Abstract
Corynebacterium halotolerans Chen et al. 2004 is a member of the genus Corynebacterium which contains Gram-positive bacteria with a high G+C content. C. halotolerans, isolated from a saline soil, belongs to the non-lipophilic, non-pathogenic corynebacteria. It displays a high tolerance to salts (up to 25%) and is related to the pathogenic corynebacteria C. freneyi and C. xerosis. As this is a type strain in a subgroup of Corynebacterium without complete genome sequences, this project describing the 3.14 Mbp long chromosome and the 86.2 kbp plasmid pCha1 with their 2,865 protein-coding and 65 RNA genes will aid the Genomic Encyclopedia of Bacteria and Archaea project.
Keywords: aerobic, non-motile, Gram-positive, mesophilic, halotolerant
Introduction
Strain YIM 70093T (= DSM 44683T) is the type strain of the species Corynebacterium halotolerans [1] and was originally isolated from saline soil in Xinjiang Province in western China. The genus Corynebacterium is comprised of Gram-positive bacteria with a high G+C content. It currently contains over 80 members [2] isolated from diverse backgrounds like human clinical samples [3] and animals [4], but also from soil [5] and ripening cheese [6].
Within this diverse genus, C. halotolerans has been proposed to form a subclade together with C. freneyi and C. xerosis [1]. Data concerning salt tolerance is not available for most corynebacteria, but C. halotolerans YIM 70093T displays the highest resistance to salt (up to 25%) described for Corynebacterium so far. Here we present a summary classification and a set of features for C. halotolerans YIM 70093T, together with the description of the genomic sequencing and annotation.
Classification and features
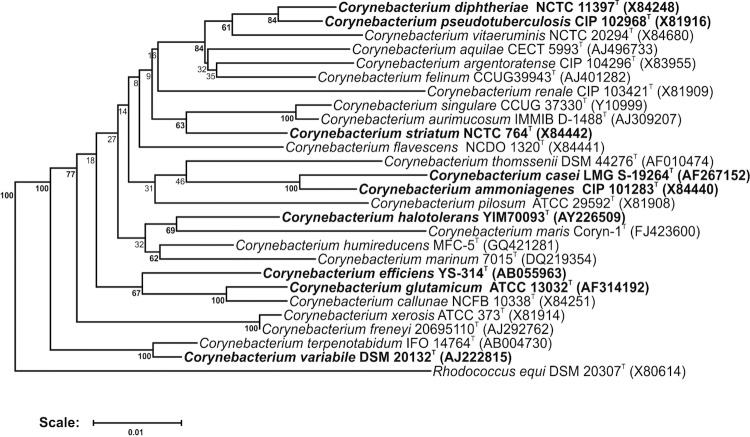
A representative genomic 16S rRNA sequence of C. halotolerans YIM 70093T was compared to the Ribosomal Database Project database [7], confirming the initial taxonomic classification. Addition of the recently published species C. maris Coryn-1T [8], C. marinum 7015T [9] and C. humireducens MFC-5T [10] as well as C. diphtheriae NCTC 11397T [11] indicates that C. halotolerans YIM 70093T, together with C. maris, C. marinum, and C. humireducens, form a distinct subclade within the genus Corynebacterium. Interestingly, C. xerosis and C. freneyi do not group closely with this subclade when C. diphtheriae is added to the comparison.
Figure 1 shows the phylogenetic neighborhood of C. halotolerans in a 16S rRNA based tree. The sequences of the four identical 16S rRNA gene copies in the genome differ by eight nucleotides from the previously published 16S rRNA sequence (AY226509), which contains two ambiguous bases.
Figure 1.
Phylogenetic tree highlighting the position of C. halotolerans relative to type strains of other species within the genus Corynebacterium as selected by Chen et al. [1]. In addition, the recently described C. maris, C. marinum, and C. humireducens were added, as they were shown to be closely related. Furthermore, the type strain of the genus, C. diphtheriae [11], was included. Species with at least one publicly available genome sequence (not necessarily the type strain) are highlighted in bold face. The tree is based on sequences aligned by the RDP aligner, utilizes the Jukes-Cantor corrected distance model to construct a distance matrix based on alignment model positions without the use of alignment inserts, and uses a minimum comparable position of 200. The tree is built with RDP Tree Builder, which uses Weighbor [12] with an alphabet size of 4 and length size of 1,000. The building of the tree also involves a bootstrapping process repeated 100 times to generate a majority consensus tree [13]. Rhodococcus equi (X80614) was used as an outgroup.
C. halotolerans YIM 70093T is Gram-positive and cells are rod-shaped, 0.5-1 μm long and 0.25-0.5 μm wide (Table 1 and Figure 2). It is described to be non-motile [1], which coincides with a complete lack of genes associated with ‘cell motility’ (functional category N). Optimal growth of YIM 70093T was shown to occur at 28°C, pH 7.2 and 100 g/l KCl, albeit the strain tolerates a wide range of salinity, between 0-250 g/l, NaCl, and MgCl2 [1]. Carbon sources utilized by strain YIM 70093T include glucose, galactose, sucrose, arabinose, mannose, mannitol, maltose, xylose, ribose, salicin, dextrin, and starch [1], although the latter is doubtful as C. halotolerans cannot hydrolize starch [1].
Table 1. Classification and general features of C. halotolerans YIM 70093T according to the MIGS recommendations [14].
| MIGS ID | Property | Term | Evidence codea) |
|---|---|---|---|
| Current classification | Domain Bacteria | TAS [15] | |
| Phylum Actinobacteria | TAS [16] | ||
| Class Actinobacteria | TAS [17] | ||
| Order Actinomycetales | TAS [17-20] | ||
| Family Corynebacteriaceae | TAS [17,18,20,21] | ||
| Genus Corynebacterium | TAS [18,22,23] | ||
| Species Corynebacterium halotolerans | TAS [1] | ||
| Type-strain YIM 70093 (=DSM 44683) | TAS [1] | ||
| Gram stain | Positive | TAS [1] | |
| Cell shape | diphtheroid, irregular rods | TAS [1] | |
| Motility | non-motile | TAS [1] | |
| Sporulation | non-sporulating | TAS [1] | |
| Temperature range | Mesophile | NAS | |
| Optimum temperature | 28°C | TAS [1] | |
| Salinity | 0-250 g/l KCl/NaCl/MgCl2 | TAS [1] | |
| MIGS-22 | Oxygen requirement | Aerobe | TAS [1] |
| Carbon source | glucose, galactose, sucrose, arabinose, mannose, mannitol, maltose, starch, xylose, ribose, salicin, dextrin |
TAS [1] | |
| Energy metabolism | Chemoorganoheterotroph | TAS [1] | |
| Terminal electron acceptor | Oxygen | NAS | |
| MIGS-6 | Habitat | saline soil | TAS [1] |
| MIGS-15 | Biotic relationship | free living | NAS |
| MIGS-14 | Pathogenicity | non-pathogenic | NAS |
| Biosafety level | 1 | TAS [24] | |
| MIGS-23.1 | Isolation | saline soil | TAS [1] |
| MIGS-4 | Geographic location | Xinjiang Province, China | TAS [1] |
| MIGS-5 | Sample collection time | Not reported | |
| MIGS-4.1 | Latitude | Not reported | |
| MIGS-4.2 | Longitude | Not reported | |
| MIGS-4.3 | Depth | Not reported | |
| MIGS-4.4 | Altitude | Not reported |
a) Evidence codes - TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [25].
Figure 2.
Scanning electron micrograph of C. halotolerans YIM 70093T.
Chemotaxonomy
The peptidoglycan of strain YIM 70093T contains meso-diaminopimelic acid, galactose, and arabinose [1], therefore it belongs to cell wall type IV, sugar type A. The menaquinones detected in the cell membrane of YIM 70093T are MK-8(H2) (35.5%) and MK-9(H2) (64.5%) [1]. Cellular fatty acids are predominantly saturated straight chain acids, C16:0 (42.1%), C14:0 (7.3%); and C18:0 (4.5%), and unsaturated acids, cis-9-C18:1 (28.9%) and cis-9-C16:1 (9.8%), in addition to 10-methyl C18:0 (7.4%) [1]. Like many, but not all corynebacteria, C. halotolerans also contains mycolic acids, predominantly of the short chain type (C32-C36): C32:0 (36.0%), C34:0 (20.8%), C34:1 (25.1%), C36:0 (3.6%), C36:1 (8.4%), and C36:2 (5.1%) [1]. The reported major polar lipids consist of diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), phosphatidylinositol (PI), glycolipid and phosphatidylinositol mannosides (PIM) [1].
Genome sequencing and annotation
Genome project history
C. halotolerans YIM 70093T was selected for sequencing as part of a project to define the core genome and pan genome of the non-pathogenic corynebacteria due to its phylogenetic position and interesting capabilities, i.e. high salt tolerance. While not being a part of the Genomic Encyclopedia of Bacteria and Archaea (GEBA) project [26], sequencing of the type strain will nonetheless aid the GEBA effort. The genome project is deposited in the Genomes On Line Database [27] and the complete genome sequence is deposited in GenBank. Sequencing, finishing and annotation were performed by the Center of Biotechnology (CeBiTec). A summary of the project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Two genomic libraries: one 454 pyrosequencing PE library (3.2 kb insert sizes), one Illumina library |
| MIGS-29 | Sequencing platforms | 454 GS FLX Titanium, Illumina GA IIx |
| MIGS-31.2 | Sequencing coverage | 22.5 × Pyrosequencing; 23.5 × SBS |
| MIGS-30 | Assemblers | Newbler version 2.3 |
| MIGS-32 | Gene calling method | GeneMark, Glimmer |
| INSDC ID | CP003697, CP003698 | |
| GenBank Date of Release | July 1, 2013 / after publication | |
| GOLD ID | Gi19308 | |
| NCBI project ID | 168616 | |
| MIGS-13 | Source material identifier | DSM 44683 |
| Project relevance | Industrial, GEBA |
Growth conditions and DNA isolation
C. halotolerans strain YIM 70093T, DSM 44683, was grown aerobically in CASO broth (Carl Roth GmbH, Karlsruhe,Germany) at 30°C. DNA was isolated from ~ 108 cells using the protocol described by Tauch et al. 1995 [28].
Genome sequencing and assembly
The genome was sequenced using a 454 sequencing platform. A standard 3k paired end sequencing library was prepared according to the manufacturers protocol (Roche). Pyrosequencing reads were assembled using the Newbler assembler v2.3 (Roche). The initial Newbler assembly consisted of 81 contigs in six scaffolds with an additional 26 lone contigs. Analysis of the six scaffolds revealed one to be an extrachromosomal element (plasmid pCha1), four to make up the chromosome with the remaining one to contain the four copies of the RRN operon which caused the scaffold breaks. The scaffolds were ordered based on alignments to the complete genomes of C. glutamicum [29] and C. efficiens [30] and subsequent verification by restriction digestion, Southern blotting and hybridization with a 16S rDNA specific probe.
The Phred/Phrap/Consed software package [31-34] was used for sequence assembly and quality assessment in the subsequent finishing process. After the shotgun stage, gaps between contigs were closed by editing in Consed (for repetitive elements) and by PCR with subsequent Sanger sequencing (IIT Biotech GmbH, Bielefeld, Germany). A total of 61 additional reactions were necessary to close gaps not caused by repetitive elements. To raise the quality of the assembled sequence, Illumina reads were used to correct potential base errors and increase consensus quality. A WGS library was prepared using the Illumina-Compatible Nextera DNA Sample Prep Kit (Epicentre, WI, U.S.A) according to the manufacturer's protocol. The library was sequenced in an 80 bp single read GAIIx run, yielding 1,497,321 total reads. Together, the combination of the Illumina and 454 sequencing platforms provided 46.0× coverage of the genome.
Genome annotation
Gene prediction and annotation were done using the PGAAP pipeline [35]. Genes were identified using GeneMark [36], GLIMMER [37], and Prodigal [38]. For annotation, BLAST searches against the NCBI Protein Clusters Database [39] were performed and the annotation was enriched by searches against the Conserved Domain Database [40] and subsequent assignment of coding sequences to COGs. Non-coding genes and miscellaneous features were predicted using tRNAscan-SE [41], Infernal [42], RNAMMer [43], Rfam [44], TMHMM [45], and SignalP [46].
Genome properties
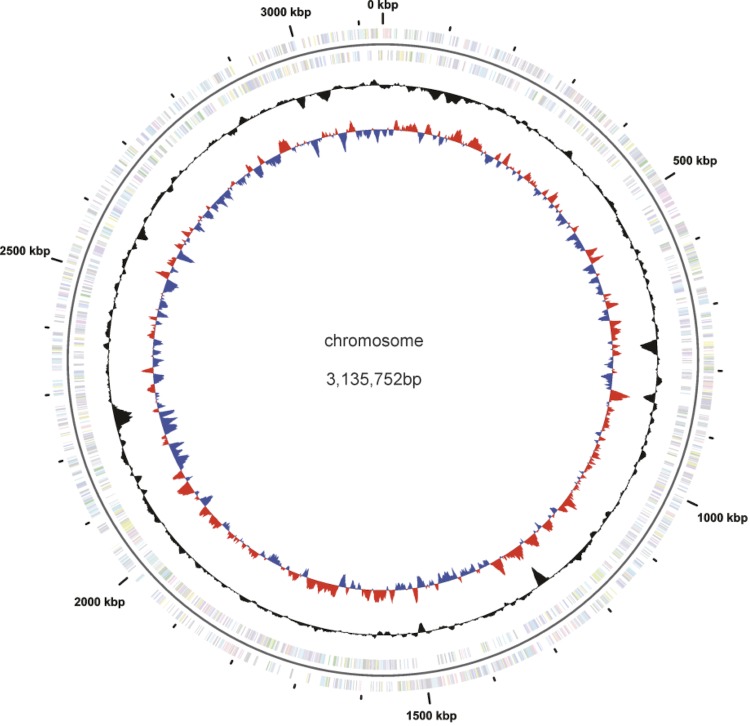
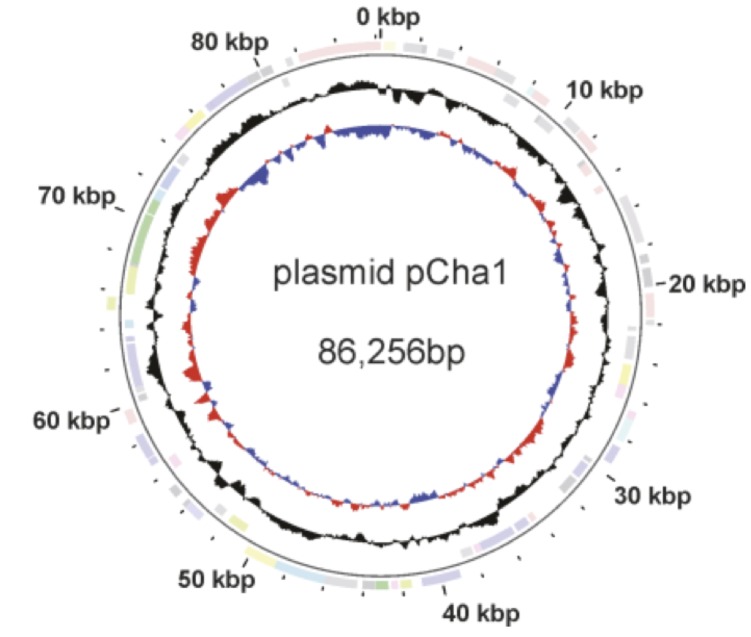
The genome includes one plasmid, for a total size of 3,222,008 bp, with one circular chromosome of 3,135,752 bp (68.44% G+C content) and one plasmid of 86,256 bp (63.20% G+C content) [Figure 3 and Figure 4]. For the main chromosome, 2,856 genes were predicted, 2,791 of which are protein-coding genes. 1,632 (57%) of the protein-coding genes were assigned to a putative function with the remaining annotated as hypothetical proteins. 1,914 protein coding genes belong to 396 paralogous families in this genome corresponding to a gene content redundancy of 66.8%. The properties and the statistics of the genome are summarized in Table 3, Tables 4 and 5.
Figure 3.
Graphical map of the chromosome (not drawn to scale with plasmid). From the outside in: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), GC content, GC skew.
Figure 4.
Graphical map of the plasmid pCha1 (not drawn to scale with chromosome). From the outside in: Genes on forward strand (color by COG categories), genes on reverse strand (color by COG categories), GC content, GC skew.
Table 3. Summary of genome: one chromosome and one plasmid.
| Label | Size (Mb) | Topology | INSDC identifier |
|---|---|---|---|
| Chromosome | 3.136 | circular | CP003697.1 |
| Plasmid pCha1 | 0.086 | circular | CP003698.1 |
Table 4. Genome Statistics.
| Attribute | Value | % of totala |
|---|---|---|
| Genome size (bp) | 3,222,008 | 100.00% |
| DNA coding region (bp) | 2,791,134 | 86.63% |
| DNA G+C content (bp) | 2,200,760 | 68.30 |
| Total genesb | 2,930 | 100.00% |
| RNA genes | 65 | 2.22% |
| rRNA operons | 4 | |
| tRNA genes | 53 | 1.81% |
| Protein-coding genes | 2,865 | 97.78% |
| Genes with function prediction (protein) | 1,632 | 56.96% |
| Genes assigned to COGs | 2,234 | 77.98% |
| Gene in paralog clusters | 1,914 | 66.81% |
| Genes with signal peptides | 251 | 8.76% |
| Genes with transmembrane helices | 686 | 23.94% |
a) The total is based on either the size of the genome in base pairs or the total number of protein coding genes in the annotated genome.
Table 5. Number of genes associated with the general COG functional categories.
| Code | Value | %age | Description |
|---|---|---|---|
| J | 155 | 5.41 | Translation, ribosomal structure and biogenesis |
| A | 1 | 0.03 | RNA processing and modification |
| K | 185 | 6.46 | Transcription |
| L | 141 | 4.92 | Replication, recombination and repair |
| B | 0 | 0.00 | Chromatin structure and dynamics |
| D | 20 | 0.70 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.00 | Nuclear structure |
| V | 44 | 1.54 | Defense mechanisms |
| T | 81 | 2.83 | Signal transduction mechanisms |
| M | 126 | 4.40 | Cell wall/membrane biogenesis |
| N | 0 | 0.00 | Cell motility |
| Z | 0 | 0.00 | Cytoskeleton |
| W | 0 | 0.00 | Extracellular structures |
| U | 25 | 0.87 | Intracellular trafficking and secretion, and vesicular transport |
| O | 88 | 3.07 | Posttranslational modification, protein turnover, chaperones |
| C | 176 | 6.14 | Energy production and conversion |
| G | 183 | 6.39 | Carbohydrate transport and metabolism |
| E | 262 | 9.14 | Amino acid transport and metabolism |
| F | 68 | 2.37 | Nucleotide transport and metabolism |
| H | 122 | 4.26 | Coenzyme transport and metabolism |
| I | 88 | 3.07 | Lipid transport and metabolism |
| P | 196 | 6.84 | Inorganic ion transport and metabolism |
| Q | 85 | 2.97 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 360 | 12.57 | General function prediction only |
| S | 214 | 7.47 | Function unknown |
| - | 631 | 22.02 | Not in COGs |
Acknowledgements
Christian Rückert acknowledges funding through a grant by the Federal Ministry for Eduction and Research (0316017) within the BioIndustry2021 initiative.
References
- 1.Chen HH, Li WJ, Tang SK, Kroppenstedt RM, Stackebrandt E, Xu LH, Jiang CL. Corynebacterium halotolerans sp. nov., isolated from saline soil in the west of China. Int J Syst Evol Microbiol 2004; 54:779-782 10.1099/ijs.0.02919-0 [DOI] [PubMed] [Google Scholar]
- 2.Euzéby JP. List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet. Int J Syst Bacteriol 1997; 47:590-592 10.1099/00207713-47-2-590 [DOI] [PubMed] [Google Scholar]
- 3.Renaud FNR, Aubel D, Riegel P, Meugnier H, Bollet C. Corynebacterium freneyi sp. nov., alpha-glucosidase-positive strains related to Corynebacterium xerosis. Int J Syst Evol Microbiol 2001; 51:1723-1728 10.1099/00207713-51-5-1723 [DOI] [PubMed] [Google Scholar]
- 4.Collins MD, Hoyles L, Foster G, Falsen E. Corynebacterium caspium sp. nov., from a Caspian seal (Phoca caspica). Int J Syst Evol Microbiol 2004; 54:925-928 10.1099/ijs.0.02950-0 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Yuan M, Tang R, Chen M, Lin M, Zhang W. Corynebacterium deserti sp. nov., isolated from desert sand. Int J Syst Evol Microbiol 2012; 62:791-794 10.1099/ijs.0.030429-0 [DOI] [PubMed] [Google Scholar]
- 6.Brennan NM, Brown R, Goodfellow M, Ward AC, Beresford TP, Simpson PJ, Fox PF, Cogan TM. Corynebacterium mooreparkense sp. nov. and Corynebacterium casei sp. nov., isolated from the surface of a smear-ripened cheese. Int J Syst Evol Microbiol 2001; 51:843-852 10.1099/00207713-51-3-843 [DOI] [PubMed] [Google Scholar]
- 7.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37(Database issue):D141-D145 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Dov E, Ben Yosef DZ, Pavlov V, Kushmaro A. Corynebacterium maris sp. nov., a marine bacterium isolated from the mucus of the coral Fungia granulosa. Int J Syst Evol Microbiol 2009; 59:2458-2463 10.1099/ijs.0.007468-0 [DOI] [PubMed] [Google Scholar]
- 9.Du ZJ, Jordan EM, Rooney AP, Chen GJ, Austin B. Corynebacterium marinum sp. nov. isolated from coastal sediment. Int J Syst Evol Microbiol 2010; 60:1944-1947 10.1099/ijs.0.018523-0 [DOI] [PubMed] [Google Scholar]
- 10.Wu CY, Zhuang L, Zhou SG, Li FB, He J. Corynebacterium humireducens sp. nov., an alkaliphilic, humic acid-reducing bacterium isolated from a microbial fuel cell. Int J Syst Evol Microbiol 2011; 61:882-887 10.1099/ijs.0.020909-0 [DOI] [PubMed] [Google Scholar]
- 11.Cerdeño-Tárraga AM, Efstratiou A, Dover LG, Holden MT, Pallen M, Bentley SD, Besra GS, Churcher C, James KD, De Zoysa A, et al. The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res 2003; 31:6516-6523 10.1093/nar/gkg874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno WJ, Socci ND, Halpern AL. Weighted neighbor joining: a likelihood-based approach to distance-based phylogeny reconstruction. Mol Biol Evol 2000; 17:189-197 10.1093/oxfordjournals.molbev.a026231 [DOI] [PubMed] [Google Scholar]
- 13.Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell DM, Bandela AM, Cardenas E, Garrity GM, Tiedje JM. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 2007; 35(Database issue):D169-D172 10.1093/nar/gkl889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrity GM, Holt JG. The Road Map to the Manual. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 119-169. [Google Scholar]
- 17.Stackebrandt E, Rainey FA, Ward-Rainey NL. Proposal for a New Hierarchic Classification System, Actinobacteria classis nov. Int J Syst Bacteriol 1997; 47:479-491 10.1099/00207713-47-2-479 [DOI] [Google Scholar]
- 18.Skerman VBD, McGowan V, Sneath PHA. Approved Lists of Bacterial Names. Int J Syst Bacteriol 1980; 30:225-420 10.1099/00207713-30-1-225 [DOI] [PubMed] [Google Scholar]
- 19.Buchanan RE. Studies in the nomenclature and classification of bacteria. II. The primary subdivisions of the Schizomycetes. J Bacteriol 1917; 2:155-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann KB, Neumann R. Lehmann's Medizin, Handatlanten. X Atlas und Grundriss der Bakteriologie und Lehrbuch der speziellen bakteriologischen Diagnostik., Fourth Edition, Volume 2, J.F. Lehmann, München, 1907, p. 270. [Google Scholar]
- 21.Zhi XY, Li WJ, Stackebrandt E. An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of the existing higher taxa. Int J Syst Evol Microbiol 2009; 59:589-608 10.1099/ijs.0.65780-0 [DOI] [PubMed] [Google Scholar]
- 22.Lehmann KB, Neumann R. Atlas und Grundriss der Bakteriologie und Lehrbuch der speziellen bakteriologischen Diagnostik, First Edition, J.F. Lehmann, München, 1896, p. 1-448. [Google Scholar]
- 23.Bernard KA, Wiebe D, Burdz T, Reimer A, Ng B, Singh C, Schindle S, Pacheco AL. Assignment of Brevibacterium stationis (ZoBell and Upham 1944) Breed 1953 to the genus Corynebacterium, as Corynebacterium stationis comb. nov., and emended description of the genus Corynebacterium to include isolates that can alkalinize citrate. Int J Syst Evol Microbiol 2010; 60:874-879 10.1099/ijs.0.012641-0 [DOI] [PubMed] [Google Scholar]
- 24.BAuA. 2010 Classification of bacteria and archaea in risk groups, TRBA 466. http://www.baua.de/de/Themen-von-A-Z/Biologische-Arbeitsstoffe/TRBA/TRBA-466.html
- 25.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 2009; 462:1056-1060 10.1038/nature08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liolios K, Chen IM, Mavromatis K, Tavernarakis N, Hugenholtz P, Markowitz VM, Kyrpides NC. The Genomes OnLine Database (GOLD) in 2009: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2010; 38:D346-D354 10.1093/nar/gkp848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauch A, Kassing F, Kalinowski J, Pühler A. The Corynebacterium xerosis composite transposon Tn5432 consists of two identical insertion sequences, designated IS1249, flanking the erythromycin resistance gene ermCX. Plasmid 1995; 34:119-131 10.1006/plas.1995.9995 [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 2003; 104:5-25 10.1016/S0168-1656(03)00154-8 [DOI] [PubMed] [Google Scholar]
- 30.Nishio Y, Nakamura Y, Kawarabayasi Y, Usuda Y, Kimura E, Sugimoto S, Matsui K, Yamagishi A, Kikuchi H, Ikeo K, et al. Comparative complete genome sequence analysis of the amino acid replacements responsible for the thermostability of Corynebacterium efficiens. Genome Res 2003; 13:1572-1579 10.1101/gr.1285603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 1998; 8:186-194 [PubMed] [Google Scholar]
- 32.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res 1998; 8:195-202 [DOI] [PubMed] [Google Scholar]
- 33.Gordon D. Viewing and editing assembled sequences using Consed. Curr Protoc Bioinformatics 2003;Chapter 11:Unit11 2. [DOI] [PubMed] [Google Scholar]
- 34.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 1998; 8:175-185 [DOI] [PubMed] [Google Scholar]
- 35.NCBI. 2010 NCBI Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP). http://www.ncbi.nlm.nih.gov/genomes/static/Pipeline.html
- 36.Borodovsky M, Mills R, Besemer J, Lomsadze A. Prokaryotic gene prediction using GeneMark and GeneMark.hmm. Curr Protoc Bioinformatics 2003;Chapter 4:Unit4 5. [DOI] [PubMed] [Google Scholar]
- 37.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res 1999; 27:4636-4641 10.1093/nar/27.23.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klimke W, Agarwala R, Badretdin A, Chetvernin S, Ciufo S, Fedorov B, Kiryutin B, O'Neill K, Resch W, Resenchuk S, et al. The National Center for Biotechnology Information's Protein Clusters Database. Nucleic Acids Res 2009; 37(Database issue):D216-D223 10.1093/nar/gkn734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, et al. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 2009; 37(Database issue):D205-D210 10.1093/nar/gkn845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eddy SR. A memory-efficient dynamic programming algorithm for optimal alignment of a sequence to an RNA secondary structure. BMC Bioinformatics 2002; 3:18 10.1186/1471-2105-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100-3108 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33 Database Issue:D121-124. [DOI] [PMC free article] [PubMed]
- 45.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 46.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783-795 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]