Abstract
Soluble complexes between the tetradecameric chaperonin GroEL and integral membrane proteins can be efficiently formed by detergent dialysis. For example, GroEL14 was found to bind a limit of two molecules of bacteriorhodopsin (BR). The GroEL-solubilized BR molecules were rapidly ejected from the chaperonin complexes on the addition of ATP or adenosine 5′-[β,γ-imido]triphosphate but not AMP, indicating that conformational changes induced by nucleotide binding eliminate a binding site for the hydrophobic transmembrane domains. BR retains its native conformation in the GroEL complexes, as judged by the spectral characteristics of the bound retinal. Moreover, the chaperonin-solubilized BR could be transferred efficiently to liposomes and used to effect a light-driven proton gradient, indicating that both native conformation and vectorial insertion were accomplished. These results suggest new approaches to the study of purified integral membrane proteins in their natural membrane environment and raise the prospect that GroEL may have a role in the integration of proteins into the cytoplasmic membrane in vivo.
With the exception of the proteins of the outer membranes of bacteria and related organelles, the transmembrane domains (TMDs) of integral membrane proteins are universally α-helical and hydrophobic. Studying the structure and function of such proteins is complicated by their insolubility in the absence of detergent. Moreover, the function of these proteins is notoriously difficult to study, because of the difficulty in integrating them into defined, preformed bilayers in vitro. An alternative to detergent-based solubilization was reported by Bochkareva et al. (1), who found that a fraction of labeled LacY made in a crude extract protein synthesis system could be kept from precipitating in the absence of detergent if the synthesis was done in the presence of excess GroEL. Moreover, the solubilized LacY comigrated with GroEL, and a fraction of it could be delivered to inverted membrane vesicles in native form, as assessed by cysteine-accessibility and partial proteolysis studies. The use of in vitro-translated, labeled LacY restricted the stoichiometry to ≈1 LacY per 25,000 added GroEL14 molecules, and <10% of the labeled LacY was finally inserted into the inverted membrane vesicles in the presence of ATP and GroES. Nevertheless, we considered these results might indicate that the chaperonin GroEL14, with its hydrophobic central cavity, could be a vehicle for a robust system for delivery of purified membrane proteins to artificial membranes. Bacteriorhodopsin (BR) represents an ideal system for testing this notion, because it is a well characterized integral membrane protein with seven α-helical TMDs, and it is a light-driven proton pump that will energize membranes if delivered in its native form and in vectorial fashion. Here we report experiments that explore the capacity of the GroEL chaperonin to solubilize BR in the absence of detergent and the possibility of its delivery in functional form to artificial membranes. The utility of GroEL for the efficient, detergent-independent delivery of proteins to membranes in vitro in general and the potential applications to structural studies of membrane proteins are discussed.
Materials and Methods
Sources. Calbiochem was the source for all detergents, Calbiosorb Bio-Beads, and ATP. BSA, AMP, ADP, and adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP) were obtained from Sigma. N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid was obtained from United States Biochemical.
General Procedures. Detergents were used at final concentration of 1%. SDS/PAGE and immunoblotting have been described (2). Antibodies against GroEL were obtained from StressGen Biotechnologies (San Diego). Polyclonal chicken antibodies against BR were produced against purified BR by Aves Labs (Tigrad, OR). Modifications to standard immunoblotting and immunoprecipitation protocols necessary to use the chicken antibodies were done according to the manufacturer's specifications. For the immunoprecipitation experiment, primary antibodies were added to the sample at 1:2,000 dilution and mixed on a roller drum at room temperature, either for 1 h or overnight as appropriate. Metal-decorated MagnaBind secondary antibodies (Pierce) were added to the sample according to the manufacturer's instructions, and the sample was again incubated overnight on a roller drum at room temperature. For each precipitation, the MagnaBind antibodies and complexes were pulled to the side with a magnet-lined tube rack and washed twice. The magnetically separated pellets were analyzed by SDS/PAGE and immunoblotting.
Protein Preparations. GroEL was either obtained from StressGen Biotechnologies or from an overexpression system as described in Kamireddi et al. (3). Results with the two preparations were identical. BR was purified from Halobacterium halobium as described by Oesterhelt and Stoeckenius (4) and delipidated as described by Huang et al. (5). The S21 holin was purified as described for the λ holin (2), except that the detergent Empigen BB (EBB) was used instead of octyl-glucoside (OG).
GroEL Solubilization of BR. GroEL at various final concentrations was added to purified BR solubilized in 1% OG/20 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, pH 7.0/150 mM NaCl. A 1-ml sample of the mixture was placed in a dialysis bag and dialyzed overnight against the same buffer lacking detergent and in the presence of Calbiosorb Bio-Beads. Dialysis was continued until there was quantitative precipitation in a control sample containing the subject protein and the same concentrations of BSA instead of GroEL. Visible adsorption spectra were obtained from both the detergent-solubilized and GroEL-solubilized samples of BR by using a Beckman Coulter DU530 spectrophotometer. To visualize the precipitate, the experiments reported in Fig. 1 contained 1 mg/ml BR and 20 mg/ml GroEL or BSA. In these experiments, 99.9% of the detergent was removed, as judged by parallel experiments in which the loss of 3H-labeled OG from the dialysis bag was monitored. In 1 d of dialysis under these conditions, >99.8% of the label was lost from the bag; after 2 d, <0.1% of the original label remained (i.e., final OG levels were <0.001%). For other detergents, we assessed the residual detergent after dialysis by using a liposome-based fluorescence-release assay. Samples were added to calcein-loaded liposomes (2), and the dye released by liposome destabilization was monitored as fluorescence; for example, EBB as low as 0.1% caused dye release. Dialysis under the conditions described removed the detergents to <10% of the critical micellar concentration.
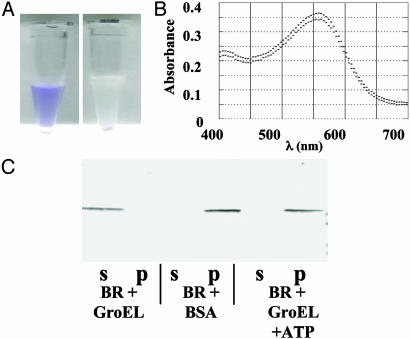
Fig. 1.
Solubilization of BR by GroEL. (A) Visualization of BR solubilized by GroEL. BR at 1 mg/ml in 1% OG was dialyzed in the presence of GroEL or BSA at 20 mg/ml as described in Materials and Methods. After dialysis, the samples were centrifuged at maximum speed for 1 min in a microcentrifuge. BR retained its normal color with GroEL, as can be seen from the spectrum, and no precipitate was observed, whereas with BSA a colorless precipitate was formed (Right). (B) BR solubilized by GroEL is native. Shown are absorption spectra of BR (0.5 mg/ml) solubilized in OG (1%; upper trace) or in GroEL (8.35 mg/ml; lower trace). (C) BR is quantitatively solubilized in an ATP-sensitive fashion by GroEL. BR at 10 μg/ml in 1% OG was dialyzed with 200 μg/ml GroEL or 200 μg/ml BSA as described. Soluble (s) and insoluble (p) pellet fractions obtained by centrifugation as described in A were analyzed by SDS/PAGE and immunoblotting with anti-BR antibodies. The two rightmost lanes represent a BR sample dialyzed with GroEL but with 5 mM ATP added to the dialysis buffer.
To test the effects of nucleotides on the solubilization process, ATP, ADP, or AMP (each at 5 mM) were added to the dialysis solution. To assess the effects of nucleotides on the stability of the complexes between the membrane proteins and GroEL, the same nucleotides or AMP-PNP were added at 5 mM final concentration directly to the solubilized protein. Precipitation, when observed, was complete within seconds for ATP, as compared with several hours for AMP-PNP and several days for ADP.
Analytical Gel Filtration Chromatography. Gel filtration analyses were performed on an AKTA (Amersham Pharmacia) workstation. Samples were filtered through a 0.22-μm sterilization filter and concentrated to 1 ml by centrifugation in an Amicon Centriplus at 3,100 rpm, according to the manufacturer's instructions. The resulting 1-ml samples were mixed with Pharmacia high- and low-molecular-weight gel filtration calibration kit markers, chromatographed on a 24-ml Superose 6 10/300 GL column, and collected in 24 1-ml fractions, all according to the manufacturer's instructions. All fractions were analyzed by immunoblotting. As with controls, samples and markers were also resolved separately on the column.
Assay of BR Function Delivered to Liposomes by GroEL Complexes. Liposomes were prepared as described (2). GroEL was loaded with BR (20 μg of BR and 400 μg of GroEL; ≈1.6:1 BR to GroEL14) by dialysis. The BR–GroEL solution or GroEL alone was added to 80 μl of the liposomes in a volume of 1.2 ml of TBS (10 mM Tris·HCl, pH7.6/150 mM NaCl) and stirred for 24 h in the dark at room temperature. Fifty microliters of TBS saturated with 9-aminoacridine (9-AA) was added to the liposome solution, and the mixtures were brought to 2 ml with TBS. Fluorescence was followed in an Aminco-Bowman Series 2 spectrofluorometer.
To assess the efficiency of this delivery, a BR/GroEL/liposome mixture in a total volume of 40 μl was placed on ice, adjusted to 50% sucrose by the addition of 73% sucrose (wt/vol in TBS), and overlaid with 150 μl of 40% sucrose and then 300 μl of 25% sucrose in a 1-ml Ultraclear centrifuge tube (Beckman). After centrifugation at 269,000 × g for 3 h at 4°C in a Beckman TLA-100.3 table top ultracentrifuge rotor, seven 100-μl fractions were drawn from the air–fluid interface. Fractions 1–3 and 5–7 were pooled as the top (floated) and bottom fractions, respectively, concentrated to 30 μl with a centrifugal concentrator (Millipore Microcon YM-3) according to the manufacturer's instructions, and analyzed for BR content by A555, the absorption maximum for bound retinal. The three top fractions contained 100% of the lipid, as judged by parallel flotation experiments with liposomes where 0.5% of the phosphatidylethanolamine was replaced with the fluorescent analog phosphatidylethanoline-N-lissamine rhodamine B (Avanti Polar Lipids).
Results
Solubilization of BR. Purified BR at 1 mg/ml solubilized in 1% OG was placed in a dialysis bag with 20 mg/ml GroEL or BSA (as a control) and subjected to dialysis. After 12 h, the control chamber showed visible precipitate (Fig. 1 A), whereas the GroEL chamber remained clear and with the characteristic purple color of native BR. The total soluble protein, measured as A280, was unchanged, and the absorption spectra from 400 nm to 700 nm, normalized for the concentration of protein, were identical for the input and solubilized BR, demonstrating that no BR was lost or denatured during the dialysis (Fig. 1B). SDS/PAGE and Western blotting showed that the BR was quantitatively solubilized in the presence of GroEL (Fig. 1C). The BR remained soluble in the presence of GroEL for at least 90 d in the cold.
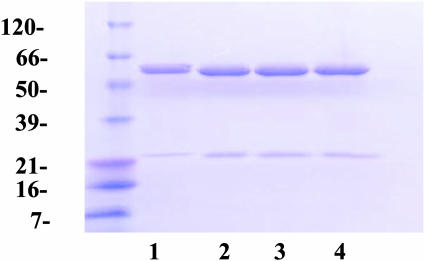
Stoichiometry of BR Solubilization by GroEL. By titrating in increasing amounts of BR into these solubilization mixtures and measuring the soluble component by densitometric analysis of Coomassie blue-stained protein gels, the capacity for solubilization was determined to be two molecules per GroEL14, or 52 kDa (Fig. 2). Interestingly, BR solubilized in EBB, where it is stable for <2 weeks, is relatively poorly retained in solution in the presence of GroEL, with only half of the BR escaping precipitation during dialysis (Table 1). This finding indicates that the GroEL-solubilization of membrane proteins depends on the original detergent, perhaps because the process of binding to GroEL competes with pathways leading to insoluble aggregates.
Fig. 2.
Capacity for BR is two molecules per GroEL14. GroEL (1 mg) was mixed with OG-solubilized BR at various ratios of BR to tetradecamer in 1 ml of final volume and then subjected to dialysis to remove detergent, as described in Materials and Methods. Insoluble material was removed by centrifugation, and the soluble material was analyzed by SDS/PAGE and staining with Coomassie brilliant blue. Ratios of BR to GroEL14 in lanes 1–4 were 1:1, 2:1, 5:1, and 10:1, respectively. Protein amounts were determined by densitometry. Mass standards in kDa are shown to the left.
Table 1. Solubilization of membrane proteins by GroEL.
| Membrane protein* | Input ratio† | % soluble‡ | No. of experiments |
|---|---|---|---|
| MalGFK2 (EBB) | 1:1§ | 100 | 1 |
| Tar (EBB) | 10:1 | 100 | 1 |
| 1:1 | 100 | 1 | |
| BR (EBB) | 1:1 | 50 | 2 |
| BR (OG) | 10:1 | 100 | 2 |
| 1:1 | 100 | >3 | |
| 1:2 | 100 | >10 | |
| 1:3 | 80 | 2 | |
| 1:10 | <40 | 2 | |
| 1:20 | <30 | 1 | |
| +ATP (5 mM) | 1:2 | 0 | 1 |
| +ADP (5 mM) | 1:2 | NA | 1 |
| +AMP (5 mM) | 1:2 | 100 | 1 |
| +AMP-PNP (5 mM) | 1:2 | 0 | 1 |
| S2168 (EBB) | 100:1 | 0 | 2 |
| 10:1 | 0 | 2 | |
| 1:1 | 0 | 4 | |
| 1:2 | 0 | 2 | |
| 1:10 | 0 | 3 | |
| 1:100 | 0 | 3 | |
| 1:300 | 0 | 2 | |
| DAGK (DM) | 1:1 | 20‡ | 1 |
| 1:10 | ≈1‡ | 1 | |
| DAGK (EBB) | 1:1 | 0 | 1 |
| 1:10 | 0 | 1 | |
| LacY (DM) | 1:1 | 20‡ | 1 |
| 1:10 | ≈1‡ | 1 |
Protein samples were purified in indicated detergent as were specified in Materials and Methods. GroEL was present at 100 μg/ml. Detergents, indicated in parentheses, were present at concentrations indicated in Materials and Methods. DM, N-dodecyl α-d-multoside.
Ratio of GroEL14 molecules to solute protein molecules.
Estimated from intensity of immunoblot bands (see Fig. 1B). For LacY and diacylglycerol kinase (DAGK), solubilization was temporary; solute began precipitating approximately 3 d after the control precipitated. NA, not applicable (see text).
Calculated as moles of the heterotetramer MalGFK2 per mole of GroEL14.
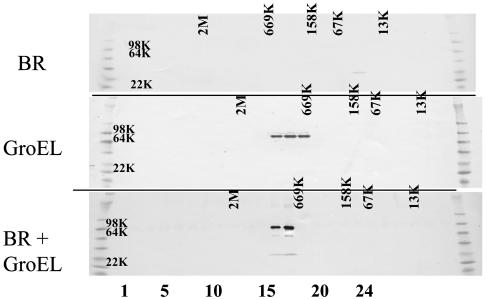
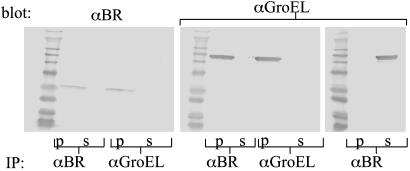
GroEL Forms a Complex with BR. To investigate the molecular basis of the GroEL-mediated solubilization, both detergent and GroEL-solubilized forms of BR were subjected to gel filtration chromatography, in parallel with GroEL itself. All of the solubilized BR migrates with GroEL in gel filtration experiments without significantly affecting the apparent mass of the tetradecamer (Fig. 3), as would be expected from the relative sizes of BR and the chaperonin. Immunoprecipitation with GroEL antibodies quantitatively coprecipitated BR (Fig. 4). Reciprocal immunoprecipitation with polyclonal anti-BR antibodies also resulted in complete precipitation of GroEL (Fig. 4) when the input ratio of BR to GroEL14 was 1:1. This result suggests that at equilibrium and under the conditions of loading by slow removal of detergent, one BR is bound per tetradecameric chaperonin. We conclude that the solubilization obtained by removing detergent in the presence of GroEL results in the formation of a complex between the membrane proteins and the GroEL chaperonin. Moreover, in the case of BR, where the solubilization does not exceed the projected capacity of the GroEL14 cavity, BR is still freely accessible to polyclonal antibodies.
Fig. 3.
Gel filtration of GroEL-solubilized BR. BR solubilized in 1% OG (Top), GroEL (Middle), and BR solubilized by GroEL (Bottom) was analyzed by gel filtration on a Superose 6 10/300 GL column. All 24 fractions were collected and analyzed by SDS/PAGE and immunoblotting with anti-BR (Top), anti-GroEL (Middle), or both antibodies (Bottom). For these experiments, 30 μgof BR and 1 mg of GroEL were used (1:1 ratio of BR to GroEL14). Positions of size standards are indicated for each chromatographic run.
Fig. 4.
Quantitative coimmunoprecipitation of GroEL and solubilized BR. A mixture of 3 μg/ml BR and 100 μg/ml GroEL (1:1 ratio of BR to GroEL14), after removal of detergent by dialysis, was subjected to immunoprecipitation and analysis by SDS/PAGE and immunoblotting with either anti-BR (Left) or anti-GroEL (Center and Right). Immunoprecipitations (IP) were done with the antibodies indicated below, with p indicating an immunoprecipitated fraction and s indicating a supernatant fraction. (Right) A control sample of GroEL subjected to immunoprecipitation with anti-BR.
Effects of Nucleotides. ATP binding by GroEL has been shown to cause a conformational change resulting in an expansion of the opening to the cavity of the chaperonin, causing a reduced affinity of the chaperonin complex for unfolded protein substrates. This change does not require ATP hydrolysis, is much less dramatic with ADP, and does not occur with AMP (6). To test whether the BR–GroEL solubilization complex was sensitive to nucleotide binding, 5 mM ATP was added to the dialysis buffer in the BR–GroEL solubilization experiment; under these conditions, a precipitate containing all of the BR formed with the same kinetics as in the control containing BSA (Fig. 1B and Table 1). Moreover, ATP added to preformed BR–GroEL complexes caused immediate precipitation of BR (data not shown). We conclude that an ATP-sensitive conformation of GroEL is required for the solubilization, suggesting that the apical domain of the chaperonin is involved and that the solubilization complex may be analogous to the complex formed on initial binding of unfolded polypeptides to GroEL. In contrast, ADP caused only a very slow release of BR, and AMP was without effect. AMP-PNP also caused release of BR from the GroEL complex, indicating that the release process does not require ATP hydrolysis (Table 1); however, with AMP-PNP the formation of the visible precipitate required 1–2 h, presumably reflecting the lower affinity of the ATP binding sites on GroEL for the analog.
Solubilization of Other Integral Membrane Proteins. The GroEL-dependent solubilization is not limited to BR. In addition, the Escherichia coli maltose-aspartate chemoreceptor Tar, with four TMDs per dimer, and the maltose permease complex consisting of the MalGFK2 heterotetramer, with 14 TMDs, also remained soluble when detergent was removed by dialysis in the presence of GroEL (Table 1). Under the same conditions, detergent removal in the presence of BSA resulted in quantitative precipitation of the proteins. Although titrations were not performed on Tar and MalGFK2, both were solubilized at ≈1:1 with the GroEL14 complex. In the membrane, Tar is a dimer of ≈120 kDa, and the Mal proteins constitute a transport complex of >170 kDa. These masses would seem to be significantly in excess of the estimated capacity of the luminal cavity of the GroEL complex, which has been reported to be ≈60 kDa (7–9), although the encapsulation of an 86-kDa heterodimer has been reported (10). However, unlike BR, the hydrophobic domains of these complexes constitute only a fraction of the total mass; thus, it is possible in these cases that only the TMDs are bound in the lumen. One integral membrane protein, E. coli lactose permease LacY, with 12 TMDs, exhibited transient solubilization, in that, during dialysis to remove the detergent DM, complete precipitation was observed in the control experiment with BSA >2 d before precipitation was detectable with GroEL present. Another protein, E. coli DAGK, showed no GroEL-dependent solubilization when the original detergent was EBB. However, DAGK in DM mirrored LacY in showing transient solubilization, again with precipitation in the BSA control >2 d before precipitation in the presence of GroEL. Finally, one of the proteins tested showed no detectable solubilization under the same conditions: the type II holin from the lambdoid phage 21, S2168, with two TMDs (Table 1).
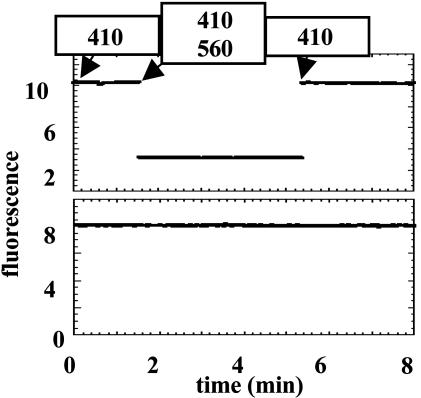
The BR–GroEL14 Complex Can Deliver Functional BR to Preformed Liposomes. Throughout the solubilization procedure, the BR–GroEL complexes retained the characteristic purple color of native BR. To determine whether these complexes formed from purified proteins could support integration of the functional BR into the bilayer, liposomes labeled with 9-AA, a fluorescent dye sensitive to the electrochemical proton gradient, were mixed with either GroEL-solubilized BR or GroEL alone and incubated for 24 h. The function of BR was assessed by its ability to generate a light-dependent proton gradient, measurable as the quenching of 9-AA fluorescence. In the liposomes exposed to the BR–GroEL complexes, excitation of BR resulted in the quenching of 9-AA fluorescence, whereas no such quenching was obtained with the GroEL-only control (Fig. 5). Addition of 0.5 mM dinitrophenol immediately abolished the quenching, even if excitation of BR was continued (data not shown). To estimate the efficiency of delivery, a parallel experiment was done in which the liposomes were separated from the GroEL mixture by flotation. The floated fraction, containing 100% of the lipid, also contained ≈29% of the BR (as judged by A555) and <10% of the GroEL, as judged by quantitative immunoblotting (data not shown). About 59% of the BR remained with the GroEL fractions; thus, ≈12% of the A555 was lost, presumably because of the loss of retinal from the BR. Moreover, washing the liposomes with 0.5 M NaCl had no effect on the amount of BR bound (data not shown), confirming that the BR molecules are integrated within the bilayer. Although no attempt was made to optimize this delivery system, these results clearly demonstrate that the BR–GroEL complexes can support efficient vectorial insertion of functional BR into a preformed bilayer.
Fig. 5.
BR delivered by GroEL complexes can form a light-dependent proton gradient. Large unilamellar liposomes prepared from 70:30 acidic:neutral phospholipids and loaded in buffer containing 9-AA were incubated with BR–GroEL complexes (formed at a 1.6:1 BR to GroEL14 ratio; Upper) or with GroEL alone (Lower). After 24 h, the liposomes were illuminated with 410-nm light to excite the 9-AA. Between 2 and 5 min after onset of illumination, the liposomes were additionally illuminated with 560-nm light to power the BR proton pump. 9-AA fluorescence was monitored throughout at 430 nm. There is a 30- to 40-sec time lapse when excitation wavelengths are changed because of a manual exchange of filters. When the experiment was repeated without liposomes, no change in the 9-AA fluorescence was observed during illumination with 560-nm light (data not shown).
Discussion
Quantitative Detergent-Free Solubilization of BR by the GroEL Chaperonin. The work of Bochkareva et al. (1) showed that ≈30% of the lactose permease (LacY) synthesized in vitro in the presence of a great molar excess of GroEL could be retained in a soluble state in the absence of detergent. Here we extend this seminal finding by reporting that high microgram to milligram quantities of a purified, native integral membrane protein, BR, with 7 α-helical TMDs, can be efficiently transferred from a detergent-solubilized state to a detergent-free complex with the chaperonin GroEL14. Moreover, this solubilization occurs efficiently and completely, even in a 2:1 molar excess of BR over the tetradecameric chaperonin and at up to low milligram concentrations of BR. As noted originally by Bochkareva et al. (1), unlike soluble proteins, which generally must be denatured to expose the hydrophobic surfaces necessary for binding in the GroEL14 cavity, integral membrane proteins could be bound in their native state. BR contains one molecule of the chromophore retinal, conferring a characteristic purple color on solutions of the purified native protein; this color is unaffected by the removal of detergent and the formation of the BR–GroEL complexes, indicating that the native conformation of BR is not compromised. By the same criterion, the BR–GroEL complex is stable for weeks in the cold. Irrespective of the molecular details of this phenomenon, it is likely to be extremely useful for the study of BR, a paradigm energy-transducing protein, and, by extension, for the study of the structure and function of integral membrane proteins in general. The ease with which GroEL can be purified in quantity (3) and its commercial availability are worth noting in this regard.
Can GroEL Be a General Vector for Integral Membrane Proteins? Our preliminary survey, which used only a narrow set of conditions, found that, although several other integral membrane proteins were solubilized if subjected to the removal of detergent in the presence of GroEL, others were not (Table 1). There is no discernible pattern for the membrane proteins that are not solubilized by this technique, either in terms of bulky hydrophilic domains, number of TMDs, or charge distribution within aqueous domains (data not shown). The GroEL-mediated retardation of the precipitation of LacY during detergent removal suggests that, for any cytoplasmic membrane protein, GroEL-mediated solubilization as detergent is removed may reflect a competition between unproductive, irreversible aggregation and a reversible partition into GroEL complexes. According to this view, the GroEL-solubilized state is kinetically trapped, possibly by a slow off-rate from the cavity of GroEL. BR is more efficiently transferred to GroEL from OG than from EBB, mirroring the increased stability of BR in the former detergent. This finding suggests that OG stabilizes BR in a conformation that is more compatible with transfer to GroEL than does the zwitterionic detergent. Some LacY associates stably with GroEL14 if DM-solubilized LacY is subjected to very rapid removal of detergent (M. Svrakic and H.R. Kaback, personal communication). Again, this result suggests that DM preserves LacY in a conformation that more rapidly associates with GroEL relative to the formation of precipitating aggregates. Whether GroEL can be made to be a general vector for solubilization and delivery of membrane proteins is still uncertain, but we think it is likely. If the binding to GroEL is a process that competes with hydrophobic aggregation as detergent is removed by dialysis, then systematically varying the detergent and the rate of dialysis may point to conditions during which any membrane protein can be efficiently partitioned into GroEL.
Structure and Assembly of the BR–GroEL Complex. Titration of BR into the GroEL14 chaperonin revealed that a maximum of two BR molecules could be bound. In principle, this could reflect complexes with both molecules of BR in the same chamber or one molecule of BR in each chamber. The binding of the second molecule of BR into the BR-containing chamber may be facilitated by interactions between the incoming BR and the BR bound near the apical domain. Alternatively, there may be steric clashes between the resident BR and a second BR molecule, leading to preferential occupancy of the other chamber. During the GroES–ATP-mediated folding cycle for misfolded soluble proteins, the binding of a folding substrate in one chamber of the tetradecamer is linked with a conformational change, leading to a narrowing of the apical domain in the other chamber (11). However, this new mode of binding of native BR to GroEL14 is GroES-independent; thus, it is unclear whether occupancy of both chambers would be unfavored in the BR2–GroEL14 complex. A preliminary electron microscopic analysis of GroE14 and BR2–GroEL14 complexes suggests that the extra density in the BR-bound structures is restricted to the chamber formed by one heptamer, irrespective of the number of BR molecules bound (J.S., J.D., C. Savva, D.S., R.Y., and A.H., unpublished data).
Delivery of BR to Membranes from the BR–GroEL Complex. Bochkareva et al. (1) showed that a fraction of in vitro synthesized LacY bound to GroEL could be transferred to inverted membrane vesicles but not to right-side out vesicles; this transfer was most efficient in the presence of ATP and GroES. Moreover, at least some of the permease molecules attained a native conformation in the membranes, as judged by protection of a sensitive cysteine thiol by a substrate analog. These studies did not allow determination of whether it was essential that the LacY cargo be cotranslationally bound to GroEL or whether the cellular machinery for the protein secretion or membrane integration and secretion was required for the insertion into the membrane. Similar findings were made by Meryandini and Drews (12), who used an in vitro translation system and membranes from Rhodobacter capsulatus. Here we have shown that purified GroEL not only efficiently binds native purified BR but also delivers it in native form to artificial membranes in the absence of ATP or GroES. In addition, we were able to demonstrate that the BR integrated into the liposomes was functional, as judged by its ability to conduct light-dependent proton pumping (Fig. 5). Thus, for BR, it is not necessary for GroEL to take up the membrane protein cotranslationally, and no proteinaceous machinery or added ATP is required for vectorial insertion of BR in its functional, native state into a bilayer from the GroEL complexes.
About 30% of the BR was delivered to the liposomes under the one set of conditions used. It seems likely that with systematic analysis of the stoichiometry, kinetics, or efficiency of the integration step, quantitative transfer to the bilayer can be obtained. With another integral membrane protein, the λ holin, we have found up to 50% associates with the liposomes after 1 h of incubation with loaded chaperonin complexes (J.D., C. Savva, J.S., A.H., and R.Y., unpublished data). Like the selection of detergent and dialysis conditions for the formation of complexes with GroEL, the efficiency of delivery to artificial membranes is likely to require the optimization of conditions, including the ionic and osmotic characteristics of the solution, lipid content of the liposomes, and concentration and stoichiometry of the chaperonin complexes and liposomes. The fluorescence of 9-AA is efficiently quenched when the liposomes inserted with BR are illuminated with visible light (Fig. 5), indicating that BR molecules are inserted vectorially in the bilayer. Our results appear to be inconsistent with one of the findings of Bochkareva et al. (1), who observed that LacY complexes with GroEL supported membrane insertion only with inverted membrane vesicles and not with right-side out vesicles derived from whole cells. Of course, it is possible that this difference reflects some intrinsic difference between LacY and BR. However, it seems more likely that their observation might reflect the small amount of labeled LacY in the incubation mixtures. Perhaps the inner leaflet of inverted membrane vesicles contains high affinity sites for GroEL complexes (i.e., the SecY–SecA translocon), as suggested by the same authors in a subsequent study (13). This possibility could account for the ATP and GroES dependency as well. In any case, the suggestion by these authors that GroEL may have a biological role in the insertion of integral membrane proteins into the bilayer is supported by the robust character of the GroEL-dependent solubilization of BR and other membrane proteins and the ability of GroEL to deliver BR to preformed bilayers.
Acknowledgments
We thank Prasad Reddy for his generous gift of a groEL overexpression strain; Ron Kaback and Maya Svracic for the communication of unpublished results, helpful discussions, and LacY protein; Mark Krebs and Hagan Bayley for invaluable advice on BR purification; Jim Sacchettini for useful suggestions and materials; the rest of the Young and Holzenburg laboratories for general support and encouragement; and Amy Davidson, Arjan Bormans and Michael Manson, and James Bowie for their generous gifts of the purified proteins MalGFK2, Tar, and DAGK, respectively. This work was supported by Public Health Service Grant GM27099 (to R.Y.) and by support from the office of the Vice President for Research at Texas A&M University (to A.H.).
Abbreviations: BR, bacteriorhodopsin; TMD, transmembrane domain; AMP-PNP, adenosine 5′-[β,γ-imido]triphosphate; EBB, Empigen BB; OG, N-octyl-β-d-glucopyranoside; 9-AA, 9-aminoacridine.
References
- 1.Bochkareva, E., Seluanov, A., Bibi, E. & Girshovich, A. (1996) J. Biol. Chem. 271, 22256–22261. [DOI] [PubMed] [Google Scholar]
- 2.Smith, D. L., Struck, D. K., Scholtz, J. M. & Young, R. (1998) J. Bacteriol. 180, 2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamireddi, M., Eisenstein, E. & Reddy, P. (1997) Protein Expression Purif. 11, 47–52. [DOI] [PubMed] [Google Scholar]
- 4.Oesterhelt, D. & Stoeckenius, W. (1971) Nat. New Biol. 233, 149–152. [DOI] [PubMed] [Google Scholar]
- 5.Huang, K.-S., Bayley, H. & Khorana, H. G. (1980) Proc. Natl. Acad. Sci. USA 77, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roseman, A. M., Ranson, N. A., Gowen, B., Fuller, S. D. & Saibil, H. R. (2001) J. Struct. Biol. 135, 115–125. [DOI] [PubMed] [Google Scholar]
- 7.Houry, W. A., Frishman, D., Eckerskorn, C., Lottspeich, F. & Hartl, F. U. (1999) Nature 402, 147–154. [DOI] [PubMed] [Google Scholar]
- 8.Ewalt, K. L., Hendrick, J. P., Houry, W. A. & Hartl, F. U. (1997) Cell 90, 491–500. [DOI] [PubMed] [Google Scholar]
- 9.Sakikawa, C., Taguchi, H., Makino, Y. & Yoshida, M. (1999) J. Biol. Chem. 274, 21251–21256. [DOI] [PubMed] [Google Scholar]
- 10.Song, J. L., Li, J., Huang, Y. S. & Chuang, D. T. (2003) J. Biol. Chem. 278, 2515–2521. [DOI] [PubMed] [Google Scholar]
- 11.Rye, H. S., Roseman, A. M., Chen, S., Furtak, K., Fenton, W. A., Saibil, H. R. & Horwich, A. L. (1999) Cell 97, 325–338. [DOI] [PubMed] [Google Scholar]
- 12.Meryandini, A. & Drews, G. (1996) Photosynth. Res. 47, 21–31. [DOI] [PubMed] [Google Scholar]
- 13.Bochkareva, E. S., Solovieva, M. E. & Girshovich, A. S. (1998) Proc. Natl. Acad. Sci. USA 95, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]