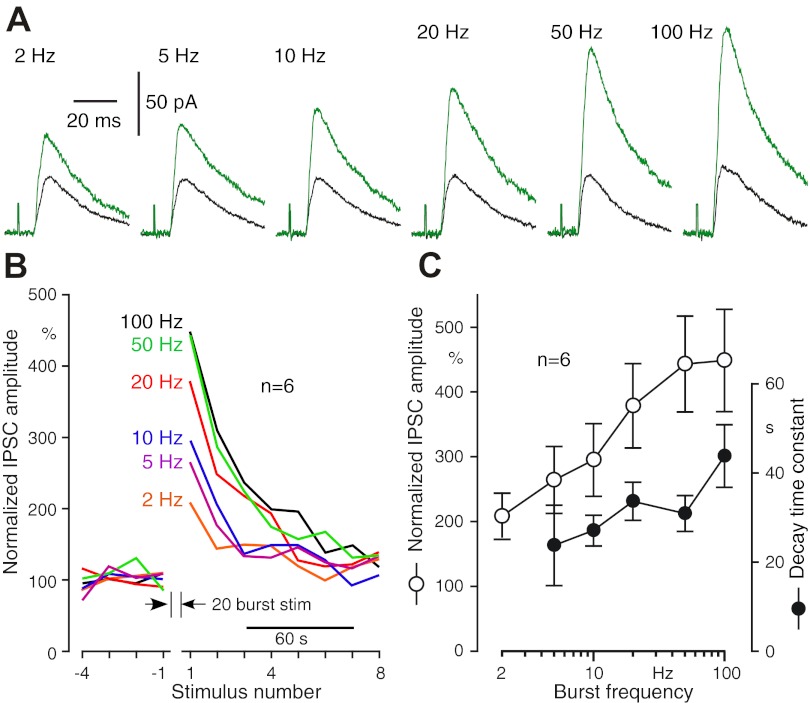
To reveal frequency-dependent plasticity of Str-GPe synapses, postsynaptic responses of GPe neurons to test and conditioning repetitive Str stimuli were recorded. For this experiment, the test stimuli were given every 15 s, and the intensity was adjusted to evoke 30–50 pA IPSCs before acquisition of data (Fig. 1). Each test stimulation was followed by a voltage-command pulse of −10 mV and 100 ms duration to monitor the input impedance of the neuron. The conditioning stimulation was 20 repetitive stimuli at 2–100 Hz with the same intensity as in the test stimulation. Figure 2 shows responses to conditioning stimulation, which resulted in frequency-dependent enhancement of IPSCs. The mean amplitude of four test IPSCs recorded prior to the conditioning was used as the baseline amplitude. With 2 Hz stimulation, weak enhancement was visible (e.g., Fig. 2A), and comparisons between the baseline and the mean of the last four IPSCs showed that the increase was significant (n = 6; P = 0.037, paired t-test). With 5–20 Hz stimuli, strong enhancement developed faster with higher stimulus frequency. With 50 Hz stimuli, IPSCs were enhanced for the initial 5–10 stimuli but decreased gradually for those evoked by later stimuli (Fig. 2E). A likely factor for the decrease is the shunting of IPSCs by a summated synaptic conductance increase, as discussed in more detail below. The enhancement may be a combination of facilitation and augmentation of synaptic transmission. The former develops in a time scale of hundreds of milliseconds during repetitive stimuli and decays rapidly after the termination of the stimuli. The latter develops and decays with a slower time constant of several to tens of seconds during repetitive stimuli [see Catterall and Few (2008), Xu et al. (2007), and Zucker and Regehr (2002) for reviews]. However, these two forms of enhancements are not readily differentiated in the present study due to the use of a wide range of conditioning frequencies.
Fig. 2.
Responses of GPe neurons to burst striatal (Str) stimulation at various frequencies. A–E: IPSCs recorded from a GPe neuron, voltage clamped at −50 mV. F: plots of IPSC amplitudes shown in A–E. G: group plots of 6 neurons. IPSC amplitudes were normalized with the mean amplitude of 4 IPSCs evoked before burst stimulation.
Figure 3 shows changes of IPSC amplitudes after termination of the conditioning stimulation. The first test stimulation was applied 5 s after the termination of a conditioning stimulation and every 15 s thereafter. In all cases, the amplitude of IPSCs to the first test stimulation was significantly larger than the preconditioning baseline amplitude (Fig. 3A). The slowly decaying enhancement of synaptic transmission observed after the termination of conditioning stimulation will be hereafter called postconditioning enhancement, although the enhancement is most likely a combination of an augmentation and a post-tetanic potentiation. These two forms have different kinetics and underlying mechanisms but often coexist. Both develop with similar time constants, but the latter decays more slowly than the former and can last for 30 s to several minutes [see Zucker and Regehr (2002) for review]. The degree of the enhancement was dependent on the frequency of the conditioning stimulation with a saturation occurring at ∼50 Hz. It should be noted that 50 and 100 Hz stimulation induced strong enhancement of IPSCs, which supported the hypothesis that a shunting could decrease the IPSC amplitude during high-frequency conditioning. The decay time constant of the postconditioning enhancement was estimated using an exponential regression to the responses to test stimuli applied every 15 s. The decay constant was also conditioning frequency dependent and was ∼25 s for the 5-Hz and 45 s for the 100-Hz conditioning stimulation. The slower decay with higher stimulus frequency may be due to an increased contribution of the post-tetanic potentiation component over the augmentation component in the higher-frequency conditioning stimulation.
Fig. 3.
Postconditioning enhancement of Str-GPe IPSCs. A: examples of the enhancement of IPSCs. An overlay plot of IPSCs recorded before and 5 s after a conditioning stimulation consisting of 20 pulses with different frequencies. B: group data from 6 neurons show that the degree of enhancement is dependent on the frequency of the conditioning stimuli. The amplitudes of IPSCs were normalized by the mean of 4 IPSCs, evoked before a conditioning stimulation. C: plots of the mean amplitude of the 1st IPSCs evoked after the conditioning and the decay time constant. The decay time constants were estimated from responses to test stimuli applied every 15 s.
Responses to repetitive burst stimuli.
It is known that some Str-GPe neurons maintain repeated burst firing after DA depletion (Kita and Kita 2011a; Mallet et al. 2006; Tseng et al. 2001; West and Grace 2002). We have characterized the bursts in DA-depleted Str of lightly anesthetized rats using the “surprise” method of Legendy and Salcman (1985) and defining the burst as having the surprise value as greater than or equal to three and the number of spikes as greater than or equal to three. The average burst duration of spontaneously active Str-GPe neurons was ∼260 ms, with nine spikes/burst (i.e., ∼30 Hz) and an average interburst interval (IBI) of 2.4 s (Kita and Kita 2011b). It is conceivable that the decay time of the postconditioning synaptic enhancement is slow enough to maintain the enhanced condition for the duration of pauses between bursts. To test this possibility, responses of GPe neurons to 10 sets of burst Str stimuli, each burst consisting of 10 pulses at 20 or 50 Hz and IBI of 2–40 s, were recorded (Fig. 4). The stimulus intensity was pre-adjusted to evoke ∼30 pA IPSCs in a 15-s interval, single-pulse trial stimuli. In both 20 and 50 Hz burst stimuli, the IPSCs were enhanced three to four times during the first burst stimulation, as described above. It was apparent that with the 20 and 50 Hz with 2-s IBI stimuli, the responses to the second and succeeding burst stimulation began at a higher level of four to seven times the baseline IPSC amplitude and then decayed during each burst (Fig. 4, A and B). The carryover of the enhancement to the next burst was still observed with 10-s IBIs (Fig. 4, C and E) but was not apparent with 40-s IBIs (Fig. 4D). We performed current clamp recordings to show that the carryover of enhanced IPSPs contributes to the generation of pauses in repetitively firing GPe neurons. The neurons recorded were maintained to fire at ∼20 Hz by current injection, and the Str stimulus intensity was adjusted to evoke small IPSPs in single stimulus mode. Four neurons were tested with both 20 and 50 Hz burst stimuli, with 2-s IBI. In both stimulus conditions, the inhibitory effect of the burst stimuli grew and reached a complete pause of firing (Fig. 4, F and G). The number of bursts needed to reach a complete pause was dependent on the strength of the stimulus. With strong, high-frequency bursts, the pause duration outlasted that of the burst stimuli due to activation of other receptors, such as GABAB (Fig. 4G) (Kaneda et al. 2007). These results demonstrate that when multiple burst activity of Str neurons occurs with intervals shorter than the decay time of the repetitive activation-induced enhancement, the enhancement developed during prior burst activation of Str-GPe inputs can be carried over to the next burst activity.
Fig. 4.
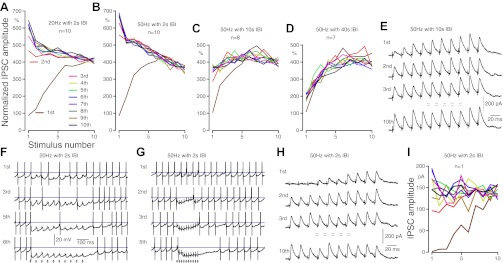
Repetitive burst activation enhanced Str-GPe synapses. A–D: group data show changes in IPSC amplitude recorded from GPe neurons during Str burst stimuli, 10 sets of 10 pulses with different frequencies and IBIs. For each neuron, the amplitudes of the IPSCs were normalized by the average of 4 IPSCs evoked by 4 single stimuli given prior to burst stimuli in 15-s intervals. A and B: IPSCs of GPe neurons to 20 and 50 Hz burst stimulation with 2-s IBIs. The IPSCs to 1st burst stimuli were greatly enhanced (brown lines). The IPSCs to succeeding burst stimuli were started from an enhanced amplitude and declined during burst, with the degree of enhancement and decline depending on the frequency of the stimuli. B–D: compare results of 50 Hz burst stimulation with 2- to 40-s IBIs. The carryover of enhanced IPSCs is clearly visible with 10-s IBIs but is very small with 40-s IBIs. E: responses of a GPe neuron to 50 Hz burst stimulation with 10-s IBIs. F and G: examples of current clamp recordings to show that repetitive burst activation of Str can induce pauses of firing in GPe neurons that were maintained to fire at ∼20 Hz by current injection. Action potentials were truncated, and horizontal lines mark −40 mV. H and I: responses of a GPe neuron to a threshold intensity 50 Hz burst stimulation with 2-s IBIs. The reduction rate of IPSC amplitude during the burst was much lower compared with that of responses to stronger burst stimulation (compare B with I). It is also clear that the threshold intensity at a single stimulus can evoke large IPSCs in burst activation conditions.
Similar to the 20-pulse conditioning stimulation experiments described earlier, the rate of the IPSC decay was stronger for the 50-Hz than the 20-Hz burst stimuli (Fig. 4, A and B). The most likely factor for this decrease was the shunting—i.e., the postsynaptic input-impedance increase generated by prior IPSCs—at the dendritic synaptic sites of Str axons. To test this possibility, responses to a low-intensity, near-threshold (to evoke IPSCs), 50-Hz burst stimulation with 2-s IBIs were recorded (Fig. 4, H and I). Because the stimulus intensity was near the threshold, original IPSC amplitudes, instead of normalized ones, were plotted in Fig. 4I. Consistent with the hypothesis, the reduction rate of IPSC amplitude during the burst was much lower compared with the responses to stronger burst stimulation (compare Fig. 4, B with I). It is also clear that the near-threshold intensity at a single stimulus can evoke large IPSCs in a burst activation condition.
Effects of D2 agonist and low extracellular Ca2+.
Application of DA D2 receptor (D2R) agonists decreases the probability of GABA release from Str-GPe terminals (Cooper and Stanford 2001; Shin et al. 2003; Watanabe et al. 2009). Lowering Ca2+ concentration in the extracellular fluid also decreases the probability by reducing Ca2+ influx into the presynaptic terminals. We have investigated whether reducing the GABA release probability affects the development of the short-term enhancement. Bath application of the D2R agonist quinpirole, 10 μM, reduced the amplitude of Str stimulation-induced IPSCs in GPe neurons, as reported previously (Cooper and Stanford 2001; Shin et al. 2003; Watanabe et al. 2009). Although the amplitude was decreased, the enhancement of IPSCs to burst Str stimulation was still observed in the presence of quinpirole. However, the speed of the enhancement development was slower in quinpirole than in the control (Fig. 5, A–C). Reduction of extracellular Ca2+ from 2.4 to 1.2 mM had similar results (Fig. 5, D–F). These results show that even in conditions that reduce the influx of Ca2+ to presynaptic terminals, the short-term enhancement of Str-GPe synapses to repetitive activation does occur but with slower development speed.
Fig. 5.
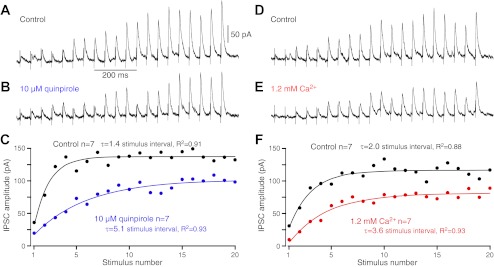
The dopamine D2 receptor agonist quinpirole (10 μM) and lowering extracellular Ca2+ from 2.4 to 1.2 mM reduced IPSC amplitude and slowed development of repetitive stimuli-induced enhancement. A and B: examples of responses of a GPe neuron show enhancement of IPSCs to 20 Hz, 20 pulse burst stimuli in the control and in quinpirole. C: group data compare the development of enhancement before and after quinpirole. The exponential regression curves were computed to compare the progress of the enhancement. The dimension of the time constants (τ) is the stimulus interval. D–F: lowering extracellular Ca2+ from 2.4 to 1.2 mM resulted in similar effects to quinpirole.
DISCUSSION
Activation pattern-dependent, short-term enhancement of Str-GPe synapses.
The primary aim of this study was to reveal how the different patterns of activation of Str-GPe synapses affect the efficacy of synaptic transmission. Str projection neurons require synaptic inputs to evoke firing and fire in a very different manner depending on the activation of afferent inputs that are dependent on the animal's condition. Unit recordings in wakeful rodents and monkeys showed that spontaneously active, presumed Str projection neurons, which had a low-frequency phasic activity (and were thus named phasically active neurons), were only occasionally encountered and that most of the neurons, which could be driven by cortical stimulation, were quiescent or only occasionally fired when animals were at rest. An intracellular recording from wakeful animals showed that some spontaneously active neurons show a depolarized membrane with slow, irregular fluctuations intermingled with high-frequency depolarizing events and trigger more random, yet still phasic, low-frequency firing (Mahon et al. 2006). This occasional Str firing in wakeful animals should evoke very weak inhibitions in GPe neurons, because Str stimulation-induced unitary IPSCs/IPSPs are very small (Kita 2007; Ogura and Kita 2000; Tecuapetla et al. 2009).
In drowsy or anesthetized animals, the membrane potential of Str projection neurons shifts between hyperpolarized and depolarized states in synchrony with cortical slow waves, suggesting that cortical activity drives the membrane oscillations. In most of the Str neurons, the depolarized stages are mostly subthreshold, and only occasional spikes are triggered from depolarized states. In a small population of Str neurons, the depolarized states are suprathreshold, and the neurons fire an oscillatory burst with several spikes (Mahon et al. 2006; Stern et al. 1998; Tseng et al. 2001; Wilson and Groves 1981). The neurons with an oscillatory burst were encountered only occasionally in unit recordings in normal, lightly anesthetized rats (Kita and Kita 2011a). However, after DA depletion, Str-GPe neurons firing with a slow oscillatory burst were encountered more frequently in both anesthetized and wakeful animals (Chen et al. 2001; Kita and Kita 2011a; Mallet et al. 2006; Pang et al. 2001; Tseng et al. 2001; West and Grace 2002). This study revealed that repetitive activation of Str-GPe synapses shows a strong short-term enhancement, similar to those reported by Sims et al. (2008). This study further revealed that the repetitive activation-induced enhancement of Str-GPe synapses decays slowly, with a decay constant of 20–40 s. Based on these results, it is likely that these oscillatory burst-firing neurons in vivo may maintain enhanced synaptic inhibition during IBI, and synchronous burst activity of a relatively small number of Str neurons may effectively generate pauses in the firing of GPe neurons. In DA-depleted animals, the pre- and postsynaptic DA suppression of Str-GPe synapses is removed (Cooper and Stanford 2001; Floran et al. 1997; Shin et al. 2003; Watanabe et al. 2009), which results in further increases in the Str-GPe inhibition, generating stronger pauses and rebound burst firing.
The short-term enhancement of Str-GPe synapses may also play significant roles in the control of movement in normal animals. Unit recordings from presumed Str projection neurons in monkeys and rodents showed that most behavioral, event-related unit activities occur as a train of firing, lasting from 100 ms to over 1 s, with a maximum frequency of up to 100 Hz (Chen et al. 2001; Kimura et al. 1990; Kubota et al. 2009). The results of this study suggest that the train of activation of Str-GPe synapses will generate very strong postsynaptic inhibitions in GPe neurons. When repetitive trains of activation occur with <40-s intervals, within which many behavioral tasks to be learned can be retried, the enhancement developed during the previous train of activation will be carried over to the subsequent trains of activation. Thus the enhancement provides a form of learning or reinforcement mechanism.
This study confirmed the result of Sims et al. (2008) that the reduction of extracellular Ca2+ concentration reduces the amplitude of Str-GPe IPSCs, probably by reducing the probability of GABA release. However, it does not eliminate the short-term enhancement of Str-GPe synapses. The effects of bath application of quinpirole on synaptic enhancement were similar to the reduction of extracellular Ca2+, suggesting that quinpirole may reduce the Ca2+ influx to presynaptic terminals in a similar manner to that of the somato-dendritic membrane [see Momiyama and Koga (2001) and Surmeier et al. (2010) for a review]. Studies in other brain sites showed that the short-term synaptic enhancement mechanisms involve Ca2+ influx to presynaptic terminals (Catterall and Few 2008; Xu et al. 2007; Zucker and Regehr 2002). The present results demonstrated that the short-term enhancement of the Str-GPe synapse could take place under normal conditions, in which the extracellular Ca2+ concentration is ∼1.2 mM, and some level of D2R activation is present, as well as in parkinsonian conditions, in which the D2R-mediated presynaptic suppression is removed.
Other possible modes of plasticity.
Str-SNr synapses also show short-term enhancement (Connelly et al. 2010). Thus both Str-GPe and Str-SNr synapses have short-term enhancement mechanisms. These studies, which demonstrated short-term enhancement, all used moderate intensities and a moderate number of repetitive stimuli. However, it is possible that different modes of stimulation or different experimental conditions might induce different modes of synaptic plasticity. Rav-Acha and associates (2005) reported that 5–33 Hz repetitive Str stimuli induced rapid, frequency-dependent suppression of IPSCs recorded from GPe neurons. It is not certain what experimental differences drive short-term suppression or enhancement, as discussed by Sims and associates (2008). Differences could include submaximal vs. moderate stimulus intensity, and the former may activate GPe-Str axons in addition to Str-GPe neurons (Ogura and Kita 2000). The synapses formed by GPe axons show short-term depression (Baufreton and Bevan 2008; Connelly et al. 2010). It is also possible that submaximal stimulation activated a larger number of axons to release more transmitters and activated other receptors, such as pre- and postsynaptic GABAB receptors. Another difference among the prior studies was the ages of animals used in the experiments. Rav-Acha et al. (2005) used 10- to 16-day-old, Sims et al. (2008) used 20- to 21-day-old, and we used 16- to 21-day-old rats, which raises the possibility of short-term plasticity changes during development, as shown in Str-SNr synapses (Connelly et al. 2010). Rueda-Orozco and associates (2009) reported another mode of plasticity. They showed that a conditioning stimulus with a large number of repetitive pulses, two trains of 100 Hz, and 1 s-long stimuli, delivered to Str with an intertrain interval of 10 s (i.e., a total of 200 stimulus pulses), induced a slowly developing, strong, long-term depression of Str-GPe IPSCs. Although responses during the conditioning stimulation were not described, the IPSC amplitude recorded soon after the conditioning stimulation was similar to the baseline, suggesting that short-term plasticity was not induced. The authors suspected that the depression may be postsynaptically mediated, because there were no changes to the paired pulse ratio (Rueda-Orozco et al. 2009). Although the stimulus conditions differed, the remaining experimental conditions between the Rueda-Orozco study (2009) and our study were similar. It is possible that the use of long trains of high-frequency conditioning stimulation may deplete the readily releasable pool of presynaptic neurotransmitter vesicles or that the development of strong, long-term depression mechanisms may have masked the short-term enhancement.
Functional implications.
Most Str-GPe synapses have been known to generate small, unitary IPSCs/IPSPs in GPe (Kita 2007; Ogura and Kita 2000; Tecuapetla et al. 2009). Thus a general consensus is that a synchronous activation of a large number of Str neurons is required to generate effective inhibition in GPe neurons. The results of the present study suggest that the strong, short-term enhancement mechanisms of Str-GPe synapses allow synchronous activation of a relatively small number of Str neurons to generate powerful behavioral, event-related inhibitions in GPe. Furthermore, the slow decay of the enhancement provides a learning or reinforcement mechanism during repeated practices, which is often attributed to Str functionality. This study also suggests that under DA-depleted conditions, overactive Str-GPe neurons will generate more powerful behavioral, event-related inhibitions and more frequent pauses and rebound burst firing in GPe, which then invokes a cascade of alterations in the activity of the basal ganglia (Kita and Kita 2011a).
GRANTS
Support for this work was provided by the National Institute of Neurological Disorders and Stroke Grants NS-47085 and NS-57236.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.K. and H.K. conception and design of research; J.K. performed experiments; J.K. and H.K. analyzed data; J.K. and H.K. interpreted results of experiments; J.K. and H.K. prepared figures; J.K. and H.K. drafted manuscript; J.K. and H.K. edited and revised manuscript; J.K. and H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank R. Kita for editing this manuscript.
Present address of J. Kim: Div. of Bio-Information Engineering, Faculty of Engineering, Univ. of Toyama, 3190 Gofuku, Toyama 930-8555, Japan.
REFERENCES
- Baufreton J, Bevan MD. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. J Physiol 586: 2121–2142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901, 2008 [DOI] [PubMed] [Google Scholar]
- Chan CS, Shigemoto R, Mercer JN, Surmeier DJ. HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons. J Neurosci 24: 9921–9932, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MT, Morales M, Woodward DJ, Hoffer BJ, Janak PH. In vivo extracellular recording of striatal neurons in the awake rat following unilateral 6-hydroxydopamine lesions. Exp Neurol 171: 72–83, 2001 [DOI] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JN. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci 30: 14854–14861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology 41: 62–71, 2001 [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro. J Physiol 527: 291–304, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol 34: 414–427, 1971 [DOI] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci 27: 2525–2538, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floran B, Floran L, Sierra A, Aceves J. D2 receptor-mediated inhibition of GABA release by endogenous dopamine in the rat globus pallidus. Neurosci Lett 237: 1–4, 1997 [DOI] [PubMed] [Google Scholar]
- Günay C, Edgerton JR, Jaeger D. Channel density distributions explain spiking variability in the globus pallidus: a combined physiology and computer simulation database approach. J Neurosci 28: 7476–7491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kita H. Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J Neurophysiol 99: 1723–1732, 2008 [DOI] [PubMed] [Google Scholar]
- Kaneda K, Kita T, Kita H. Repetitive activation of glutamatergic inputs evokes a long-lasting excitation in rat globus pallidus neurons in vitro. J Neurophysiol 97: 121–133, 2007 [DOI] [PubMed] [Google Scholar]
- Kimura M, Kato M, Shimazaki H. Physiological properties of projection neurons in the monkey striatum to the globus pallidus. Exp Brain Res 82: 672–676, 1990 [DOI] [PubMed] [Google Scholar]
- Kita H. Globus pallidus external segment. Prog Brain Res 160: 111–133, 2007 [DOI] [PubMed] [Google Scholar]
- Kita H, Kita T. Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J Neurosci 31: 10311–10322, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kita T. Role of striatum in the pause and burst generation in the globus pallidus of 6-OHDA-treated rats. Front Syst Neurosci 5: 42, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res 564: 296–305, 1991 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Liu J, Hu D, DeCoteau WE, Eden UT, Smith AC, Graybiel AM. Stable encoding of task structure coexists with flexible coding of task events in sensorimotor striatum. J Neurophysiol 102: 2142–2160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci 26: 12587–12595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 26: 3875–3884, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama T, Koga E. Dopamine D(2)-like receptors selectively block N-type Ca(2+) channels to reduce GABA release onto rat striatal cholinergic interneurones. J Physiol 533: 479–492, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura M, Kita H. Dynorphin exerts both postsynaptic and presynaptic effects in the globus pallidus of the rat. J Neurophysiol 83: 3366–3376, 2000 [DOI] [PubMed] [Google Scholar]
- Pang Z, Ling GY, Gajendiran M, Xu ZC. Enhanced excitatory synaptic transmission in spiny neurons of rat striatum after unilateral dopamine denervation. Neurosci Lett 308: 201–205, 2001 [DOI] [PubMed] [Google Scholar]
- Rav-Acha M, Bergman H, Yarom Y. Pre- and postsynaptic serotoninergic excitation of globus pallidus neurons. J Neurophysiol 100: 1053–1066, 2008 [DOI] [PubMed] [Google Scholar]
- Rav-Acha M, Sagiv N, Segev I, Bergman H, Yarom Y. Dynamic and spatial features of the inhibitory pallidal GABAergic synapses. Neuroscience 135: 791–802, 2005 [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Mendoza E, Hernandez R, Aceves JJ, Ibanez-Sandoval O, Galarraga E, Bargas J. Diversity in long-term synaptic plasticity at inhibitory synapses of striatal spiny neurons. Learn Mem 16: 474–478, 2009 [DOI] [PubMed] [Google Scholar]
- Shin RM, Masuda M, Miura M, Sano H, Shirasawa T, Song WJ, Kobayashi K, Aosaki T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J Neurosci 23: 11662–11672, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RE, Woodhall GL, Wilson CL, Stanford IM. Functional characterization of GABAergic pallidopallidal and striatopallidal synapses in the rat globus pallidus in vitro. Eur J Neurosci 28: 2401–2408, 2008 [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature 394: 475–478, 1998 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res 183: 149–167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Koos T, Tepper JM, Kabbani N, Yeckel MF. Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 29: 8977–8990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci 21: 6430–6439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kita T, Kita H. Presynaptic actions of D2-like receptors in the rat cortico-striato-globus pallidus disynaptic connection in vitro. J Neurophysiol 101: 665–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22: 294–304, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res 220: 67–80, 1981 [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage 55: 204–215, 2011 [DOI] [PubMed] [Google Scholar]
- Xu J, He L, Wu LG. Role of Ca(2+) channels in short-term synaptic plasticity. Curr Opin Neurobiol 17: 352–359, 2007 [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002 [DOI] [PubMed] [Google Scholar]