Abstract
Background
Nitric oxide (NO) seems to play an important role during renal ischemia/reperfusion (I/R) injury. We investigated whether rutin inhibits inducible nitric oxide synthase (iNOS) and reduces 3-nitrotyrosine (3-NT) formation in the kidneys of rats during I/R.
Methods
Wistar albino rats were nephrectomized unilaterally and, 2 weeks later, subjected to 45 minutes of left renal pedicle occlusion followed by 3 hours of reperfusion. We intraperitoneally administered L-N6-(1-iminoethyl)lysine (L-NIL; 3 mg/kg) for 30 minutes or rutin (1 g/kg) for 60 minutes before I/R. After reperfusion, kidney samples were taken for immunohistochemical analysis of iNOS and 3-NT. We measured plasma nitrite/nitrate and cyclic guanosine monophosphate (cGMP) to evaluate NO levels.
Results
Ischemia/reperfusion caused plasma cGMP to increase significantly. Similarly, plasma nitrite/nitrate was elevated in the I/R group compared with the control group. Histochemical staining was positive for iNOS and 3-NT in the I/R group. Pretreatment with L-NIL or rutin significantly mitigated the elevation of plasma cGMP and nitrite/nitrate. These changes in biochemical parameters were also associated with changes in immunohistochemical appearance. Pretreatment with L-NIL or rutin significantly decreased the incidence and severity of iNOS and 3-NT formation in the kidney tissues.
Conclusion
Our findings suggest that high activity of iNOS causes renal I/R injury, and that rutin exerts protective effects, probably by inhibiting iNOS.
Abstract
Contexte
L’oxyde nitrique (NO) semble jouer un rôle important durant la lésion d’ischémie/reperfusion (I/R) rénale. Nous avons vérifié si la rutine inhibe l’oxyde nitrique synthase inductible (iNOS) et réduit la formation de 3-nitrotyrosine (3-NT) dans les reins de rats durant l’I/R.
Méthodes
Des rats albinos Wistar ont subi une néphrectomie unilatérale avant d’être soumis 2 semaines plus tard à une occlusion du pédicule rénal gauche d’une durée de 45 minutes, suivie de 3 heures de reperfusion. Nous avons administré de la L-N6-(1-iminoéthyl)lysine (L-NIL; 3 mg/kg) par voie intrapéritonéale pendant 30 minutes ou de la rutine (1 g/kg) pendant 60 minutes avant l’I/R. Après la reperfusion, des échantillons de tissu rénal ont été prélevés pour analyse immunohistochimique de l’iNOS et de la 3-NT. Nous avons mesuré les taux plasmatiques de nitrite/nitrate et de guanosine monophosphate cyclique (cGMP) pour évaluer les taux de NO.
Résultats
L’ischémie/reperfusion a causé une augmentation significative du cGMP plasmatique. De même, les taux de nitrite/nitrate plasmatiques ont augmenté dans le groupe soumis à l’I/R, comparativement au groupe témoin. Les épreuves de coloration histochimique ont donné des résultats positifs pour l’iNOS et la 3-NT dans le groupe soumis à l’I/R. Un prétraitement par L-NIL ou rutine a significativement atténué l’élévation des taux de cGMP plasmatique et de nitrite/nitrate. Ces changements des paramètres biochimiques ont aussi été associés à des changements de l’aspect immunohistochimique. Le prétraitement au moyen de L-NIL ou de rutine a significativement réduit l’incidence et l’ampleur de la formation d’iNOS et de 3-NT dans les tissus rénaux.
Conclusion
Nos observations donnent à penser qu’une forte activité de l’iNOS provoque la lésion I/R rénale et que la rutine confère une protection, probablement en inhibant l’iNOS.
Ischemia/reperfusion (I/R) of an organ or tissue is cellular injury triggering a complex cascade of biochemical events that affect the structure and function of almost every organelle and subcellular system of affected cells.1 Many scientists report that renal I/R injury is a common cause of renal cell death, acute renal failure and, in the case of transplantation, delayed graft function or graft rejection.2–4 Many mediators are involved in the pathophysiology of I/R injury, including reactive oxygen species (ROS), reactive nitrogen species (RNS), purine metabolites, neutrophil accumulation, vasoactive substance (endothelin, angiotensin II) and subsequent release of lytic enzymes.5–7
Nitric oxide (NO), a soluble, free radical gas, has an astounding range of biological roles, including modulation of vascular tone and inflammation.8,9 Nitric oxide usually achieves these effects by binding to the heme group of the soluble form of an enzyme called guanylate cyclase, but rarely through irreversible chemical modifications of other molecules.10 Guanylate cyclase comprises an important class of enzymes that, on activation, synthesize the second messenger cyclic guanosine monophosphate (cGMP).11 For this reason, cGMP accumulation is an indirect marker of NO levels in tissue or blood plasma. Nitric oxide is produced from l-arginine by nitric oxide sythase (NOS).12,13 This enzyme is expressed as 3 isoforms, all of which have been isolated from the kidney: endothelial NOS, neuronal NOS and inducible NOS (iNOS). The endothelial and neuronal isoforms have been identified in the renal vasculature and macula densa, respectively,14,15 whereas iNOS is expressed in several segments of the renal tubule and in the glomerulus and interlobar artery of a healthy rat kidney.16 Inducible NOS has low basal expression until activated by an immune response, such as cytokines and oxidative stress. Endothelial and neuronal NOS are expressed constitutively.17 Several researchers have suggested that NO produced from iNOS is detrimental in I/R,18–20 because iNOS is responsible for the production of large bursts of NO during I/R. Chatterjee and colleagues21 have shown that selective inhibition of iNOS by using L-N6-(1-iminoethyl)lysine (L-NIL) can reduce renal dysfunction and injury associated with I/R of the kidney via inhibition of iNOS activity and subsequent reduction of NO generation. Also, Mark and colleagues22 reported that blockade of iNOS after I/R resulted in a salutary effect on renal function. Furthermore, NO itself can combine with the superoxide radical to form a powerful cytotoxic metabolite, peroxynitrite, causing tissue injury by protein tyrosine nitration or by decomposition into hydroxyl radicals and NO.23,24 3-Nitrotyrosine is a reaction product of peroxynitrite with susceptible tyrosine residues. Specifically, in the kidney 3-NT generation has been implicated in the pathophysiology of both renal I/R and hypoxia/reoxygenation injury.18,25,26
Flavonoids occur naturally in plants and cannot be synthesized by humans.27 In vitro studies have shown that flavonoids, including rutin, possess anti-inflammatory, anti-allergic and anticancerogenic properties.28 Rutin (quercetin-3-rhamnosyl glucoside) is a kind of flavonoid glycoside found in buckwheat and many other vegetables, fruits and plant-derived beverages, such as tea and wine.29 The health benefits of flavonoids attributed to polyphenols are usually linked to 2 properties: antioxidant activity and inhibition of certain enzymes.30 Rutin is an important antilipoperoxidant agent and has been found to be a strong scavenger of superoxide and hydroxyl radicals.31,32 Furthermore, Robak and Gryglewski33 have shown that rutin is a scavenger of the superoxide dismutase–sensitive free radicals. It has been reported that flavonoids inhibit xanthine-oxidase, cyto-chrome P450–dependent estrogen synthase, lipoxygenase and some other enzymes. Various types of NO production inibitors, such as polyacetylenes, coumarines, flavonoids and diterpernes, were isolated from several natural medicines. Although flavonoids, which are widely distributed in the plant kingdom, have been recognized to show various biological activities, the effects of specific iNOS inhibition of rutin have not been discussed extensively.31,33,34
Thus, we designed the present study to determine whether rutin can inhibit iNOS activity and reduce 3-NT formation in the kidneys of rats during I/R. We examined plasma nitrite/nitrate and cGMP concentrations in all experimental groups. The presence of 3-NT and iNOS in the kidneys was also examined using immunohistochemical analysis.
Methods
Experimental animals
All experimental procedures and animal use protocols were approved by the Ethics Committee of Hacettepe University. Moreover, all experimental procedures conformed with the National Research Council Guidelines35 for the handling and care of laboratory animals. We performed the study on 50 male Wistar rats weighing 250–300 g that we obtained from the Production Centre of Experimental Animals in Hacettepe University, Ankara, Turkey. All animals were housed in polycarbonate cages with stainless steel covers in an air-conditioned room (12-h light/dark cycle with a mean temperature of 21°C ± 4°C and a relative mean humidity of 50% ± 10%. Throughout the experimental period, all animals were provided, ad libitum, with tap water and standard rat pellet food.
Experimental groups
We separated the animals into the following experimental groups (n = 10 per group).
Sham-operated control group (sham)
The right renal pedicle of rats was dissected without performing a nephrectomy. The left renal pedicle of rats was not dissected.
Right nephrectomy control group (SH+NP)
The animals underwent right nephrectomy, but the left renal pedicle was not dissected.
Right nephrectomy + I/R group (NP+I/R)
The animals received 0.5 mL of saline 1 hour before ischemia, and then the left renal pedicle was occluded for 45 minutes to induce ischemia followed by 3 hours of reperfusion.
Right nephrectomy + L-NIL + I/R group (NP+L-NIL+IR)
The animals received L-NIL (3 mg/kg in 0.5 mL of saline) intraperitoneally 30 minutes before ischemia, and then the left renal pedicle was occluded for 45 minutes to induce ischemia followed by 3 hours of reperfusion. The treatment dose of L-NIL for this group was chosen according to previous studies.18,21
Right nephrectomy + rutin + I/R group (NP+RU+IR)
The animals received rutin (1 g/kg in 0.5 mL of saline) intraperitoneally 1 hour before ischemia, and then the left renal pedicle was occluded for 45 minutes to induce ischemia followed by 3 hours of reperfusion.
Surgical procedures
For nephrectomy, we anesthetized the animals with an intramuscular injection combining 70 mg/kg of ketamine with 10 mg/kg of xylazine. The depth of anesthesia was monitored continuously. The animals were placed ventrally on a heated surgical pad, and rectal temperature was maintained at 37°C. The abdominal region of each rat was shaved and sterilized with a povidone-iodine solution. We made a dorsoventral incision into the abdominal cavity, continuing down the side of the rat near the costal border of the thorax. A single thread ligature was placed around the renal blood vessels and the ureter as far from the kidney as possible. The ligature was tied securely with a double knot, and the blood vessels were transected next to the kidney, which was removed. After the surgical procedures, the animals were given 2 mL of 0.9% (w/v) NaCl in the abdominal cavity, and then the incision was closed in the usual way. All of the rats were allowed to recover for 2 weeks before groups 3, 4 and 5 were subjected to I/R injury.
On the fifteenth day after nephrectomy, rats were fasted overnight. One hour before ischemia, we intraperitoneally administered 0.5 mL of saline alone to the rats in groups 1, 2 and 3. Thirty minutes before ischemia, L-NIL in 0.5 mL of saline was administered intraperitoneally to the rats in group 4. Also 1 hour before ischemia, rutin in 0.5 mL of saline was administered intraperitoneally to the rats in group 5. We anesthetized the animals with an intramuscular injection combining 70 mg/kg−1 of ketamine and 10 mg/kg−1 of xylazine before the surgical procedure. After surgery preparation, the rats were placed on a heated table to maintain constant body temperature. For I/R surgery, the anesthetized rat was laid on its back with its tail toward the investigator. A midline abdominal incision was made extending posteriorly for about half the length of the abdomen. We isolated and occluded the left renal pedicle. A small padded vascular bulldog clamp was placed around the left renal artery and vein at the level of the hilum for a total of 45 minutes of reversible occlusion. We considered occlusion to be confirmed when we observed a substantially pallid change of the kidney colour and a return to a red shade upon reperfusion. We removed the microsurgical clamps 45 minutes after reperfusion. After that, the animals were given 2 mL of 0.9% (w/v) NaCl in the abdominal cavity, the midline incision was sutured, and the povidone-iodine solution was applied locally. Three hours after removing the vascular clamp, we euthanized the animals by cervical dislocation. The surgical procedures were devised based on the methods of Waynforth and Flecknell.36
Immediately after the animals were euthanized, we isolated the left kidney and washed it with ice-cold saline. The kidney samples were cut in 2 and immediately placed in a Bouin solution for immunohistochemical evaluation of iNOS and 3-NT levels. We collected blood samples from the heart in heparinized centrifuge tubes after euthanization. The blood samples were centrifuged, and plasma was collected. We stored the plasma samples at −20°C until we completed the analysis for plasma cGMP and nitrite/nitrate levels.
Measurement of plasma nitrite/nitrate concentrations
Nitrite and nitrate are the stable degradation products of NO subsequent to reaction with oxygen; therefore, we used nitrite/nitrate concentration in the plasma as an indicator of NO synthesis. Plasma samples were assayed for nitrite/nitrate concentration using a colorimetric nonenzymatic NO assay kit (Oxford Biomedical Research).
Measurement of plasma cGMP concentrations
We measured plasma cGMP levels using a competitive enzyme immunoassay kit (Cayman Chemical Co.). The assay is based on the competition between free cGMP and a cGMP tracer (cGMP linked to an acetylcholinesterase molecule) for a limited number of cGMP-specific rabbit antiserum binding sites.
Immunohistochemical localization of 3-NT and iNOS
3-Nitrotyrosine may serve as a biomarker for the effects of NO based on tyrosine residues becoming nitrated in proteins at sites of inflammation-induced tissue injury. Inducible NOS is a common indicator of NO production in tissue.37 The specimens of the left kidney cortex in all groups were used for immunohistochemical staining. Paraffin-embedded kidney tissue samples were used for iNOS and 3-nitrotyrosine immunohistochemistry. Tissue sections 4 μm thick were deparaffinized twice in xylene for 10 minutes each and rehydrated in ethanol followed by water and phosphate buffered saline. To improve the antigen demonstration, the slides were then incubated in a water bath with unmasking solution (Vector Laboratories Inc.) for 1 hour at 95ºC. Subsequently, endogenous peroxidase was blocked by immersion in 10% hydrogenper-oxide and methanol for 20 minutes, and the tissue was washed twice in phosphate buffered saline for 10 minutes each. Nonspecific protein binding was blocked by incubation with normal horse serum (for 3-NT) or normal goat serum (for iNOS) for 20 minutes. The tissue sections were drained well and then incubated with 3-NT antibody (1:100 dilution in phosphate buffered saline; Chemicon International) or with iNOS antibody (1:1000 dilution in phosphate buffered saline) for 1 hour at room temperature. The immunostain was developed with diamino-benzidinetetrahydrochloride for 5 minutes (Vectorlabs). The sections were counterstained with methyl green, rehydrated in ethanol followed by xylene, and cover slipped. Negative control experiments involved omitting the incubation with the primary antibody. Immunohistochemical staining for 3-NT and iNOS was evaluated by light microscopy.
Two experienced pathologists graded specific 3-NT and iNOS staining on a semiquantitative scale. Whenever there was a disagreement, the slides were reviewed, and consensus was reached. They scored immunostaining based on the intensity of staining and the percentage of cells that stained positively. Staining scores were calculated by multiplying the percentage of positive cells per section (0%–100%) by the immunohistochemical staining intensity. We classified the sections according to staining intensity as negative (total absence of staining), 1+ (weak staining), 2+ (moderate staining) or 3+ (strong staining), and scores ranged from 0 to 300. The staining scores obtained for 2 slides from the same specimen were calculated, and the result was recorded as the score for that case. We calculated the staining scores for the specimens that contained iNOS combined with 3-NT using the intensity of staining and the percentage of each component stained on the entire slide.
Statistical analysis
Data are expressed as means and standard errors of the mean. We detected between-group differences using 1-way analysis of variance (ANOVA) with Bonferroni post hoc analysis. We analyzed differences in immunohistochemical staining among groups using the Fisher exact test. We considered results to be significant at p < 0.05.
Results
Effect of rutin and L-NIL on I/R-mediated increase in plasma nitrite/nitrate levels
We measured plasma concentration of nitrite/nitrate as a marker of NOS activity. Table 1 shows the level of nitrite/nitrate and its percent decrease or increase in the plasma of all experimental groups. The renal I/R groups had significantly higher plasma levels of nitrite/nitrate than the SH and SH+NP groups. Increased plasma nitrite/nitrate levels mediated by renal I/R were significantly reduced after administration of L-NIL or rutin before renal I/R. Groups that received L-NIL or rutin had similar plasma nitrite/nitrate levels to sham-operated rats that received saline only.
Table 1.
Effect of rutin and L-NIL on plasma nitrite/nitrate and cyclic guanosine monophosphate production mediated by renal ischemia/reperfusion
| Group | Plasma cGMP, mean (SD) pmol/mL | Percent change | Plasma nitrite/nitrate, mean (SD) μmol/L | Percent change |
|---|---|---|---|---|
| Sham | 5.56 (0.09)*† | — | 5.6 (0.31)* | — |
| Sham+NP | 5.78 (0.09)*† | + 3.9 | 5.3 (0.83)* | −5.3 |
| NP+I/R | 9.73 (0.05)†‡ | + 75 | 13.21 (0.8)†‡ | + 135 |
| NP+L-NIL+I/R | 6.86 (0.10)*‡ | + 23 | 5.9 (0.61)* | + 5.3 |
| NP+rutin+I/R | 6.56 (0.10)*‡ | + 17 | 5.6 (0.46)* | — |
cGMP = cyclic guanosine monophosphate; I/R = ischemia/reperfusion; L-NIL = L-N6-(1-iminoethyl)lysine; NP = nephrectomy; SD = standard deviation.
p < 0.05 v. NP+I/R group.
p < 0.05 v. NP+L-NIL+I/R group; p < 0.05 v. NP+rutin+I/R group.
p < 0.05 v. sham group; p < 0.05 v. sham+NP group.
Effect of rutin and L-NIL on I/R-mediated increase in plasma cGMP levels
Plasma cGMP is an appropriate marker of NO bioactivity. Table 1 shows the level of plasma cGMP and its percent decrease or increase in all experimental groups. The NP+IR group showed a marked increase of plasma cGMP compared with the SH and SH+NP groups. In contrast, rutin pretreatment in the I/R group significantly decreased plasma cGMP concentrations, which was found to be different from the NP+IR group. Moreover, plasma cGMP levels significantly decreased in rats subjected to L-NIL pretreatment before I/R.
Effect of rutin and L-NIL on iNOS and 3-NT formation during renal I/R
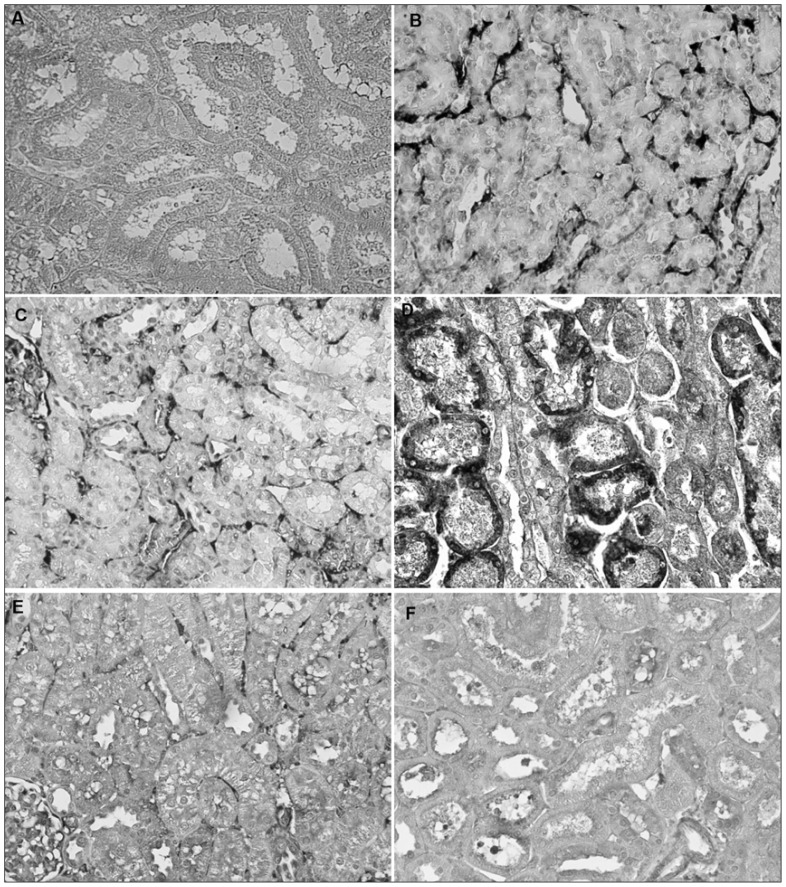
Immunohistochemical detection of iNOS was used as a marker of NO formation in the kidney. Formation of 3-NT protein adducts is a reliable biomarker of peroxynitrite formation. The iNOS and 3-NT staining scores for each antibody are summarized in Table 2 and depicted in Figures 1 and 2. The cross-reactivity of antinitrotyrosine antibody to erythrocyte components was detected in the kidney slides of all groups, as indicated by the dark staining in the interstitial space of the cortex seen in Figure 2D. The staining should not be evaluated as an antibody with specific antigen reaction.
Table 2.
Immunohistochemical staining intensity and score for 3-nitrotyrosine and inducible nitric oxide synthetase in the kidney specimens of rats in all experimental groups
| Group | Intensity of staining* | Staining score | ||
|---|---|---|---|---|
|
|
|
|||
| 3-NT | iNOS | 3-NT | iNOS | |
| Sham | — | — | 0 | 0 |
|
| ||||
| Sham+NP | — | — | 0 | 1 |
|
| ||||
| NP+I/R | 3+ | 3+ | 289† | 293† |
|
| ||||
| NP+L-NIL+I/R | 1+ | 1+ | 15‡ | 20‡ |
|
| ||||
| NP+rutin+I/R | 1+ | 1+ | 23‡ | 27‡ |
3-NT = 3-nitrotyrosine; iNOS = inducible nitric oxide synthase; I/R = ischemia/reperfusion; L-NIL = L-N6-(1-iminoethyl) lysine; NP = nephrectomy.
Where – indicates no staining, + weak staining, 2+ moderate staining, 3+ intense staining.
p < 0.05 v. sham group; p < 0.05 v. sham+NP group; p < 0.05 v. NP+L-NIL+I/R group; p < 0.05 v. NP+rutin+I/R group.
p < 0.05 v. NP+I/R group.
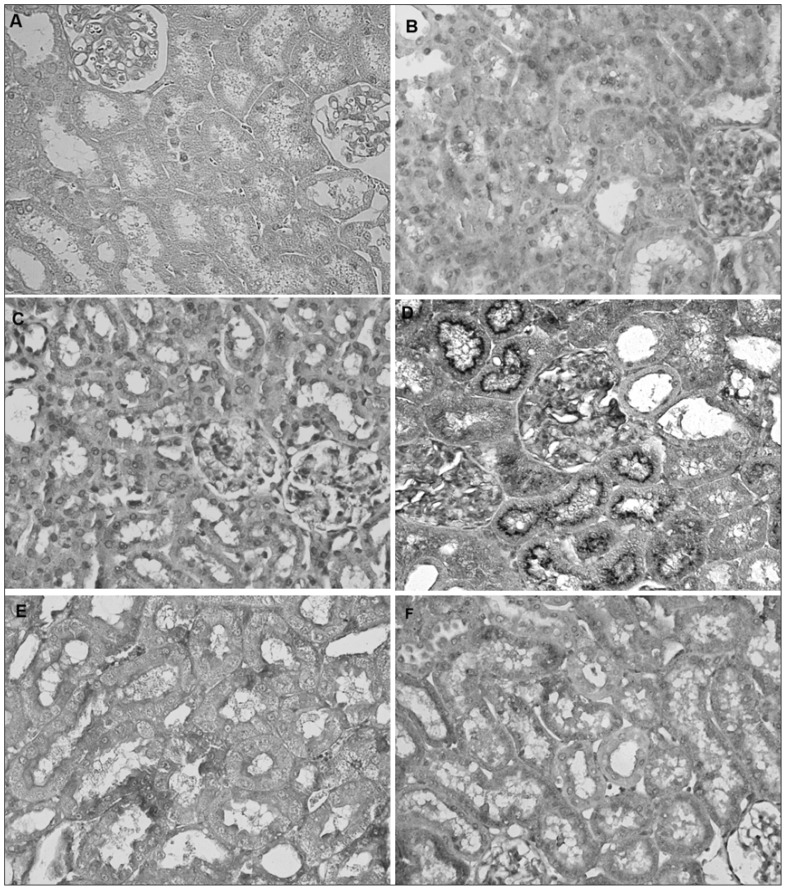
Fig. 1.
Immunohistochemical evidence of expression of inducible nitric oxide synthase in the cortex of a rat kidney following ischemia/reperfusion (I/R). Panels refer to the following treatment groups: (A) negative control without primary antibody, (B) sham, (C) right nephrectomy control, (D) right nephrectomy + I/R, (E) right nephrectomy + L-N6-(1-iminoethyl)lysine + I/R, and (F) right nephrectomy + rutin + I/R. Magnification ×400.
Fig. 2.
Immunohistochemical evidence of 3-nitrotyrosine formation in the cortex of a rat kidney following ischemia/reperfusion (I/R). Panels refer to the following treatment groups: (A) negative control without primary antibody, (B) sham, (C) right nephrectomy control, (D) right nephrectomy + I/R, (E) right nephrectomy + L-N6-(1-iminoethyl)lysine + I/R, and (F) right nephrectomy + rutin + I/R. Magnification ×400.
Figures 1 and 2 show the immunostaining without primer antibody for 3-NT and iNOS, respectively, in the kidney. No evidence of staining for iNOS (Fig. 1B and C) and 3-NT (Fig. 2B and C) was observed in kidney sections obtained from the SH and SH+NP groups. In comparison, immunohistochemical analysis of renal sections obtained from the SH and SH+NP rats subjected to renal I/R revealed strong positive staining for iNOS (Fig. 1D). In addition, staining in the kidney samples of the NP+I/R group was strongly positive for 3-NT (Fig. 2D), whereas specimens from the SH and SH+NP groups were weakly positive. In contrast, the kidney specimens pretreated with L-NIL (Fig. 1E and Fig. 2E) or rutin (Fig. 1F and Fig. 2F) before I/R were weakly positive for iNOS and 3-NT, whereas specimens from the NP+IR group were strongly positive.
Discussion
The acute renal failure induced by I/R is an important syndrome characterized by decreased renal blood flow and glomerular filtration rate, extensive tubular damage, and vasoconstriction and glomerular injury.38 We have previously shown that uninephrectomy did not alter the renal parameters (serum creatinine, blood urea nitrogen).39,40 Furthermore, we showed that the renal I/R treatment (45 min of ischemia followed by 3 h of reperfusion) impaired glomerular function by increasing serum blood urea nitrogen and creatinine levels in rats.39,40 Therefore, the experimental model of renal I/R that we used in the present study is suitable to demonstrate the potential iNOS inhibiting role of rutin in I/R injury.
The pathophysiology of renal injury after I/R is very complex, as numerous potential mediators of injury have been demonstrated.2,39,41–43 Reactive oxygen and nitrogen species are believed to play a central role in I/R injury in addition to several other factors, such as neutrophils, platelets, the coagulation system and the xanthine–oxido–reductase enzyme system. Our previous report demonstrated that renal I/R worsened the renal dysfunction, oxidative stress and histopathologic features in rats.39 We determined oxidative injury in the kidney during I/R by measuring malondialdehyde concentration, reduced glutathine concentration and manganese superoxide dismutase activity.39 In addition, it was observed that reactive oxygen and nitrogen species, such as NO, play an important role in the pathogenesis of I/R.24,44
Basal production of NO is necessary for maintaining adequate glomerular function, but after I/R the NO level is increased due to the activity of iNOS.45 Noiri and colleagues46 have demonstrated the deleterious effect of NO derived from iNOS in ischemic acute renal failure. We have demonstrated that the expression of iNOS protein is increased after I/R. The results of immunohistochemical staining of iNOS suggest that the increased level of NO produced as a result may be a causative factor in the process of renal injury. Further supporting this notion is our demonstration of increased plasma levels of nitrite/nitrate and cGMP after reperfusion in ischemic compared with nonischemic rats. Therefore, our data strongly support the hypothesis that excessive NO produced in response to an increase in iNOS after I/R in the kidney is deleterious to renal function. In addition, the present results show that L-NIL pretreatment was effective in decreasing nitrite/nitrate and cGMP levels in the plasma and in decreasing the intensity and incidence of immunohistochemical staining of iNOS in the kidneys of rats following I/R exposure. Similarly, Chatterjee and colleagues19 found that administration of selective inhibitors of iNOS significantly reduced plasma nitrite/nitrate levels, renal dysfunction and injury caused by renal IR.
Weight and colleagues42 reported that early in reperfusion, NO and superoxide quench to form peroxynitrite, which is more toxic than its components. Peroxynitrite is a potent oxidant that can react with cellular lipids, proteins and DNA to produce several end products, including 3-NT.47 Based on this information, we hypothesized that induction of iNOS and subsequent NO production would result in protein damage in the renal cells of ischemic rats. We also focused on 3-NT, a main end product of protein nitration, as an indicator of cell damage. Our findings strongly support the hypothesis that iNOS activity increases NO production and peroxynitrite formation, which occurs during I/R. The increased production of peroxynitrite, as measured by the increased expression of 3-NT, was also very evident in our model, predominantly in the renal tubules after 3 hours of reperfusion. Our results of immunohistochemical staining showed that renal I/R caused an increase in generation of 3-NT, indicating increased nitration of proteins. Also, the presence of 3-NT residues implies a high level of activity of the expressed iNOS. Furthermore, weakly positive stains for 3-NT were detected in the kidney specimens of rats pretreated with L-NIL owing to iNOS inhibition. Similarly, other studies have shown that 3-NT-protein adducts were detected in renal tubules after I/R injury.18,22
There are various endogenous mechanisms, such as superoxide dismutase, catalase, glutathione peroxidase and glutathine, that protect against kidney injury induced by reactive oxygen and nitrogen species.2,48 The imbalance between reactive species production and endogenous anti-oxidant activity may lead to renal injury during I/R. In this circumstance, exogenous antioxidants may act as scavengers for reactive oxygen and nitrogen species, inhibiting their accumulation and enhancing endogenous antioxidant defenses. Previous experiments have shown that various exogenous antioxidant agents have the potential to reduce I/R-induced oxidative damage in the kidney. Di Mari and colleagues49 observed that N-acetylcysteine inhibited reactive oxygen species generation and decreased lipid peroxidation in an ischemic kidney. Our previous study suggested that ascorbic acid plays a role in decreasing I/R-induced reactive oxygen species generation in the renal tissue of rats.40 Inal and colleagues50 and Shoskes51 have shown that administration of quercetin has an antioxidative effect against renal I/R-induced oxidative damage in the kidney. Similarly, naringin (another bioflavonoid) has been demonstrated to play an important role in preventing renal I/R injury.52
Flavonoids are plant phytochemicals that cannot be synthesized by humans.27 The health benefit of flavonoids attributed to polyphenols is usually linked to 2 properties: inhibition of certain enzymes, such as xanthine oxidase, and antioxidant activity.30 Our previous findings showed that pretreatment with rutin significantly inhibited lipid peroxidation and protected against the severe depletion of glutathione content and manganese superoxide dismutase activity in the kidneys of I/R rats pretreated with rutin.37 Although it has been shown that rutin has radical scavenging activity, which prevents the pathogenesis of I/R, to our knowledge rutin has not previously been examined for potential iNOS inhibition in an experimental model of I/R. This flavonoid is an important antilipoperoxidant agent and a strong scavenger of hydroxyl and superoxide radicals.53 It has also been shown that rutin inhibits xanthine oxidase enxymes, which play a role in I/R pathogenesis.54 Neumayer and colleagues55 reported that a flavonoid tablet containing rutin was able to prevent I/R injury in rabbit skeletal muscle.
In the present study, we observed that pretreatment with rutin significantly reduced plasma nitrite/nitrate and cGMP levels in the kidneys of I/R rats. Moreover, the positive staining for iNOS and 3-NT in their kidney specimens was considerably reduced by pretreatment with rutin. Our results demonstrate that, besides its reactive oxygen species scavenging properties, rutin is a powerful iNOS inhibitor, like L-NIL, against renal I/R injury. Although our results do not address all the potential mechanisms for attenuation of renal I/R injury, the results of nitrite/nitrate and cGMP levels and the immunohistochemical staining scores for iNOS and 3-NT suggest that rutin plays a role in I/R-induced NO formation and assists in the recovery from renal injury after I/R.
Conclusion
Our results demonstrate that high activity of iNOS, which leads to excessive NO generation, occurs during renal I/R and ultimately causes renal injury. Most importantly, the results show that rutin, with its potent iNOS inhibiting activity, may protect against nitrosative kidney damage and may be a highly promising agent in preventing renal dysfunction due to I/R. Given the clinical relevance of renal I/R, these data suggest that if and when rutin becomes available for clinical use, it may be of therapeutic value in patients undergoing vascular surgery or renal transplantation.
Acknowledgements
This work was supported by grants from the Hacettepe University Research Foundation. We thank Müfide (Aydogan) Ahbab and Cansin Güngörmüs for their support in immunostaining. We also thank to Karen McDonough for her critical reading of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: Both authors designed the study and approved its publication. A. Korkmaz acquired and analyzed the data and wrote the article. D. Kolankaya reviewed the article.
References
- 1.Eldaif SM, Deneve JA, Wang N. Attenuation of renal ischemia–reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl Int. doi: 10.1111/j.1432-2277.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 2.McMichael M, Moore RM. Ischemia-reperfusion injury pathophysiology. Part I. J Vet Emerg Crit Care. 2004;14:231–41. [Google Scholar]
- 3.Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- 4.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–64. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waz WR, Van Liew JB, Feld LG. Nitric oxide methabolism following unilateral renal ischemia/reperfusion injury in rats. Pediatr Nephrol. 1998;12:26–9. doi: 10.1007/s004670050397. [DOI] [PubMed] [Google Scholar]
- 6.Honda N, Hishida A. Pathophysiology of experimental nonoliguric acute renal failure. Kidney Int. 1993;43:513–21. doi: 10.1038/ki.1993.78. [DOI] [PubMed] [Google Scholar]
- 7.Bonventre JV. Mediators of ischemic renal injury. Annu Rev Med. 1988;39:531–44. doi: 10.1146/annurev.me.39.020188.002531. [DOI] [PubMed] [Google Scholar]
- 8.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 9.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–8. [PubMed] [Google Scholar]
- 10.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–5. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 11.De Vente J, Hopkins DA, Markerin-Van Ittersum M, et al. Distribution of nitric oxide synthase and nitric oxide-receptive, cyclic GMP-producing structures in the rat brain. Neuroscience. 1998;87:207–41. doi: 10.1016/s0306-4522(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 12.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–64. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhoden EL, Rhoden CR, Mauri M, et al. Experimental model of renal ischemia-reperfusion in rats: study of the stress oxidative induced by oxygen-derived free radicals. Braz J Urol. 1999;25:431–6. [Google Scholar]
- 14.Roczniak A, Levine DZ, Burns KD. Localization of protein inhibitor of neuronal nitric oxide synthase in rat kidney. Am J Physiol Renal Physiol. 2000;278:F702–7. doi: 10.1152/ajprenal.2000.278.5.F702. [DOI] [PubMed] [Google Scholar]
- 15.Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis. 1997;30:311–33. doi: 10.1016/s0272-6386(97)90275-4. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension. 2000;35:337–41. doi: 10.1161/01.hyp.35.1.337. [DOI] [PubMed] [Google Scholar]
- 17.Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stres and progression of chronic renal failure. Semin Nephrol. 2004;24:354–65. doi: 10.1016/j.semnephrol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Walker LM, Walker PD, Imam SZ, et al. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N6-(-1-iminoethyl)lysine. J Pharmacol Exp Ther. 2000;295:417–22. [PubMed] [Google Scholar]
- 19.Chatterjee PK, Patel NSA, Sivarajah A. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853–65. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 20.Joles JA, Vos IH, Grone HJ, et al. Inducible nitric oxide synthase in renal transplantation. Kidney Int. 2002;61:872–5. doi: 10.1046/j.1523-1755.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee PK, Patel NSA, Kvale EO, et al. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–71. doi: 10.1046/j.1523-1755.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 22.Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005;129:236–41. doi: 10.1016/j.jss.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Liaudet L, Soriano FG, Szabó C. Biology of nitric oxide signaling. Crit Care Med. 2000;28(4 Suppl):N37–52. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 24.Goligorsky MS, Brodsky SV, Noiri E. NO bioavailability, endothelial dysfunction and acute renal failure: new insights into pathophysioloy. Semin Nephrol. 2004;24:316–23. doi: 10.1016/j.semnephrol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Lieberthal W. Biology of ischemic and toxic renal tubular injury: role of nitric oxide and the inflammatory response. Curr Opin Nephrol Hypertens. 1998;7:289–95. doi: 10.1097/00041552-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Paller MS. Nitric-oxide-mediated renal epithelial cell injury during hypoxia and reoxygenation. Ren Fail. 1998;20:459–69. doi: 10.3109/08860229809045135. [DOI] [PubMed] [Google Scholar]
- 27.Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res. 1998;18:1995–2018. [Google Scholar]
- 28.Manach C, Morand C, Demigné C, et al. Bioavalibility of rutin and quercetin in rats. FEBS Lett. 1997;409:12–6. doi: 10.1016/s0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 29.Middleton E. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998;439:175–82. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- 30.Cotelle N. Role of flavonoids in oxidative stress. Curr Top Med Chem. 2001;1:569–90. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 31.Nègre-Slvayre A, Affany A, Hariton CR. Additional antilipoperoxidant activities of alpha-tocopherol and ascorbic acid on membrane-like systems are potentiated by rutin. Pharmacology. 1991;42:262–72. doi: 10.1159/000138807. [DOI] [PubMed] [Google Scholar]
- 32.Bombardelli E, Morazzoni P. The flavonoids: new perspectives in biological activities and therapeutics. Chim Oggi. 1993;11:25–8. [Google Scholar]
- 33.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–41. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Milde J, Elstner EF, Graßmann J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid. Phytomedicine. 2004;11:105–13. doi: 10.1078/0944-7113-00380. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council. Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- 36.Waynforth HB, Flecknell PA. Experimental and surgical technique in the rat. 2nd ed. New York: Academic Press; 1992. [Google Scholar]
- 37.Girault I, Karu AE, Schaper M, et al. Immunodetection of 3-nitrotyrosine in the liver of zymosan-treated rats with a new monoclonal antibody: comparison to analysis by HPLC. Free Radic Biol Med. 2001;31:1375–87. doi: 10.1016/s0891-5849(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 38.McCord JM. Oxygen derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 39.Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309–15. doi: 10.1016/j.jss.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Korkmaz A, Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren Fail. 2009;31:36–43. doi: 10.1080/08860220802546271. [DOI] [PubMed] [Google Scholar]
- 41.Bonventure JV, Kelly KJ. Ischemia/reperfusion injury in transplantation. In: Tilney NL, Strom TB, Paul LC, editors. Transplantation biology: cellular and molecular aspects. Philadelphia (PA): Lippincott-Raven Publishers; 1996. p. 257. [Google Scholar]
- 42.Weight SC, Furness PN, Nicholson ML. Nitric oxide generation is increased in experimental warm ischemia-reperfusion injury. Br J Surg. 1998;85:1663–8. doi: 10.1046/j.1365-2168.1998.00960.x. [DOI] [PubMed] [Google Scholar]
- 43.Weight SC, Furness PN, Nicholson ML. Biphasic role for nitric oxide in experimental renal warm ischemia-reperfusion injury. Br J Surg. 1999;86:1039–46. doi: 10.1046/j.1365-2168.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 44.Viñas JL, Sola A, Genescà M, et al. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006;40:992–1003. doi: 10.1016/j.freeradbiomed.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Looney CG, Qi WN. Reperfusion injury is reduced in skeletal muscle by inhibition of inducible nitric oxide synthase. J Appl Physiol. 2003;94:1473–8. doi: 10.1152/japplphysiol.00789.2002. [DOI] [PubMed] [Google Scholar]
- 46.Noiri E, Peresleni T, Miller F, et al. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996;97:2377–83. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268(5 Pt 1):L699–722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 48.Halliwell B, Aeschbach R, Loliger L, et al. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–17. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 49.DiMari J, Megyesi J, Udvarhelyi N, et al. N-acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272(3 Pt 2):F292–7. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- 50.Inal M, Altinisik M, Bilgin D. The effect of quercetin on renal ischemia and reperfusion injury in the rat. Cell Biochem Funct. 2002;20:291–6. doi: 10.1002/cbf.953. [DOI] [PubMed] [Google Scholar]
- 51.Shoskes DA. Effect of bioflavonoid quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation. 1998;66:147–52. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- 52.Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia-reperfusion induced renal injury in rats. Pharmacol Res. 2004;50:187–93. doi: 10.1016/j.phrs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Nègre-Salvayre A, Mabile L, Delchambre J, et al. alpha-Tocopherol, ascorbic acid, and rutin inhibit synergistically the copper promoted LDL oxidation and the cytotoxicity of oxidized LDL to cultured endothelial cell. Biol Trace Elem Res. 1995;47:81–91. doi: 10.1007/BF02790104. [DOI] [PubMed] [Google Scholar]
- 54.Cos P, Calome M, Hu JP, et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–6. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 55.Neumayer C, Fügl A, Nanobashvili J, et al. Combined enzymatic and antioxidative treatment reduces ischemia-reperfusion injury in rabbit skeletal muscle. J Surg Res. 2006;133:150–8. doi: 10.1016/j.jss.2005.12.005. [DOI] [PubMed] [Google Scholar]