Abstract
Background
Lymphatic filariasis (LF) affects more than 120 million people worldwide. Efforts to eliminate this disease require sustained community participation. This study explores community valuation of LF elimination efforts by estimating household and community willingness to pay (WTP) for the prevention of transmission and treatment of filarial lymphedema in the community of Leogane, Haiti.
Methods
A contingent valuation survey was used to assess individual WTP for specific prevention and treatment interventions. A 2-dimensional Monte Carlo simulation was developed to estimate confidence limits in mean WTP and to generate a distribution of WTP for the community, accounting for uncertainty in regression coefficients and variability within the population.
Results
Mean WTP was estimated to be $5.57/month/household (95% CL: $4.76, $6.72) to prevent disease transmission, and $491/yr (95% CL: $377, $662) for treatment of lymphedema for one person. Based on the estimated distributions, 7% and 39% of households were not willing to pay for prevention and treatment, respectively.
Conclusions
These results suggest that the majority of the community places a positive value on both prevention and treatment of LF. Mean WTP provides a useful monetary estimate of overall societal benefit of LF prevention and treatment programs. However, for interventions which require broad and sustained community participation, the lower end of the distribution of WTP has additional implications. Cost recovery policies may result in inadequate participation and longer program duration.
Keywords: Lymphatic filariasis, willingness to pay, cost, contingent valuation
Background
Lymphatic filariasis, a mosquito-transmitted parasitic disease, affects an estimated 120 million people worldwide, causing lymphatic dysfunction in virtually all those infected and recurrent acute adenolymphangitis (ADL), hydrocele, and lymphedema in millions of affected persons. Infection with the parasite results in damage to lymphatic vessels, leading to swelling of the limbs and genitals and painful bacterial infections. As the swelling progresses, it becomes difficult for individuals to carry out their household and job-related activities [1]. The disfigurement caused by the disease can also lead to social stigma and difficulty in fulfilling social roles. The burden of the disease includes medical expenses, lost productivity, value of familial assistance, and the value associated with diminished social function and quality of life [2].
Following the 1997 declaration by the World Health Assembly and the subsequent drug donations by SmithKline Beecham (now GlaxoSmithKline) and Merck & Co. Inc., global and local efforts to eliminate LF as a public health problem were intensified. These efforts are directed toward interrupting transmission of the parasite and providing treatment for people with clinical filarial disease. In selecting and allocating resources, local and national decision makers along with international donors need information on the value that filariasis-endemic communities place on prevention and treatment services. Several studies have estimated the economic value of certain aspects of disease burden, particularly the cost of medical attention and the productivity losses associated with the disease [1,4-6]. However anthropological studies of communities with endemic LF suggest that the more intangible benefits of preventing and treating filarial disease (e.g. reduced social stigma) may provide a greater contribution to social wellbeing [7]. This study uses a contingent valuation method to estimate the economic value of both the direct and intangible benefits of preventing and treating lymphatic filariasis in a Haitian community. Contingent valuation studies are typically used to characterize the mean or total value placed on an improvement in health within a community, and compare it to the costs of the improvement. Here the approach also is used to examine distribution of household willingness to pay (WTP) for interventions within the community and the expected impact on participation rates, to provide a more complete measure of the benefits of these projects.
Methods
Lymphatic filariasis is endemic to parts of Haiti, including the town of Leogane and its surrounding communities. The descriptions of the treatment and prevention programs in the valuation exercises are modeled after projects conducted in the Leogane area. The treatment intervention includes the management of lymphedema and prevention of adenolymphangitis (ADLs) through improved hygiene, skin care, and physical measures, such as elevation and movement. Two prevention strategies can be used to interrupt transmission of LF, either regular use of table salt fortified with diethylcarbamazine (DEC) [8] or mass administration with a 2-drug combination: DEC plus albendazole or ivermectin plus albendazole [9]. Both kill the microfilaria produced by adult parasites, preventing further transmission. For this study DEC-fortified salt was the intervention used in the valuation exercise.
Survey Instrument
Individual WTP was elicited using an in-person survey conducted between November and December 1997, prior to the implementation of a DEC-fortified salt distribution program [8]. The survey was translated into Haitian Creole and was developed and pre-tested with the help of bilingual (Haitian Creole and English) residents of Leogane. The survey's four sections included information on demographics, knowledge and attitudes about LF, and two dichotomous choice WTP exercises (one for a prevention intervention and the other for a lymphedema treatment intervention). Demographic information included sex, age, household size, monthly household cash income, and type of house construction (cement, wood, or thatch). The latter was used as an index of wealth. The questions regarding knowledge and attitudes towards filariasis were based on earlier anthropological work on community perceptions of the disease in Leogane [7]. The section included questions on familiarity with the different disease-related conditions, perceived risk of being infected with the filarial parasite, and the respondents' beliefs about transmission of the disease.
In the prevention exercise, individuals were asked about WTP for the prevention of infection in the entire household. Respondents were asked whether they would be willing to pay a predetermined amount to purchase DEC-fortified salt for their family, in order to prevent new filarial infections among family members. The respondents replied "yes" or "no" to the questions. Dichotomous choice questions are recommended over open-ended questions that ask individuals directly how much they are willing to pay [10]. Although the latter are statistically easier to analyze, they are often difficult for respondents to answer and may not result in individuals reporting their true maximum willingness to pay.
The valuation exercise for the treatment intervention followed the same basic format as that used to value prevention. Respondents were asked to imagine that they had lymphedema. A hypothetical condition was described, along with a course of treatment and expected improvement in health status from the treatment. Improvement was described as including cessation of acute attacks, reduced swelling, and improvement in mobility. The amount of time each day required for treatment was also described. Respondents were then asked whether they would pay different predetermined amounts for the supplies required for the lymphedema treatment (e.g., soap, towel, basin, antiseptics). The treatment exercise elicited WTP for treatment of one individual. In both exercises, bid amounts were presented in Haitian gourdes. They are converted to U.S. dollars in the results presented here.
For both exercises, each respondent was offered a single bid amount. The bids for each respondent were chosen randomly from 5 and 4 possible values for the prevention and treatment interventions, respectively. Following the general approach outlined by Alberini [11], bids were selected using limited data from pilot testing. Bid amounts were intended to cover the expected range of true underlying WTP values of respondents based on data from pilot testing with 25 individuals. Using a single bid value for all respondents can result in inefficient and biased parameter estimates [12].
The study area has an estimated population of approximately 20,000 individuals. The survey was administered to a random sample of adults in the town of Leogane and its immediate surroundings. Sampling was based on the division of area into four geographically defined regions: two in central Leogane (east and west) and two immediately adjacent communities (one to the north and one to the south). Within each area houses were selected by starting at a random corner and choosing every third house on the street. When the end of the street was reached, the process was begun on an adjacent street. Sampling within each area continued until 150 sample households were identified and one adult household member was interviewed. The study protocol and data collection instrument were approved by the human subjects protection committees of the Centers for Disease Control and Prevention and the Hospital Ste. Croix (Leogane, Haiti).
Data analysis
The data were analyzed from three perspectives: the distribution of household WTP within the community, the community's total WTP, and the expected participation of households in the interventions. Data were analyzed in two stages. In the first stage, data from the survey were used in two regression models to estimate household WTP for prevention of infection in the entire household and treatment of one individual, based on household characteristics. Willingness to pay for treatment (WTPt) and prevention (WTPp) interventions were each estimated with a single bound probit model using the responses to the valuation question. The probit model estimates the likelihood that the household would be willing to pay different amounts for the interventions, rather than estimating a single WTP for each household. The shape and location of the likelihood function depend on several household characteristics.
The model for WTPt included sex, age, income, whether someone in the household had lymphedema or hydrocele, accurate knowledge of the causes of filariasis, household construction type (cement vs. wood/thatch) as a proxy for wealth, and the starting bid. The model for WTPp included these same variables plus two additional ones: household size and perceived risk. The model was specified as
Pr {'yes'} = Φ(α + βA),
where Φ is the cumulative distribution function of the standard normal distribution, α and β are the estimated regression parameters, and A is the vector of household characteristics described above. The LIMDEP econometric software package was used to obtain the maximum likelihood estimates for the regression parameters [13]. The order in which the WTP questions were presented did not influence valuation responses in the initial model and this was not included in the final model.
In the second stage of the data analysis the results of the regression model were used to estimate the distribution of household WTP for prevention and treatment within the community (rather than that of individual households). Several approaches can be used to estimate population mean willingness to pay from the estimated household likelihood functions. The traditional approach of estimating WTP for each respondent in the sample to estimate the mean and distribution was not used. This approach was not used in effort to adjust for potential selection bias in our sample. Because a disproportionate number of respondents were women and approximately 35% did not provide their income, to adjust for potential selection bias in our sample a Monte Carlo simulation was used to estimate the mean and the distribution of household WTP, using Crystal Ball software [14]. The simulation model captures the variability within the population by repeatedly calculating the function estimated in the regression, using different combinations of household characteristics (income, number of members, etc.). This approach allowed us to use the expected gender distribution in the community and an estimated income distribution (based on those who responded), in order to adjust for potential biases within our sample. The model also incorporates uncertainty in the individual regression coefficient estimates.
Variability in respondent and household attributes was characterized by fitting distributions to the different independent variables (income, household members, age, etc.), using the survey data. Continuous distributions were fit for income, age, and number of household members using Crystal Ball's distribution fitting procedures. Discrete distributions were used for categorical data (e.g. sex, knowledge, house type). Uncertainty in the regression coefficients was characterized based on the estimated variances and covariances of the parameters estimated in the regression model.
A 2-dimensional Monte Carlo simulation model was used to separate variability in household characteristics from uncertainty in the estimated coefficients of the determinants of WTP. For both prevention and treatment, the model operates by first selecting a random set of regression coefficients from their respective distributions. Holding these coefficient values constant, the model then randomly selects 1,000 combinations of household characteristics, based on the distribution of each. These are used to describe a likelihood distribution (based on the probit regression results) from which each household's WTP is estimated. A new set of regression coefficients is then selected and the process is repeated (250 iterations). The result provides a description of the distribution of household WTP for prevention and treatment within the community, and confidence intervals for these estimates. The model was also run allowing all variables to change simultaneously to develop an overall estimate of the distribution (using 100,000 iterations), including variability within the population and uncertainty regarding the fitted distribution.
Participation rates for both interventions were then estimated by calculating the percentage of the population willing to pay more than a series of different amounts. For both interventions, mean household WTP was estimated among the entire population and those willing to pay a positive amount. For the prevention intervention, community willingness to pay was estimated based on estimates of population, household size, percentage of the population willing to participate, and mean WTP.
Results
A total of 583 useable surveys were collected, of which 346 respondents reported monthly household income. The latter surveys were used to estimate WTP, while all surveys were used for descriptive statistics and to fit distributions for age, income, and household size (Table 1). Approximately 71% of respondents were women, presumably due to their greater likelihood of being at home during the times when the surveys were conducted (Table 1). The mean reported monthly household income was 624 Haitian Gourdes, (US$35, [18 Gourdes = US$1 in 1999]). The majority of respondents reported knowing someone with lymphedema or hydrocele (84% and 76% respectively). Approximately 20% reported having a family member with lymphedema or hydrocele. Half of the respondents did not have an opinion as to how likely they were to get filariasis. However, 62% of those who did described it as likely or very likely. Only 22% of the people surveyed correctly identified potential sources of filarial infection. Fifty-two percent of respondents identified magical powder as a cause of lymphedema. Differences in respondent familiarity and knowledge about filariasis did not significantly differ between men and women.
Table 1.
Demographic And Other Characteristics Of Survey Respondents – Leogane, Haiti
| Demographic Characteristics | N | Mean | Fitted Distribution Information | |
| Distribution | Goodness of Fit* | |||
| Age | 583 | 39.1 | Gamma, Location 0.31, Scale 1.74, Shape 2.97 | Chi-Square 87.63 K-S 0.043 A-D 1.50 |
| Monthly Income (Gourdes)** | 346 | 624.0 | Weibull, Location -1.03, Scale 417.28, Shape 0.60 | Chi-Square 351.11 K-S 0.205 A-D 19.87 |
| Household Size | 583 | 5.5 | Gamma, Location 15.98, Scale 11.55, Shape 2.00 | Chi-Square 1067.81 K-S 0.0867 A-D 4.52 |
| N | Percent | |||
| Sex (percent female) | 583 | 71.4 | ||
| House Construction | 583 | |||
| Thatch | 4.5 | |||
| Wood | 51.1 | |||
| Cement | 44.4 | |||
| Knowledge and Attitudes | ||||
| Knowledge of causes | 583 | 22.3 | ||
| Belief in magical etiology | 583 | 52.1 | ||
| High perceived risk (risky or very risky) | 274 | 37.2 | ||
| Familiarity | ||||
| Know someone with lymphedema | 581 | 84 | ||
| Know someone with hydrocele | 580 | 76 | ||
| Family member with lymphedema | 577 | 19.2 | ||
| Family member with hydrocele | 580 | 10.7 | ||
*Distributions fit using Crystal Ball software (Decisioneering, 2000). K-S and A-D refer to the Kolmogorov-Smirnov and Anderson-Darling goodness-of-fit tests, respectively. **18 Gourdes =US$1 in 1999
Regression model results
Estimation results for prevention and treatment interventions are shown in Table 2. These parameters reflect the impact of a change in each of the variables on the probability of a 'yes' response. For each household, these parameters define a cumulative distribution function based on the household's characteristics, representing the likelihood that the household is willing to pay a given amount. Willingness to pay for treatment was positively affected by income, and negatively affected by age, a belief in a magical etiology, living in a wood or thatch home, having someone in the household with lymphedema or hydrocele, and appropriate knowledge about the causes of filariasis. Willingness to pay for prevention was positively affected by income, and negatively affected by living in a wood or thatch home and having someone in the household with lymphedema or hydrocele.
Table 2.
Single Bounded Probit Model Maximum Likelihood Estimates
| Variable | Treatment | Prevention | |||||
| Name | Explanation | Coefficient | S.E. | P | Coefficient | S.E. | P= |
| Intercept | 2.3831 | 0.408 | 0.0001 | 2.5192 | 0.454 | 0.0001 | |
| Bid | Gourdes | -0.0001 | 0.00001 | 0.0001 | -0.0169 | 0.0024 | 0.0001 |
| Income | Gourdes/month | 0.0004 | 0.0001 | 0.0001 | 0.0004 | 0.0002 | 0.0116 |
| Sex | (0 = female, 1 = male) | -0.0562 | 0.178 | 0.7517 | -0.0473 | 0.186 | 0.7993 |
| Age | Years | -0.0184 | 0.006 | 0.0012 | -0.0083 | 0.006 | 0.1443 |
| House Type | (0 = cement, 1 = wood or thatch) | -0.6615 | 0.145 | 0.0001 | -0.4318 | 0.149 | 0.0039 |
| Knowledge | Correctly identify causes of filariasis (0 = yes, 1 = no) | -0.5014 | 0.188 | 0.0077 | -0.0983 | 0.199 | 0.6214 |
| Familiarity | Family member with lymphedema or hydrocele (0 = no, 1 = yes) | -0.4476 | 0.181 | 0.0133 | -0.3865 | 0.197 | 0.0498 |
| Magic | Belief in magic etiology of lymphedema (0 = no, 1 = yes) | -0.5692 | 0.163 | 0.0005 | -0.0124 | 0.170 | 0.9420 |
| Household Number | Number of people living in household | 0.0014 | 0.025 | 0.9559 | |||
| Perceived Risk | 0 = not or somewhat risky, 1 = risky or very risky | 0.3283 | 0.216 | 0.1283 | |||
Note: The probit model describes the likelihood that a household with specific characteristics would be willing to pay different amounts, rather than predicting a single deterministic WTP value
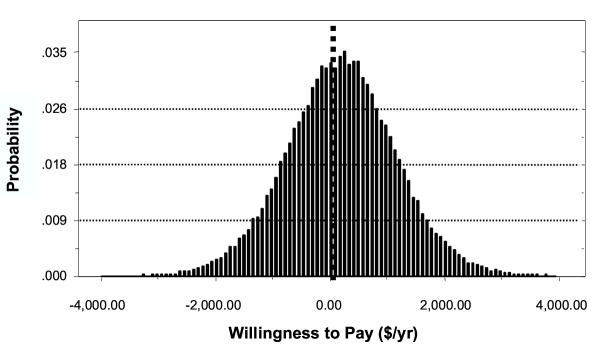
Figure 1 shows the estimated overall distribution of household WTP for treatment within the community based on the Monte Carlo simulation. Approximately 61% [95% C.I.: 52–69%] of the population would be willing to pay some positive amount for lymphedema treatment. Among those individuals who would be willing to pay for treatment, the mean was $805 [$724 – $959]/year. This represents the maximum amount of income the household would be willing to give up for cessation of acute attacks, reduced swelling, and improved social function associated with treatment of lymphedema for one year as presented in the survey scenario. Since individuals with negative WTP would choose not to participate in such a program, the value of the program to them is zero. Assuming a WTP of zero for these individuals, results in a mean WTP of $491/yr (95% CL: $377 – $662) for the community as a whole.
Figure 1.
Distribution Of Willingness To Pay For Individual Lymphedema Treatment In Leogane, Haiti ($/Year)
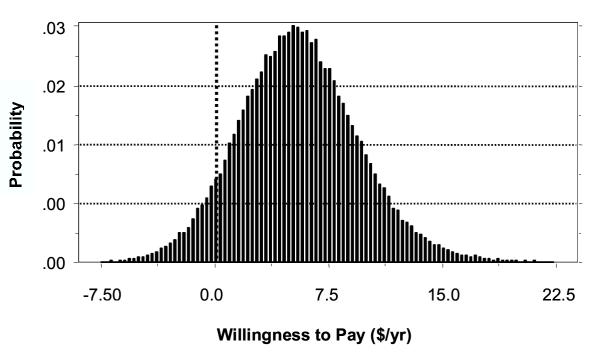
For LF prevention the mean WTP for all households was $5.57 per month [$4.76 – $6.72]. This represents the maximum amount of income the household would be willing to give up each month to eliminate the risk of any household member developing LF. This mean monthly household WTP is equivalent to $12.15 annually per person, assuming 5.5 people per household. Figure 2 shows the estimated distribution of household WTP for prevention within the community. Approximately 93% [88–97%] of the population would be willing to pay some positive amount for DEC-fortified salt. Assuming a WTP of zero for other households, the mean was $5.77 per month, with a 95% confidence interval of $4.91 – $7.01.
Figure 2.
Distribution Of Household Willingness To Pay For LF Prevention In Leogane, Haiti ($/Month)
The annual economic value of preventing LF is estimated based on the expected number of households willing to pay for the intervention and the mean WTP for these households. Given a total population of 20,000 individuals and an average of 5.5 individuals per household, total annual monetary value of preventing lymphatic filariasis is approximately $252,000 per year. This is the amount the community would be willing to pay each year for prevention and hence it estimates the annual value of the program benefits. This does not include the value of reduced likelihood of infection for individuals who choose not to participate in the intervention but may nonetheless benefit by the reduced risk resulting from a community-wide program.
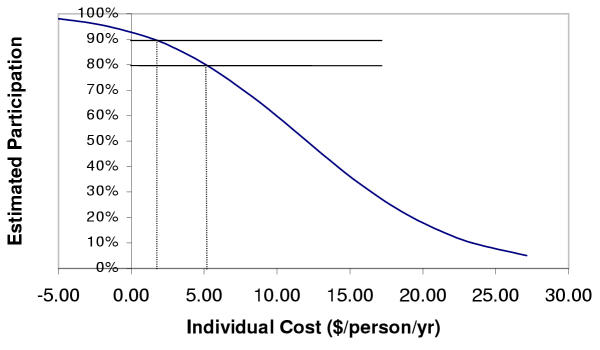
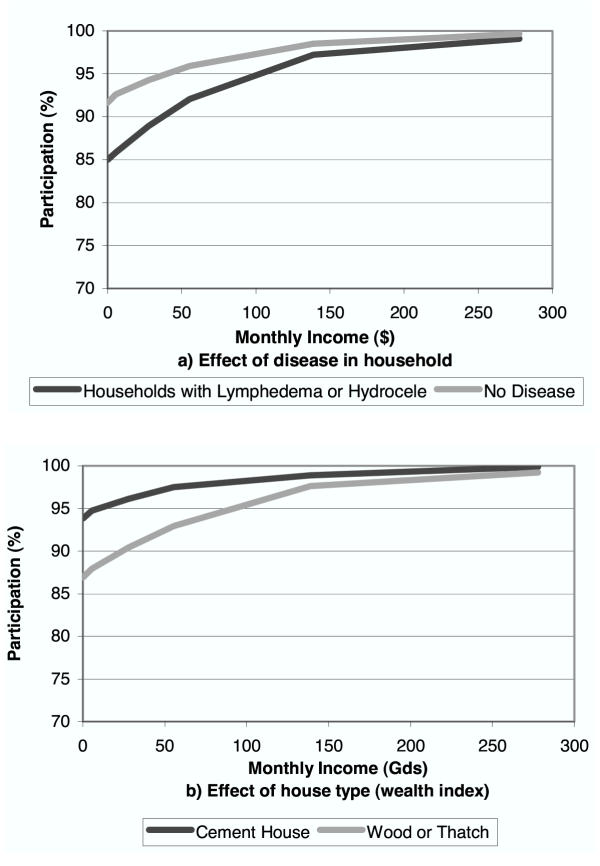
Estimated rates of participation for different levels of individual cost are shown in Figure 3. Potential individual costs for the DEC salt intervention would include any increase in the price of table salt resulting from the fortification program. Figure 4 shows the influence of income, house type, and whether a family member has lymphedema or hydrocele on expected participation rates (assuming no costs to the household).
Figure 3.
Effects of Participant Costs on Estimated Participation in Prevention Activities (simulation results)
Figure 4.
Effect of Income, House type, and Household Members with Clinical Disease on Expected Participation in Prevention (simulation results)
Discussion
Willingness to pay studies are usually used to estimate the economic value of the burden created by a disease or the benefit of reducing that burden as a result of a specific intervention. Although no studies have directly estimated the medical costs or productivity losses from filariasis in this community, the estimated WTP for LF prevention in this community ($252,000 / year) is likely to exceed actual medical expenses and productivity losses. The WTP values estimated in this study reflect the value of avoided pain, suffering, and reduced social functioning, as well as any expected medical expense savings or lost earnings.
The monetary value of improved health associated with an intervention, when combined with other benefit measures, can be compared to the costs of the intervention in a cost-benefit analysis. Estimating the costs of LF treatment and prevention programs is beyond the scope of this paper. However, the estimated annual value of $12 per person greatly exceeds initial estimates of the annual per capita costs of mass treatment with anti-filarial drugs. Estimates from the first three years of mass drug administration in Leogane, Haiti with DEC and albendazole suggest an annual cost of $1–2 per person [15]. These initial cost estimates are within the wide range of costs ($0.05 – $3) in studies published elsewhere [16,17]. This suggests that programs to interrupt LF transmission in Haiti are likely to have very high net value, with the monetary value of benefits exceeding costs. Similarly, the mean WTP for lymphedema treatment of $805 per year greatly exceeds the actual annual costs of clinic-based treatment of individuals with lymphedema in this community, estimated at $22–78 per person depending on the stage of disease (S. Kanjilal, personal communication, 2003).
Respondents' knowledge and/or attitudes regarding filariasis affected willingness to pay for treatment and prevention. However the results do not necessarily indicate that improved information through health education will increase participation. It was initially expected that individuals who identified magical powders as a source of lymphedema would be willing to pay less for prevention or treatment interventions which they may see as ineffective. WTP for prevention was not influenced by beliefs about etiology. However WTP for treatment was negatively influenced by both the belief in magical etiology and correct knowledge about the mechanisms of filarial infection. A trend toward higher WTP for prevention was observed among people who perceived themselves to be at higher risk of getting LF. Although the result was not statistically significant, it may suggest that health education efforts that focus on informing individuals about disease risks and the opportunity for prevention and treatment may be more effective than those aimed at improving knowledge about the biology or causes of LF.
Individuals' familiarity with lymphedema or hydrocele (whether they or a family member have one of the conditions) significantly negatively affected WTP for treatment and prevention. This finding is consistent with valuation studies for other health conditions [18]. Having the condition (or having a family member with it) may lead to coping mechanisms and increased acceptance, which in turn reduce an individual's willingness to pay to treat the condition. In the case of treatment, an alternative hypothesis may be that individuals with lymphedema may be more cognizant of the immediate financial constraints on paying for treatment, or they may have felt that the prices asked about were not 'fair', given their assessment or knowledge of the actual costs of the treatment services. Another factor may be that they do not believe that the treatment would be effective. Individuals who already have filarial lymphedema also may consider it too late to prevent it.
As expected, household income positively affected estimates of mean WTP for both lymphedema treatment and LF prevention. Willingness-to-pay studies often restrict WTP estimates to be less than reported income, given that households cannot expect to be willing to pay more than their actual income [12]. In this study we did not artificially restrict WTP to be less than reported income. For some households estimated WTP for lymphedema treatment exceeded their reported income. However this may not actually represent a violation of the logical restrictions on WTP. Although households may not realistically be able to pay their entire income to prevent LF or treat lymphedema, there are other resources that contribute to household ability to pay. Respondents may base their WTP on past savings or expected future earnings, rather than their current monthly income (which may be highly variable). In addition, WTP for treatment and prevention may be strongly influenced by resources of the extended family (including individuals living and working abroad). This is supported by the influence of household construction type (as a proxy for wealth) on WTP estimates. Having a house constructed with cement (as opposed to wood or thatch) was positively related to WTP for both prevention and treatment.
In this study we used a 2-dimensional Monte Carlo model to estimate the distribution of WTP, reflecting the fact that some individuals may be willing to pay a great deal to participate in programs, while others have negative WTP. This suggests that even if the interventions were offered free of charge, they would choose not to participate. Negative WTP estimates suggest that some people may see the interventions as ineffective or not worth the individual costs associated with participating in them, such as the time, travel, and other costs. It may also reflect a sense of fatalism about their condition [7]. Their participation would require an incentive or subsidy of some sort, to offset these costs.
Such a distribution of household willingness to pay may have important program implications. For lymphedema treatment, if the cost of the intervention exceeds a household's WTP, then that household may choose not to participate. For example, if the annual cost of treatment were the equivalent of $100, the model estimates that 45% would choose not to seek treatment, if they had lymphedema. For the majority of those who would choose to participate, the value of the service (their WTP) would exceed the actual cost to them, resulting in a net benefit or surplus.
For the prevention intervention, the distribution of WTP has important implications for the ability of the program to achieve transmission interruption. The distribution suggests that as the cost of the intervention to households increases the rate of participation is likely to decline. It is believed that a minimum coverage level must be sustained for several years in order to interrupt transmission, although the precise level is unknown and is likely to differ between settings [19].
For an individual to decide to participate, the value of prevention must be perceived to be at least equal the cost incurred by participation. For a DEC-fortified salt program this could include any additional costs for the fortified salt. Programs that distribute anti-filarial drugs through mass drug distribution also may introduce costs to participating individuals. In some countries cost recovery practices are used for similar programs (e.g. onchocerciasis) to cover program costs. Households may encounter a variety of costs including transportation to distribution points or lost time from work.
Based on Figure 3, if it is assumed that 80% of households need to participate in order to interrupt transmission, the expected cost (or household contribution) for the intervention (e.g. cost recovery fees, transportation costs, or additional cost of DEC-fortified salt) would have to be below the 20th percentile of the WTP distribution ($5.09/person/yr). Above that value, it is estimated that less than the required 80% would participate. If the actual cost per household were greater than the appropriate threshold WTP, then a subsidy may be required to reach the needed coverage rate. If 90% participation were needed, then the cost to households would have to be less than $1.53/person/yr. Other factors may also affect participation, including illness or pregnancy (contraindications to treatment), fear of adverse drug reactions, and not being available at the time of drug distribution.
Cost recovery policies may provide short-term revenue, but may also reduce participation levels, leading to the need for longer intervention periods to interrupt transmission. Policies that introduce other costs to households, such as the limiting number of drug distribution posts or not providing medication for adverse reactions free of charge, may have a similar effect. Reducing these costs may increase short-term participation, resulting in lower long-term costs. While cost recovery policies will almost always result in reduced participation, the consequences may be particularly important for LF elimination and other programs that require very high levels of participation.
Subsidies, whether financial or in the form of other health services, may be useful in increasing participation, and decreasing long-term program costs. The anthelminthic benefit resulting from co-administration of albendazole with DEC or ivermectin can be viewed as such a subsidy. Efforts to improve participation in these ways or through educational campaigns may be particularly important in specific settings – including poor communities (low income or lower quality housing) and households of individuals with clinical disease (Figure 4).
While WTP estimates capture many of the components of disease burden and program impact, the method may miss other societal benefits from treatment and prevention programs. For example, other individuals who travel into the region might be willing to pay for protection during short visits. For prevention activities, our WTP estimates do not capture the benefit to future generations who would no longer be at risk (only the value that these current residents put on future benefits to others). For treatment, we may not completely capture benefits to other household members who may benefit from another family member's reduced morbidity. Alternative measures of burden of disease and program benefits include health metrics (Disability-Adjusted Life Years, Quality-Adjusted Life Years) and monetary measures of medical costs and lost productivity. Each of these captures different aspects of a disease on individuals and society. These approaches provide burden information which complements WTP estimates of burden.
Conclusions
The WTP estimates generated in this study expand our understanding of the economic impact of the disease in two ways. First, WTP measures provide an estimate of welfare gain that includes changes in morbidity, economic production, and other intangible values such as social function. All of these aspects of welfare are affected by lymphatic filariasis, and should be considered when assessing the burden of the disease. Second, WTP studies help identify factors that, if addressed through social mobilization, subsidies, or other strategies, may improve program participation rates critical to an elimination goal.
List of Abbreviations Used
ADL Adenolymphangitis
DEC Diethylcarbamazine
LF Lymphatic filariasis
WTP Willingness to pay
Competing Interests
None declared.
Authors' Contributions
All authors were involved in the design of the study and interpretation of results. RR developed the study design, developed the instrument, participated in data analysis, and drafted the manuscript. ACH conceived the study, developed study design, reviewed instrument, and helped in writing the paper. MLM helped in design of the study and instrument, assisted in data analysis, and helped in writing the paper. MM helped in study and instrument design and in writing of paper. GM assisted in design, adaptation, and implementation of survey instrument and helped in interpretation of results. DA assisted in design of study and writing of paper.
Acknowledgments
Acknowledgements
The authors would like to thank Dr. Jack LaFontant and Jackie Louis-Charles of Hospital Ste. Croix in Leogane, Haiti for their assistance in carrying out the study. The study was funded in part by a research grant from the CDC Office of Women's Health.
Contributor Information
Richard D Rheingans, Email: rrheing@sph.emory.edu.
Anne C Haddix, Email: achaddi@sph.emory.edu.
Mark L Messonnier, Email: qzm3@cdc.gov.
Martin Meltzer, Email: qzm4@cdc.gov.
Gladys Mayard, Email: gladysmayard@hotmail.com.
David G Addiss, Email: dga1@cdc.gov.
References
- Gyapong JO, Gyapong M, Evans DB, Aikins MK, Adjei S. The economic burden of lymphatic filariasis in northern Ghana. Ann Trop Med Parasitol. 1996;90:39–48. doi: 10.1080/00034983.1996.11813024. [DOI] [PubMed] [Google Scholar]
- Evans DB, Gelband H, Vlassoff C. Social and economic factors and the control of lymphatic filariasis: a review. Acta Trop. 1993;53:1–26. doi: 10.1016/0001-706X(93)90002-S. [DOI] [PubMed] [Google Scholar]
- Haddix AC, Kestler A. Lymphatic filariasis: economic aspects of the disease and programmes for its elimination. Trans R Soc Trop Med Hyg. 2000;94:592–593. doi: 10.1016/s0035-9203(00)90199-8. [DOI] [PubMed] [Google Scholar]
- Ramaiah KD, Guyatt H, Ramu K, Vanamail P, Pani SP, Das PK. Treatment costs and loss of work time to individuals with chronic lymphatic filariasis in rural communities in south India. Trop Med Int Health. 1999;4:19–25. doi: 10.1046/j.1365-3156.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- Ramaiah KD, Das PK, Michael E, Guyatt H. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16:251–3. doi: 10.1016/S0169-4758(00)01643-4. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy K. Estimated costs of acute adenolymphangitis to patients with chronic manifestations of bancroftian filariasis in India, Indian J Public Health. 1999;43:58–63. [PubMed] [Google Scholar]
- Coreil J, Mayard G, Louis-Charles J, Addiss D. Filarial elephantiasis among Haitian women: social context and behavioural factors in treatment. Trop Med Int Health. 1998;3:467–473. doi: 10.1046/j.1365-3156.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Freeman AR, Lammie PJ, Houston R, Jooste PL, LaPointe MD, Brissau JM, Lafontant JG, Addiss DG. A community-based trial for the control of lymphatic filariasis and iodine deficiency using salt fortified with diethylcarbamazine and iodine. Am J Trop Med Hyg. 2001;65:865–871. doi: 10.4269/ajtmh.2001.65.865. [DOI] [PubMed] [Google Scholar]
- No authors listed Lymphatic filariasis. Wkly Epidemiol Rec. 2001. pp. 149–54. [PubMed]
- Arrow K, Solow R, Portney PR, Leamer EE, Radner R, Schuman H. Report of the NOAA Panel on Contingent Valuation. Federal Register. 1993;58:4601–4614. [Google Scholar]
- Alberini A. Optimal designs for discrete choice contingent valuation surveys: Single-bound, double-bound, and bivariate models. J Environ Econ Manage. 1995;28:287–306. doi: 10.1006/jeem.1995.1019. [DOI] [Google Scholar]
- Hanemann WJ, Kanninen B. Statistical Analysis of Discrete-Response CV Data. In: Bateman and Willis, editor. Valuing Environmental Preferences. Oxford, Oxford University Press; 1999. [Google Scholar]
- Economic Software Inc Limdep 7.0. Plainview, NY. 1999.
- Decisioneering, Inc Crystal Ball 2000. Denver, CO. 2000.
- McLaughlin SI, Radday J, Michel MC, Addiss DG, Beach MJ, Lammie PJ, Lammie J, Rheingans R, LaFontant J. Frequency, severity, and costs of adverse reactions following mass treatment for lymphatic filariasis using diethylcarbamazine and albendazole in Leogane, Haiti, 2000. Am J Trop Med Hyg. 2003;68:568–573. doi: 10.4269/ajtmh.2003.68.568. [DOI] [PubMed] [Google Scholar]
- Michael E, Meyrowitsch DW, Simonsen PE. Cost and cost effectiveness of mass diethylcarbamazine chemotherapy for the control of bancroftian filariasis: comparison of four strategies in Tanzania. Trop Med Int Health. 1996;1:414–426. doi: 10.1046/j.1365-3156.1996.d01-82.x. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy K, Ramu K, Srividya A, Appavoo NC, Saxena NB, Lal S, Das PK. Cost of mass annual single dose diethylcarbamazine distribution for the large scale control of lymphatic filariasis. Indian J Med Res. 2000;111:81–89. [PubMed] [Google Scholar]
- Sloan FA, Viscusi WK, Chesson HW, Conover CJ, Whetten-Goldstein K. Alternative approaches to valuing intangible health losses: the evidence for multiple sclerosis. J Health Econ. 1998;17:475–497. doi: 10.1016/S0167-6296(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Plaiser AP, Stolk WA, van Oortmarssen GJ, Habbema JD. Effectiveness of annual ivermectin treatment for Wuchereria bancrofti infection. Parasitol Today. 2000;16:298–302. doi: 10.1016/S0169-4758(00)01691-4. [DOI] [PubMed] [Google Scholar]