Abstract
Background
Epidemiological studies have evaluated the association between nicotinamide adenine dinucleotide phosphate (NADPH) oxidase p22phox C242T polymorphism and risk of ischemic cerebrovascular disease (ICVD), but the results remain inconclusive. This meta-analysis was therefore designed to clarify these controversies.
Methodology/Principal Findings
Systematic searches of electronic databases Embase, PubMed and Web of Science, as well as hand searching of the references of identified articles and the meeting abstracts were performed. Statistical analyses were performed using software Review Manager (Version 5.1.7) and Stata (Version 11.0). The pooled odds ratios (ORs) with 95% confidence intervals (95%CIs) were performed. Fixed or random effects model was separately used depending on the heterogeneity between studies. Publication bias was tested by Begg's funnel plot and Egger's regression test. A total of 6 studies including 1,948 cases and 2,357 controls were combined showing no statistical evidence of association between NADPH oxidase p22phox C242T polymorphism and overall ICVD (allelic model: OR = 1.08, 95%CI = 0.93–1.26; additive model: OR = 1.33, 95%CI = 0.81–2.17; dominant model: OR = 1.00, 95%CI = 0.86–1.15; recessive model: OR = 1.06, 95%CI = 0.77–1.45). Significant association was found in large-artery atherosclerotic stroke subgroup (allelic model: OR = 1.12, 95%CI = 0.88–1.41; additive model: OR = 1.36, 95%CI = 0.60–3.09; dominant model: OR = 1.25, 95%CI = 0.74–2.11; recessive model: OR = 2.17, 95%CI = 1.11–4.23). No statistical evidence of significant association was observed for small-vessel occlusive stroke, as well as Asian subgroup and Caucasian subgroup. Statistical powers on the combined sample size (total and subgroup) were all lower than 80%.
Conclusions/Significance
This meta-analysis indicates that NADPH oxidase p22phox C242T polymorphism is more associated with large-artery atherosclerotic stroke than small-vessel occlusive stroke. However, this conclusion should be interpreted with caution due to the small sample size. Larger sample-size studies with homogeneous ICVD patients and well-matched controls are required.
Introduction
Reactive oxygen species (ROS) has been suggested to play a major role in vascular disease [1]–[7]. The most significant sources of ROS in the vascular system are nicotinamide adenine dinucleotide phosphate (NADPH) oxidases [8], which include two membrane-bound subunits Nox2 and p22phox and the cytosolic components p47phox, p67phox, p40phox and Rac-1 [9], [10]. The p22phox subunit, which binding to Nox proteins leads to protein stabilization, is essential for the activation of NADPH oxidase [11]–[13]. The p22phox is encoded by the CYBA gene, which is located on the long arm of chromosome 16 at position 24 [14]. Several polymorphisms of the CYBA gene have been reported, which could lead to significant functional variation among individuals in oxidative stress by influencing gene expression and NADPH oxidase activation [15]–[17]. Among them, the C242T polymorphism, which is located in exon 4 at position 214 from the ATG codon, is a well studied one. The C242T polymorphism, resulting from functional C-to-T substitution, has been reported to go along with a reduction in the generation of superoxide anions in the vascular wall [18] and closely with various diseases including renal disease [19], hypertension [20], diabetes [21], cardiovascular disease [22], [23] and cerebrovascular disease [24], [25]. As for ischemic cerebrovascular disease (ICVD), a variety of epidemiological studies have evaluated the role of NADPH oxidase p22phox C242T polymorphism, but the results were inconclusive [24], [26]–[30]. It is likely that NADPH oxidase p22phox C242T polymorphism may influence the susceptibility of ICVD. The present meta-analysis was therefore designed to derive a more precise estimation of the association between NADPH oxidase p22phox C242T polymorphism and ICVD.
Methods and Materials
Data sources
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria [31] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [32]. We selected possibly relevant articles in Embase (1966–June 2012), PubMed (up to June 2012) and Web of Science (1950–June 2012) (last search was update on June 1, 2012) with search strategy: “NADPH oxidase” AND “mutation OR variant OR polymorphism OR genotype” AND “stroke OR cerebrovascular disease OR cerebrovascular disorder OR cerebral infarction OR cerebral ischemia OR brain infarction”. Other relevant studies were identified by hand-searching the references of included articles identified by electronic search and the abstracts presented at related scientific societies meetings. The search was limited to English and Chinese language papers and human subject studies. Two investigators (Li BH and Zhang LL) screened each of the titles, abstracts and full texts to determine inclusion independently. The results were compared and disagreements were resolved by consensus. Detailed search strategy is available at the Appendix S1.
Inclusion criteria
Studies were included in the meta-analysis if: (1) Studies on the relationship between NADPH oxidase C242T polymorphism and ICVD; (2) ICVD includes ischemic stroke and transient ischemic attack; (3) Published case-control, nested case-control or cohort designs studies; (4) Studies with full text articles; (5) Studies reporting odds ratios (ORs) with 95% confidence intervals (CIs) or raw data for their calculations. Studies deviating from Hardy-Weinberg equilibrium (HWE) were not removed.
Data extraction
Information was carefully extracted from all included publications independently by two of the authors (Li BH and Zhang LL) according to the inclusion criteria listed above. Disagreement was resolved by consensus. If these two authors could not reach a consensus, another author (Li JC) was consulted. The following data were collected from each study: first author's name, publication date, country, ethnicity, study design (source of controls), phenotype (type of ICVD), diagnoses of ICVD (clinical or imaging diagnosis), total number of cases and controls, frequency of C242T polymorphism in cases and controls or published crude ORs derived from these data and evidence of HWE (P value less than 0.05 of HWE was considered significant), respectively. Different ethnicities were categorized as Caucasian, Asian, African and mixed. Study design was stratified to population-based (PB) studies and hospital-based (HB) studies. According to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification, there are five subtypes of ischemic stroke: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology [33]. When studies reported genotype distributions for ischemic stroke subtype, we also extracted data of each subtype separately for subgroup analyses. If necessary data were not reported in the primary manuscripts, we contacted the corresponding authors by email to request the missing data.
Quality score assessment
The qualities of included studies were assessed independently by the same two investigators using the Newcastle-Ottawa Scale (NOS) [34]. The NOS uses a ‘star’ rating system to judge quality based on 3 aspects of the study: selection, comparability, and exposure (case-control studies) or outcome (cohort studies). Scores were ranged from 0 star (worst) to 9 stars (best). Studies with a score ≥7 were considered to be of high quality. Disagreement was settled as described above.
Statistical analysis
The strength of association between C242T polymorphism and ICVD risk was measured by ORs and 95% CIs. The combined ORs and 95% CIs were calculated respectively for allelic model (T vs. C), additive model (TT vs. CC), dominant model (TT+TC vs. CC) and recessive model (TT vs. TC+CC) [35]. We used the generic inverse variance method to obtain pooled ORs and 95% CIs, weighting each study by the inverse of the square of the standard error of its study-specific OR. We only used the crude ORs and 95%CIs for meta-analysis. If the studies did not provide crude ORs and 95%CIs, we calculated the ORs and 95%CIs by the total number of cases and controls, and frequency of C242T polymorphism in cases and controls. A fixed effects model was adopted when no heterogeneity was observed among studies. Otherwise, a random effects model was adopted. Between-study heterogeneity was assessed by the Q-test and I2 statistic, P<0.10 and I2>50% indicated evidence of heterogeneity [36], [37]. Subgroup analyses were performed by ischemic stroke subtypes. Sensitivity analysis was performed by including studies of one ethnic group and by limiting the meta-analysis to studies in agreement with HWE to identify if different results were seen. Publication bias was examined by plotting a Begg's funnel plot and Egger's regression test (P<0.05 was considered representative of statistically significant publication bias) [38]. All above statistical analyses were performed using Review Manager 5.1.7 and Stata 11.0. Power analysis was performed using Quanto software package (Version 1.2.4, http://hydra.usc.edu/gxe/) [39]. We assumed an unmatched case-control design and considered a two-sided p-value of 0.05.
Results
Study characteristics
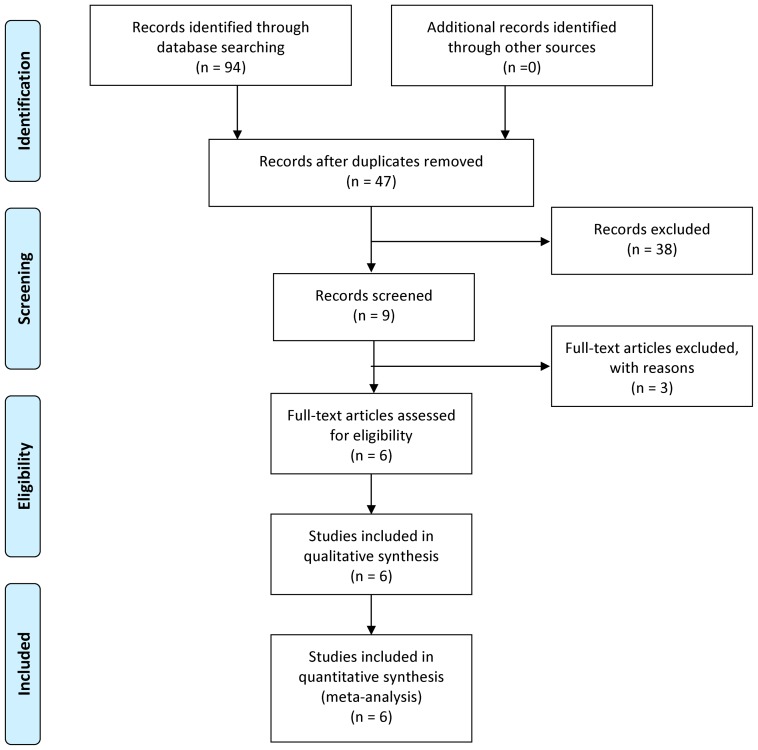
The present study met the PRISMA statement requirements (Appendix S2) and MOOSE guidelines (Appendix S3). The study selection process is detailed in Figure 1 . Based on our preliminary search criteria, a total of nine publications were eligible [24], [26]–[30], [40]–[42]. Among these articles, one study was review article [40]. Two studies reported the p47phox C923T rather than p22phox C242T polymorphism [41], [42]. Hence, six studies were included in the final meta-analysis, including 1,948 cases and 2,357 controls. Khan et al. provided no frequency of C242T polymorphism in cases and controls, and we failed to obtain these data by contacting the corresponding author, which limited our calculation of OR and 95%CI for allelic model and additive model. All studies were case-control in design. Table 1 shows the studies identified and their main characteristics. The NOS results showed that the average score was 8.5 (range 8 to 9), indicating that the methodological quality was generally good. Statistical powers based on the given sample size of each study ranged from 5.1% to 70.8%, which were all lower than 80%. Among the six articles, three focused on Asians, and three on Europeans. The countries of these studies included Japan, UK, Germany and Poland. Five studies characterized ischemic stroke subtypes in their analyses, allowing subtype specific meta-analysis. However, because of the limited available data on cardioembolic, other determined etiology and undetermined subtypes, we only made meta-analysis for small-vessel occlusive ischemic stroke and large-artery atherosclerotic ischemic stroke, in which five studies were combined for small-vessel occlusive subtype [24], [26]–[29] and four for large-artery atherosclerotic subtype [24], [26]–[28].
Figure 1. Flow diagram of the selection of eligible studies.
Table 1. Main characteristics of studies included in the meta-analysis.
| First author | Year | Country | Ethnicity | SOC | Genotypes distribution case/control, N(%) | MAF case/control, N(%) | Subtype of ICVD (diagnostic methods) | Adjustment for confounders | HWE Y/N(P) | Score | Sample size (power) | ||
| CC | TC | TT | T | ||||||||||
| Ito | 2000 | Japan | Asian | PB | 177(78.3)/261(86.7) | 46(20.4)/38(12.6) | 3(1.3)/2(0.7) | 52(11.5)/42(7.0) | SVO, LAA and TIA (brain CT and/or MRI) | age and sex matched | Y(0.63) | 8 | 226/301 (70.8%) |
| Shimo-Nakanishi | 2004 | Japan | Asian | PB | 102(85.0)/154(87.0) | 18(15.0)/23(13.0) | 0(0.0)/0(0.0) | 18(7.5)/23(6.5) | SVO and LAA (brain CT and/or MRI) | age, sex and ethnicity matched | Y(0.36) | 9 | 120/177 (47.5%) |
| Kuroda | 2007 | Japan | Asian | PB | 851(80.7)/840(79.6) | 189(17.9)/198(18.8) | 15(1.4)/17(1.6) | 219(10.4)/232(11.0) | SVO,LAA, CE and SUE (brain CT and/or MRI) | age, sex and ethnicity matched | Y(0.18) | 9 | 1055/1055 (9.5%) |
| Khan | 2007 | UK | Caucasian | PB | - | - | - | - | SVO (brain CT and/or MRI) | age, sex and ethnicity matched | NE | 9 | 316/638 (19.21%) |
| Genius | 2008 | Germany | Caucasian | PB | 73(45.3)/62(45.6) | 66(41.0)/68(50.0) | 22(13.7)/6(4.4) | 110(34.2)/80(29.4) | SVO,LAA, CE, SUE and TIA (brain CT and/or MRI) | sex and ethnicity matched | N(0.02) | 8 | 161/136 (27.2%) |
| Niemiec | 2010 | Poland | Caucasian | PB | 35(50.0)/26(52.0) | 27(38.6)/18(36.0) | 8(11.4)/6(12.0) | 43(30.7)/30(30.0) | Total ICVD (neuroimaging) | age and sex matched | Y(0.31) | 8 | 70/50 (5.1%) |
SOC: source of controls; PB: population-based.
MAF: minor allele frequency.
HWE: Hardy-Weinberg equilibrium; Y: yes; N: no; NE: not estimate.
TIA: transient ischemic attack; ICVD: ischemic cerebrovascular disease.
SVO: small-vessel occlusion; LAA: large-artery atherosclerosis; CE: cardioembolism; SUE: stroke of undetermined etiology.
CT: computed tomography; MRI: magnetic resonance imaging.
Quantitative synthesis
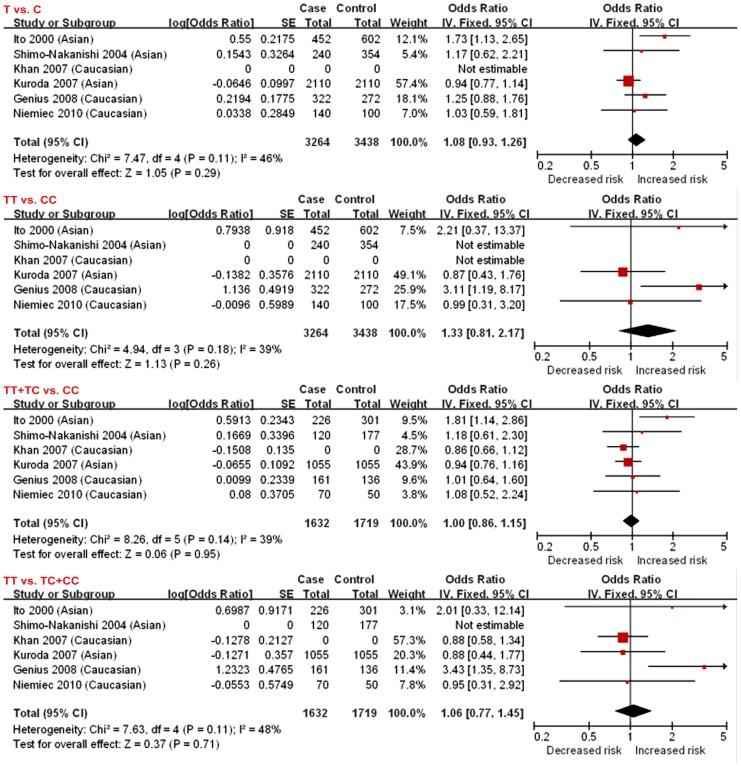
Fixed effect models were adopted as no heterogeneity was observed among studies for all the genetic models (I2 = 46%, P = 0.11; I2 = 39%, P = 0.18; I2 = 39%, P = 0.14; I2 = 48%, P = 0.11, respectively). When all six studies including 1,948 cases and 2,357 controls were pooled into the meta-analysis, there was no statistical evidence of association between p22phox C242T polymorphism and overall ICVD (allelic model: OR = 1.08, 95%CI = 0.93–1.26; additive model: OR = 1.33, 95%CI = 0.81–2.17; dominant model: OR = 1.00, 95%CI = 0.86–1.15; and recessive model: OR = 1.06, 95%CI = 0.77–1.45) ( Figure 2 ). Power calculation on the pooled sample size showed that the statistical power was 21.4%, which was lower than 80%.
Figure 2. Forest plots for overall studies.
Fixed effects models were used as no statistical heterogeneity across studies were observed (I2 = 46%, p = 0.11; I2 = 39%, p = 0.18; I2 = 39%, p = 0.14; I2 = 48%, p = 0.11, respectively). There was no statistical evidence of association (T vs. C: OR = 1.08, 95%CI = 0.93–1.26; TT vs. CC: OR = 1.33, 95%CI = 0.81–2.17; TT+TC vs. CC: OR = 1.00, 95%CI = 0.86–1.15; and TT vs. TC+CC: OR = 1.06, 95%CI = 0.77–1.45). se: standard error; IV: inverse variance; CI: confidence interval.
Four studies including 526 cases and 1,669 controls were pooled into the meta-analysis for large-artery atherosclerotic subtype. Statistically significant association was found in recessive model (allelic model: OR = 1.12, 95%CI = 0.88–1.41; additive model: OR = 1.36, 95%CI = 0.60–3.09; dominant model: OR = 1.25, 95%CI = 0.74–2.11; and recessive model: OR = 2.17, 95%CI = 1.11–4.23) (Appendix S4). Power calculation on the combined sample size showed that the statistical power was 40.8%, which was lower than 80%. Five studies including 959 cases and 2,307 controls were pooled into the meta-analysis for small-vessel occlusive subtype. There was no statistical evidence of significant association (allelic model: OR = 1.16, 95%CI = 0.94–1.44; additive model: OR = 1.32, 95%CI = 0.28–6.12; dominant model: OR = 1.11, 95%CI = 0.84–1.47; and recessive model: OR = 0.90, 95%CI = 0.62–1.31) (Appendix S5). Power calculation on the pooled sample size showed that the statistical power was 38.6%, which was lower than 80%.
Sensitivity analysis
The combined minor allele frequency (MAF) was 33.1% for Caucasian cases and 10.3% for Asian cases. Considering ethnic variations, sensitivity analysis was firstly performed by including studies of one ethnic group. Three studies including 547 cases and 824 controls were pooled into the meta-analysis for Caucasian subgroup. The combined ORs and 95%CIs were: for allelic model: OR = 1.18, 95%CI = 0.88–1.59; for additive model: OR = 1.85, 95%CI = 0.60–5.65; for dominant model: OR = 0.91, 95%CI = 0.73–1.13; and for recessive model: OR = 1.36, 95%CI = 0.57–3.22 (Appendix S6). Power calculation on the pooled sample size showed that the statistical power was 50.3%, which was lower than 80%. Three studies including 1,401 cases and 1,533 controls were pooled into the meta-analysis for Asian subgroup. The combined ORs and 95%CIs were: for allelic model: OR = 1.20, 95%CI = 0.79–1.84; for additive model: OR = 0.98, 95%CI = 0.51–1.89; for dominant model: OR = 1.22, 95%CI = 0.78–1.91; and for recessive model: OR = 0.98, 95%CI = 0.51–1.88 (Appendix S7). Power calculation on the pooled sample size showed that the statistical power was 57.0%, which was lower than 80%.
Secondly, we performed sensitivity analysis by limiting the meta-analysis to studies in agreement with HWE. One study [27] deviating from HWE was excluded. The corresponding pooled ORs and 95%CIs were not substantively altered (allelic model: OR = 1.15, 95%CI = 0.84–1.58; additive model: OR = 0.99, 95%CI = 0.56–1.74; dominant model: OR = 1.07, 95%CI = 0.83–1.37; and recessive model: OR = 0.91, 95%CI = 0.65–1.27), indicating that our results were robust.
Publication bias
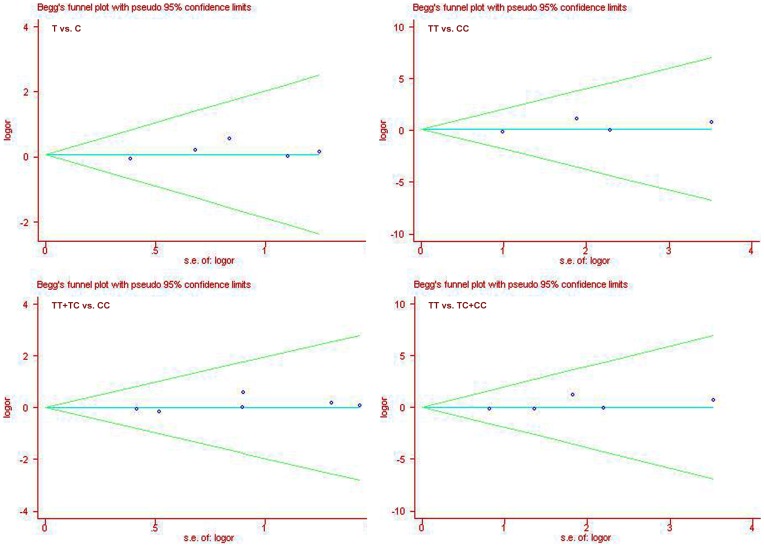
The shapes of the funnel plots did not reveal any evidence of obvious asymmetry visually ( Figure 3 ). Also there was no statistical evidence of publication bias among studies by using Egger's regression test (P = 0.28 for allelic model; P = 0.38 for additive model; P = 0.24 for dominant model; and P = 0.32 for recessive model, respectively).
Figure 3. Funnel plots for overall studies.
The shapes of the funnel plots did not reveal any evidence of obvious asymmetry visually. se: standard error; OR: odds ratio.
Discussion
ICVD is a multifactorial disease, leading to a high mortality and disability rate [43]. It is well known that stroke is associated closely with conventional vascular risk factors [44] and genetic factors. Increasing evidences from evidence-based studies support the critical role of genetic factors in the development of ICVD [45], [46]. Recently, a variety of studies have focused on the association between NADPH oxidase p22phox C242T mutation and ICVD. However, the observed associations of these studies were inconclusive and a single study may be too underpowered to detect a possible small effect of the gene polymorphism on ICVD, especially when the sample size is relatively small. Meta-analysis has the benefit to overcome this limitation by increasing the sample size and may generate more precise results, which has been widely used in genetic association studies [47], [48]. The present meta-analysis was therefore carried out.
Based on our study selection process, a total of six publications were included in our meta-analysis. Ito et al. reported that the C242T polymorphism was a novel pathogenetic risk factor for ICVD with a fact that TC+TT genotype was significantly higher in the ICVD patients [24], which was confirmed by Genius et al., demonstrating that homozygosity for the T variant was associated with an enhanced risk for cerebral ischemia [27]. Our meta-analyses did not show statistical evidence of association between the NADPH oxidase p22phox C242T polymorphism and ICVD in the overall study population. This finding is consistent with most of the included studies.
Theoretically, an impaired NADPH oxidase activity was linked to procoagulant state [49] and hypercholesterolemia [50] and thus increase the risk of ICVD. However, a negative result is reached. Possible explanation may be that: (1) The combined sample sizes in our study were still inadequate to detect the association between C242T polymorphism and ICVD because power calculations for the combined sample size demonstrated that all the meta-analyses were underpowered; (2) Other enzyme systems such as the xanthine oxidase also contributes to the generation of ROS [51]. The C242T polymorphism on ICVD might be confounded by the genetic variation in these systems, so further studies were required; (3) The C242T polymorphism may be associated with the specific subtypes of ischemic stroke as some studies showed that genetic factors were more associated with small- and large-vessel stroke than cardioembolic stroke [52], [53]. Regarding C242T polymorphism, Ito et al. reported that the TC+TT genotype was higher in the ICVD patients with atherothrombotic infarction than those with lacunar infarction and transient ischemic attack. We also analyzed this potential association in our study based on available data. Results showed that C242T polymorphism was more associated with large-artery atherosclerotic stroke than small-vessel occlusive stroke. In the subgroup analysis of large-artery atherosclerotic stroke, statistically significant association was found in recessive model, in which the OR was 2.17. It indicated that TT genotype could increase the risk of large-artery atherosclerotic stroke of 2.17 fold, suggesting that individual with homozygous TT genotype could have higher risk of large-artery atherosclerotic stroke than CC and TC genotype. However, based on the combined sample size (526 cases and 1,669 controls for large-artery atherosclerotic subtype and 959 cases and 2,307 controls for small-vessel occlusive subtype), power calculation showed that it was underpowered to detect the association between C242T polymorphism and both subtype.
With respect to MAF of the p22phox C242T, it is quite different between Caucasian population and Asian population. Data of International HapMap Project shows that the MAF of the p22phox C242T polymorphism is common among Utah residents with Northern and Western European ancestry (CEU) (MAF = 0.314), whereas it is very low among Han Chinese in Beijing, China (CHB) and Japanese in Tokyo, Japan (JPT) (MAF = 0.084 and 0.062, respectively) [54]. Our study also showed that the combined MAF was 33.1% for Caucasian cases and 10.3% for Asian cases. Considering ethnic variations, sensitivity analysis was performed. However, both subgroups revealed no statistical evidence of significant association between p22phox C242T polymorphism and ICVD.
For better interpreting the results, some limitations of this meta-analysis should be acknowledged. Firstly, the small number of studies and sample size limited the ability to draw more solid conclusions. Secondly, lacking of the original data limited our further evaluation of potential interactions among gene-gene and gene-environment.
Nonetheless, to the best of our knowledge, the present study is the first meta-analysis of the relationship between C242T polymorphism and ICVD. Our meta-analysis suggests that C242T polymorphism is more associated with large-artery atherosclerotic stroke than small-vessel occlusive stroke. However, this conclusion should be interpreted with caution due to the small sample size. Larger sample-size studies with homogeneous ICVD patients and well-matched controls are required.
Supporting Information
Search Strategy.
(DOC)
PRISMA 2009 Checklist.
(DOC)
MOOSE Checklist.
(DOC)
Forest plots for large-artery atherosclerotic subtype.
(TIF)
Forest plots for small-vessel occlusive subtype.
(TIF)
Forest plots for Caucasian subgroup.
(TIF)
Forest plots for Asian subgroup.
(TIF)
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (30970998, 30800444), and a grant from the Chongqing Key Project of Science and Technologies (CSTC2010GGC502). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Frey RS, Ushio-Fukai M, Malik AB (2009) NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Custodis F, Baumhakel M, Schlimmer N, List F, Gensch C, et al. (2008) Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 117: 2377–2387. [DOI] [PubMed] [Google Scholar]
- 3. Chan PH (1996) Role of oxidants in ischemic brain damage. Stroke 27: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 4. He YY, Hsu CY, Ezrin AM, Miller MS (1993) Polyethylene glycol-conjugated superoxide dismutase in focal cerebral ischemia-reperfusion. Am J Physiol 265: H252–256. [DOI] [PubMed] [Google Scholar]
- 5. Imaizumi S, Woolworth V, Fishman RA, Chan PH (1990) Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke 21: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 6. Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38. [DOI] [PubMed] [Google Scholar]
- 7. Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY (1989) Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol 256: H589–593. [DOI] [PubMed] [Google Scholar]
- 8. Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313. [DOI] [PubMed] [Google Scholar]
- 9. DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, et al. (1998) Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest 101: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griendling KK (2004) Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK (1996) p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271: 23317–23321. [DOI] [PubMed] [Google Scholar]
- 12. Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, et al. (2002) Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 13. Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–297. [DOI] [PubMed] [Google Scholar]
- 14. Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH (1990) Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 86: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zafari AM, Davidoff MN, Austin H, Valppu L, Cotsonis G, et al. (2002) The A640G and C242T p22(phox) polymorphisms in patients with coronary artery disease. Antioxid Redox Signal 4: 675–680. [DOI] [PubMed] [Google Scholar]
- 16. Moreno MU, San Jose G, Orbe J, Paramo JA, Beloqui O, et al. (2003) Preliminary characterisation of the promoter of the human p22(phox) gene: identification of a new polymorphism associated with hypertension. FEBS Lett 542: 27–31. [DOI] [PubMed] [Google Scholar]
- 17. Moreno MU, San Jose G, Fortuno A, Beloqui O, Redon J, et al. (2007) A novel CYBA variant, the -675A/T polymorphism, is associated with essential hypertension. J Hypertens 25: 1620–1626. [DOI] [PubMed] [Google Scholar]
- 18. Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, et al. (2000) Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation 102: 1744–1747. [DOI] [PubMed] [Google Scholar]
- 19. Perianayagam MC, Liangos O, Kolyada AY, Wald R, MacKinnon RW, et al. (2007) NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 255–263. [DOI] [PubMed] [Google Scholar]
- 20. Moreno MU, San Jose G, Fortuno A, Beloqui O, Diez J, et al. (2006) The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. J Hypertens 24: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 21. Lim SC, Goh SK, Lai YR, Tee WW, Koh A, et al. (2006) Relationship between common functional polymorphisms of the p22phox gene (−930A>G and +242C>T) and nephropathy as a result of Type 2 diabetes in a Chinese population. Diabet Med 23: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 22. Shimokata K, Yamada Y, Kondo T, Ichihara S, Izawa H, et al. (2004) Association of gene polymorphisms with coronary artery disease in individuals with or without nonfamilial hypercholesterolemia. Atherosclerosis 172: 167–173. [DOI] [PubMed] [Google Scholar]
- 23. Nasti S, Spallarossa P, Altieri P, Garibaldi S, Fabbi P, et al. (2006) C242T polymorphism in CYBA gene (p22phox) and risk of coronary artery disease in a population of Caucasian Italians. Dis Markers 22: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito D, Murata M, Watanabe K, Yoshida T, Saito I, et al. (2000) C242T polymorphism of NADPH oxidase p22 PHOX gene and ischemic cerebrovascular disease in the Japanese population. Stroke 31: 936–939. [DOI] [PubMed] [Google Scholar]
- 25. Krex D, Ziegler A, Konig IR, Schackert HK, Schackert G (2003) Polymorphisms of the NADPH oxidase P22PHOX gene in a Caucasian population with intracranial aneurysms. Cerebrovasc Dis 16: 363–368. [DOI] [PubMed] [Google Scholar]
- 26. Kuroda J, Kitazono T, Ago T, Ninomiya T, Ooboshi H, et al. (2007) NAD(P)H oxidase p22phox C242T polymorphism and ischemic stroke in Japan: the Fukuoka Stroke Registry and the Hisayama study. Eur J Neurol 14: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 27. Genius J, Grau AJ, Lichy C (2008) The C242T polymorphism of the NAD(P)H oxidase p22phox subunit is associated with an enhanced risk for cerebrovascular disease at a young age. Cerebrovasc Dis 26: 430–433. [DOI] [PubMed] [Google Scholar]
- 28. Shimo-Nakanishi Y, Hasebe T, Suzuki A, Mochizuki H, Nomiyama T, et al. (2004) Functional effects of NAD(P)H oxidase p22(phox) C242T mutation in human leukocytes and association with thrombotic cerebral infarction. Atherosclerosis 175: 109–115. [DOI] [PubMed] [Google Scholar]
- 29. Khan U, Bevan S, Markus HS (2007) NADPH oxidase polymorphisms in cerebral small vessel disease. Cerebrovasc Dis 24: 135–138. [DOI] [PubMed] [Google Scholar]
- 30. Niemiec P, Zak I, Emich-Widera E, Balcerzyk A, Kopyta I, et al. (2010) The C242T polymorphism of the gene encoding cytochrome b-245 alpha is not associated with paediatric ischaemic stroke: family-based and case-control study. Neurol Neurochir Pol 44: 453–458. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 33. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, et al. (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 34. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2011 Dec 1 [Google Scholar]
- 35. Lewis CM (2002) Genetic association studies: design, analysis and interpretation. Brief Bioinform 3: 146–153. [DOI] [PubMed] [Google Scholar]
- 36. Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10: 101–129. [Google Scholar]
- 37. Higgins JPTS (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 38. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer-Rosinsky A, Fisher E, Kovacs P, Bluher M, Mohlig M, et al. (2008) Lack of association between the tagging SNP A+930→G of SOCS3 and type 2 diabetes mellitus: meta-analysis of four independent study populations. PLoS One 3: e3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. San Jose G, Fortuno A, Beloqui O, Diez J, Zalba G (2008) NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci (Lond) 114: 173–182. [DOI] [PubMed] [Google Scholar]
- 41. Huang XS, Yang QD, Long XY, Liu YH, Liu HF, et al. (2007) [The relationship of P47phox C923T (Ala308Val) polymorphism to cerebral infarction and its effect on plasma lipid levels]. Zhonghua Yi Xue Za Zhi 87: 2062–2064. [PubMed] [Google Scholar]
- 42. Huang XS, Yang QD, Guo TS, Long XY, Yang J, et al. (2007) [The relationship between the exon 10 of the p47(phox) polymorphism and stroke and the effect of the polymorphism on plasma lipid]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 24: 524–528. [PubMed] [Google Scholar]
- 43. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. (2010) Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: 948–954. [DOI] [PubMed] [Google Scholar]
- 44. Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 45. Dahabreh IJ, Kitsios GD, Kent DM, Trikalinos TA (2010) Paraoxonase 1 polymorphisms and ischemic stroke risk: A systematic review and meta-analysis. Genet Med 12: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu X, Li X, Li J, Ou R, Sheng W (2010) Meta-analysis of association between variation in the PDE4D gene and ischemic cerebral infarction risk in Asian populations. Neurogenetics 11: 327–333. [DOI] [PubMed] [Google Scholar]
- 47. Shi TY, He J, Qiu LX, Zhu ML, Wang MY, et al. (2012) Association between XPF Polymorphisms and Cancer Risk: A Meta-Analysis. PLoS One 7: e38606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, et al. (2012) Posttraumatic Stress Disorder Prevalence and Risk of Recurrence in Acute Coronary Syndrome Patients: A Meta-analytic Review. PLoS One 7: e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorlach A, Brandes RP, Bassus S, Kronemann N, Kirchmaier CM, et al. (2000) Oxidative stress and expression of p22phox are involved in the up-regulation of tissue factor in vascular smooth muscle cells in response to activated platelets. FASEB J 14: 1518–1528. [PubMed] [Google Scholar]
- 50. Corsetti JP, Ryan D, Moss AJ, Zareba W, Sparks CE (2008) NAD(P)H oxidase polymorphism (C242T) and high HDL cholesterol associate with recurrent coronary events in postinfarction patients. Atherosclerosis 196: 461–468. [DOI] [PubMed] [Google Scholar]
- 51. Harrison R (2004) Physiological roles of xanthine oxidoreductase. Drug Metab Rev 36: 363–375. [DOI] [PubMed] [Google Scholar]
- 52. Schulz UG, Flossmann E, Rothwell PM (2004) Heritability of ischemic stroke in relation to age, vascular risk factors, and subtypes of incident stroke in population-based studies. Stroke 35: 819–824. [DOI] [PubMed] [Google Scholar]
- 53. Jerrard-Dunne P, Cloud G, Hassan A, Markus HS (2003) Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke 34: 1364–1369. [DOI] [PubMed] [Google Scholar]
- 54.International HapMap Project (2012) Available: http://hapmapncbinlmnihgov. Accessed 2012 Nov 28.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy.
(DOC)
PRISMA 2009 Checklist.
(DOC)
MOOSE Checklist.
(DOC)
Forest plots for large-artery atherosclerotic subtype.
(TIF)
Forest plots for small-vessel occlusive subtype.
(TIF)
Forest plots for Caucasian subgroup.
(TIF)
Forest plots for Asian subgroup.
(TIF)