Abstract
Aims
To determine the rate of diffusion of myoglobin and bovine serum albumin (BSA) through the human cornea. These small proteins have hydrodynamic diameters of approximately 4.4 nm and 7.2 nm, and molecular weights of 16.7 kDa and 66 kDa, for myoglobin and BSA, respectively.
Methods
Diffusion coefficients were measured using a diffusion chamber where the protein of interest and balanced salt solution were in different chambers separated by an ex vivo human cornea. Protein concentrations in the balanced salt solution chamber were measured over time. Diffusion coefficients were calculated using equations derived from Fick’s law and conservation of mass in a closed system.
Results
Our experiments demonstrate that the diffusion coefficient of myoglobin is 5.5±0.9×10−8 cm2/s (n=8; SD = 1.3×10−8 cm2/s; 95%CI: 4.6×10−8 to 6.4×10−8 cm2/s) and the diffusion coefficient of BSA is 3.1±1.0×10−8 cm2/s (n=8; SD = 1.4×10−8 cm2/s; 95%CI: 2.1×10−8 to 4.1×10−8 cm2/s).
Conclusions
Our study suggests that molecules as large as 7.2 nm may be able to passively diffuse through the human cornea. With applications in pharmacotherapy and the development of an artificial cornea, further experiments are warranted to fully understand the limits of human corneal diffusion and its clinical relevance.
Keywords: Cornea, diffusion, proteins, human cornea, myoglobin, BSA
INTRODUCTION
A potential complication of keratoprosthesis is infection and tissue melt or necrosis at the recipient tissue and artificial cornea junction.[1] Epithelialization of the keratoprosthesis may reduce the incidence of such a complication. Corneal epithelium and keratocytes receive their nutrients from the aqueous chamber via diffusion through the corneal stroma, thus, in order to develop an artificial cornea that best mimics the human cornea, it is important to understand the diffusional properties of the human cornea. The diffusional properties of the cornea also have clinical implications for drug delivery of topical medications into the eye.[2]
Maurice et al. has previously shown that fluorescein-labeled IgG injected into the rabbit corneal stroma is able to diffuse away from the injection site.[3] Pharmaceutical studies have demonstrated small molecule drug delivery to the aqueous chamber following topical administration, and other studies have determined the permeability of corneal stroma to small molecules such as fluorescein, propanolol, sucrose, and inulin.[4–6] In 2006, Myung et al. reported the diffusion coefficient of glucose (diameter less than 1.0 nm) through the human cornea to be 3.0 ± 0.2×10−6 cm2/s.[7]
Most recently, topical treatment with antibodies as large as 26 kDa have been demonstrated to penetrate through the cornea and provide treatment efficacy.[8] To the best of our knowledge, the diffusion of larger proteins has not yet been demonstrated and measured.
The goal of our study is to determine the rate of diffusion of myoglobin and bovine serum albumin (BSA) through the ex vivo human cornea. These small proteins were chosen based on their hydrodynamic diameters, of approximately 4.4 nm and 7.2 nm and molecular weights of 16.7 kDa and 66 kDa, respectively.
MATERIALS AND METHODS
Diffusion Chamber Experimentation
Diffusion experiments were set up in Neuro Probe Blind Well Chemotaxis Chambers Model BW200S (diffusion chambers) obtained from NeuroProbe, Gaithersburg, MD. These chambers have a 200 μl top and bottom chamber (Figure 1A). Human research corneas used for experiments were obtained from North Carolina Eye Bank within one-week post-mortem and stored in Optisol media. A trephine was used to excise a 6 mm diameter circle from the central cornea for experimentation.
Figure 1.
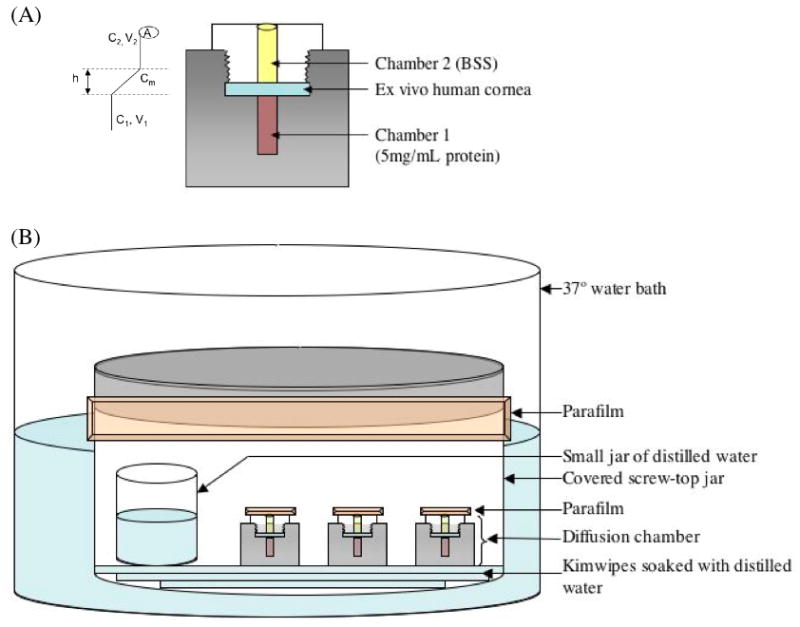
(A) Initial setup of diffusion chamber apparatus. Protein concentration in chamber 2 (C2), which is initially filled with balanced salt solution (BSS), is measured over course of experiment. Variables used for calculation of diffusion coefficient include C1 (protein concentration in bottom chamber), C2 (protein concentration in top chamber), V1 (volume of bottom chamber), V2 (volume in top chamber), h (thickness of barrier to diffusion), and A (surface area of cornea through which diffusion is possible) as illustrated on the left. (B) Humidity chamber set-up as described in text.
As shown in Figure 1A, in the first set of experiments, the test cornea was placed between the top and bottom chambers and served as the barrier to diffusion of nutrients. This is the “cornea experimental study.” The bottom chamber was filled with 200 μl of myoglobin (Sigma-Aldrich, St. Louis, MO) (5 mg/ml) or BSA (Sigma-Aldrich, St. Louis, MO) (5 mg/ml) and the top chamber with 180 μl of balanced salt solution (BSS). In another set of experiments, human corneas were allowed to swell in BSS for 24 hours prior to serving as the barrier to the diffusion studies. This is labeled the “pre-swollen cornea study.”
Both positive and negative control studies were conducted in parallel. The positive control had an ultrafiltration membrane with a 100 kDa cut-off (Spectra Laboratories, Milipitas, CA) as the barrier to diffusion. The negative control experiment used an impermeable polyethylene membrane cut out of a Ziploc bag (S.C. Johnson & Son, Racine, Wisconsin) as the barrier to diffusion. In both of these control studies, the bottom chamber was filled with 200 μl of myoglobin (5 mg/ml) or BSA (5 mg/ml) and the top chamber with 180 μl of BSS, similar to the cornea experiment.
The cornea itself may release protein over time. Therefore, a control was performed where the human cornea was placed between the top and bottom chambers filled with 200 μl and 180 μl of BSS, respectively, without any myoglobin or BSA. This is labeled the “cornea negative control.” All of these control groups are summarized in Table 1.
Table 1.
Experimental setup of all diffusion chambers throughout experimentation where number of experiments performed at each condition is indicated in the left column.
| Condition (number) | Barrier | Bottom Chamber | Top Chamber |
|---|---|---|---|
| Negative control (2) | 6–8 kDa membrane | 200 μl of 5 mg/ml BSA | 180 μl BSS |
| Positive control (2) | 100 kDa membrane | 200 μl of 5 mg/ml myoglobin | 180 μl BSS |
| Corneal negative control (5) | Human cornea | 200 μl of BSS | 180 μl BSS |
| Corneal experimental variables (8) | Human cornea | 200 μl of 5 mg/ml myoglobin | 180 μl BSS |
| Corneal experimental variables (8) | Human cornea | 200 μl of 5 mg/ml BSA | 180 μl BSS |
During experimental set-up of the diffusion chambers, care was taken to avoid any air bubbles between the barrier to diffusion and the top and bottom chamber solutions. Once the chambers were set up and tightened by hand, an additional 1/4 to 1/2 turn was made using pliers to avoid any leakage between the two solutions. Chambers were then sealed with Parafilm (Structure Probe Inc., West Chester, PA) and placed in a humidity chamber to avoid evaporation. Humidity chambers consisted of a covered screw-top jar (Nalgene, Rochester, NY) with a small jar (Nalgene) of distilled water and lined with Kimwipes (Kimberly-Clark, Neenah, WI) soaked in distilled water. Humidity chambers were then sealed with Parafilm and placed in a water bath of 37 °C (Figure 1B).
Over the course of one week, 2 μl samples were removed daily from the top chamber and protein concentrations were measured by the Qubit Protein Quant-It system (Invitrogen, Carlsbad, CA). For a subset of BSA experiments, Enzyme-Linked Immunosorbant Assay (ELISA) was performed using a Bovine Albumin (BSA) ELISA Kit (Alpha Diagnostic International, San Antonio, Texas) to confirm positive diffusion.
In addition, liquid chromatography matrix-assisted laser desorption/ionization (LC-MALDI) was performed on corneal negative control samples and corneal myoglobin experimental variables at the Stanford University Protein and Nucleic Acid Facility in order to confirm the presence of myoglobin in the experimental group and identify the proteins that were found in the corneal negative controls. An Applied Biosystems (Foster City, CA) software package was used to interpret the LC-MALDI results.
Data Analysis
Diffusion of myoglobin and BSA was measured indirectly by determining the concentration of protein in the top chamber. In order to quantify the true protein concentrations that had diffused through the cornea and that were not released from the cornea itself, the protein concentrations measured from corneal negative controls were subtracted from the corneal experimental variable protein concentrations. These remaining protein concentrations were then analyzed to determine the diffusion coefficients using basic diffusion equations derived from Fick’s law and conservation of mass in a closed system, as previously described by Lee CJ, et al. (Figure 2).[9] The equation of interest was ln(1−(C2(t)/(N/V))) = −(D/h)*(t/τ) where C2(t) = protein concentration in the top chamber at given time, N = total solute mass in the system, V = total volume of the system, D = diffusion coefficient, h = thickness of the barrier to diffusion, t = time, and τ= scaling factor specific to the individual diffusion chamber setup.
Figure 2.
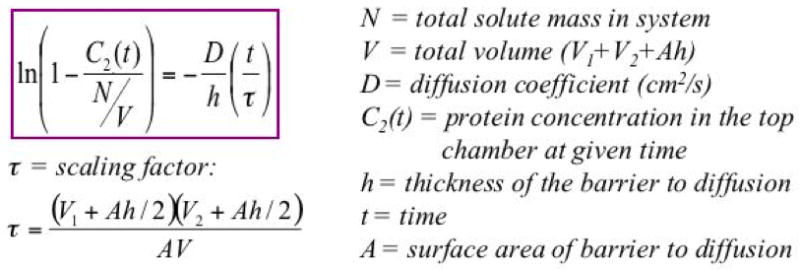
Equation used to determine diffusion coefficients: combines Fick’s law with law of mass conservation.[9]
RESULTS
Controls
In our positive control experiments (n = 2), after 4 days, we found that the initially negative BSS chamber had 2.3 mg/mL of myoglobin within it, which had diffused through the ultrafiltration membrane. In our negative control experiments (n = 2), we found that no protein (<0.1 mg/ml BSA) had diffused across the dialysis membrane over the course of one week.
Myoglobin
The diffusion coefficient for myoglobin (4.4 nm, 16.7 kDa) through the human cornea (average thickness = 570 μm) was 5.5 ± 0.9×10−8 cm2/s (SD = 1.3×10−8 cm2/s; 95%CI: 4.6×10−8 to 6.4×10−8 cm2/s) (Table 2, Figure 3). Following a 24-hour soak in BSS to pre-swell the cornea, the diffusion coefficient dropped to 2.2×10−8 cm2/s in a cornea with thickness of 1235 μm. This decrease in the diffusion coefficient was linearly correlated to the increase in corneal thickness. All linear regressions of protein concentrations over time had R2 values over 0.75.
Table 2.
Diffusion coefficients of experimental variable corneas for both the myoglobin and BSA experiments reported in 10−8 cm2/s.
| Cornea | Myoglobin | BSA |
|---|---|---|
| 1 | 5.7 | 2.4 |
| 2 | 4.7 | 2.7 |
| 3 | 4.4 | 4.4 |
| 4 | 4.0 | 2.5 |
| 5 | 4.7 | 2.1 |
| 6 | 6.1 | 6.0 |
| 7 | 6.8 | 2.7 |
| 8 | 8.0 | 1.9 |
|
Average SD |
5.5 1.3 |
3.1 1.4 |
Figure 3.
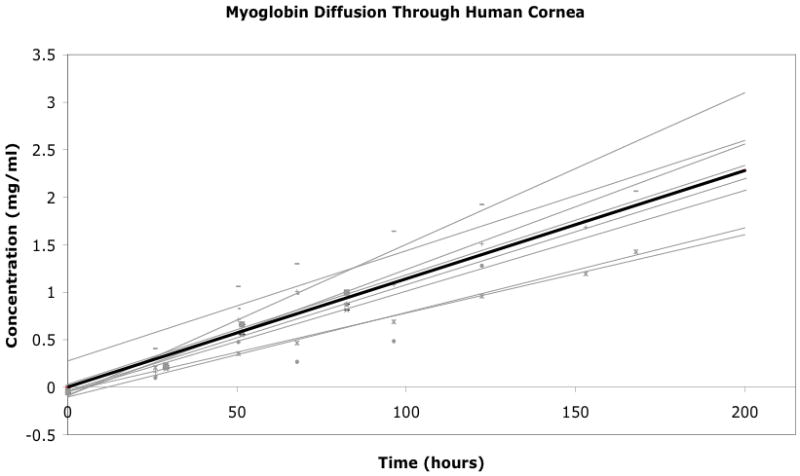
Graph of myoglobin concentration in chamber 2 over time for the eight experimental variable corneas. The black line represents the average trendline for the entire dataset.
Bovine Serum Albumin
The diffusion coefficient for BSA (7.2 nm, 66 kDa) through the human cornea (average thickness = 566 μm) was 3.1 ± 1.0×10−8 cm2/s (SD = 1.4×10−8 cm2/s; 95%CI: 2.1×10−8 to 4.1×10−8 cm2/s) (Table 2, Figure 4). All linear regressions of protein concentrations over time had R2 values over 0.75. BSA diffusion through the cornea was confirmed via ELISA with anti-BSA antibodies. ELISA results confirmed that there was no diffusion of BSA through the corneal negative controls and that there was positive diffusion of BSA through the human cornea in experimental variables (data not shown). The measured BSA diffusion coefficient was statistically different from the measured myoglobin diffusion coefficient with a p-value of 0.0032 (Figure 5).
Figure 4.
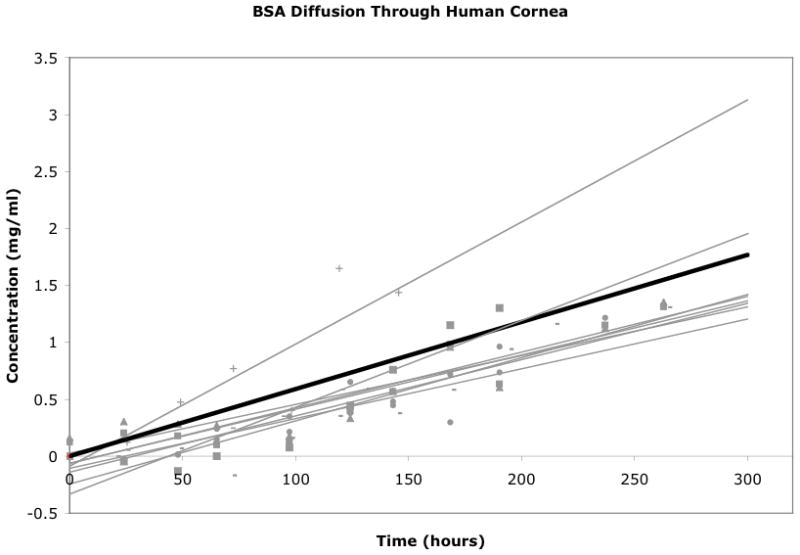
Graph of BSA concentration in chamber 2 over time for the 8 experimental variable corneas. The black line represents the average trendline for the entire dataset.
Figure 5.
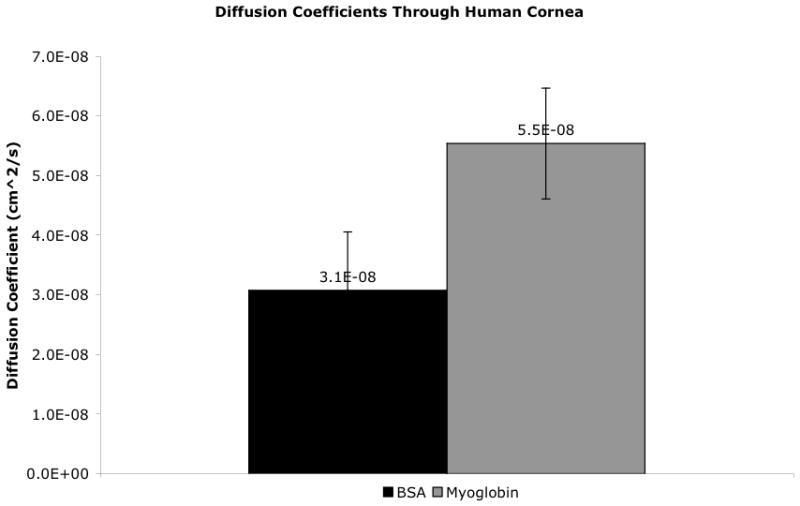
Average diffusion coefficients of myoglobin as compared to bovine serum albumin (BSA). Error bars represent 95% CI. The diffusion coefficient of myoglobin and BSA are significantly different from each other with p-value of 0.0032.
LC-MALDI
LC-MALDI of the corneal myoglobin experimental variable confirmed the presence of myoglobin. LC-MALDI of the corneal negative controls revealed over 200 proteins that were likely to have been in the top chamber. Of these, the top 70 were likely to have been in the solution given their high best ion score. These proteins include: human serum albumin precursor, TGF-β-induced protein Ig-h3 precursor, and aldehyde dehydrogenase (dimeric NADP-preferring). Table 3 lists the top ten proteins that were likely to have diffused out from the human cornea itself in the corneal negative control group.
Table 3.
LC-MALDI results displaying the top 10 proteins that were likely to be released from the human cornea in order of likelihood based on best ion score.
| Protein | Best Ion Score |
|---|---|
| TGF-beta-induced protein Ig-H3 precursor | 154 |
| Serum albumin precursor | 147 |
| IgG-1 chain C region | 133 |
| Lumican precursor | 125 |
| Aldehyde dehydrogenase | 123 |
| Alpha-enolase | 120 |
| Keratin, type II cytoskeletal 5 | 119 |
| Transketolase | 114 |
| Complement factor H precursor | 100 |
| Collagen alpha-3(VI) chain precursor | 87 |
DISCUSSION
In our study evaluating the ability of proteins to diffuse through the human cornea, we used several controls to verify the validity of our experimental setup. Our positive permeable membrane control verified that our setup did not contain any air bubbles or other barriers to diffusion aside from our intended membrane, while our negative impermeable membrane control verified that the setup did not allow for leakage of our protein of interest around the intended barrier. Furthermore, most of the proteins we identified in our corneal negative control experiments via LC-MALDI have been previously associated with the cornea using similar techniques, suggesting that the identified proteins were released from the cornea itself.[10] Taken together, these results imply that our corneal experimental results represent true diffusion of the intended protein through the human cornea.
Our findings suggest that proteins up to 66 kDa in mass and hydrodynamic diameters of 7.2 nm can diffuse through an ex vivo human cornea. The diffusion coefficient of myoglobin and BSA in our experiments was 5.5 ± 0.9×10−8 cm2/s and 3.1 ± 1.0×10−8 cm2/s, respectively. The rate of diffusion for these two proteins is about 100-fold slower than the diffusion rate of glucose (3.0 ± 0.2×10−6 cm2/s), which has a diameter of less than 1.0 nm.
Despite the integrity of our experimental setup, our results are limited by the uncertainty of corneal integrity throughout the experiment. Pristine preservation of the epithelial and endothelial layers is challenging to control given many factors that are both intrinsic and extrinsic to the experiment. External limiting factors include the status of the donor, time from donor expiration to corneal preservation, and the length of time in storage prior to experimentation. These factors were minimized by the exclusion of any donor tissue that had visible damage to the epithelial layer or had been in storage for over a week. During the experiment, the cornea was removed from its Optisol media and stored in BSS, leading to epithelial and endothelial cell death over time despite care to avoid mechanical damage to these layers during trephination and experimental setup. Given the numerous and difficult to control factors, it is likely that both the epithelial and endothelial layers, which contain active transport mechanisms, may have been damaged. As a result, our measurements are more reflective of corneal stromal diffusion, rather than diffusion through the entire cornea. Future in vivo experiments are warranted to evaluate the integrity of the cornea epithelium and compare our ex vivo diffusion rates to the true diffusion rate of proteins in vivo.
Though further testing is necessary to determine whether our ex vivo experiments have clinical significance, the recent report of topical anti-TNF-α single-chain antibody fragment administration reaching therapeutic levels in the anterior and posterior segments of the eye is supportive of our findings.[8] In their initial experiments, penetration of the anti-TNF-α antibody fragment through rabbit corneas was demonstrated in ex vivo experiments. These ex vivo experiments, were later validated in vivo within rabbits. Although we used a different diffusion chamber and species of corneal origin, in principle, our experimental setups are very similar given that in both experiments, diffusion of the protein of interest was measured between two chambers containing a BSS-based solution, separated by a cornea. Thus, validation of their ex vivo experiments with in vivo experiments, suggests that our experiments may be similarly valid in vivo. Furthermore, the anti-TNF-α antibody fragment used in previously reported experiments, has a molecular weight of 26kDa, which is within the molecular weight range of myoglobin and BSA and again, consistent with our findings.
We hope that our results elucidating the human cornea’s natural diffusion coefficients will prove useful for many corneal applications including pharmacotherapy and an artificial cornea. Our results imply that topical antibodies and other small proteins applied directly to the human corneal surface may be able to penetrate through the cornea into the aqueous chamber. Additionally, our results suggest that it may be necessary for an artificial cornea to have pore sizes greater than 7.2 nm in order to allow diffusion of proteins up to 66 kDa in molecular weight. Though it is unclear whether such large pores are clinically necessary for corneal epithelial nourishment or whether the corneal epithelium is able to synthesize its own protein nutrients from acquired amino acids, protein diffusion may be necessary to create the most biomimetic artificial cornea. Thus, although our study is limited by its ex vivo nature, it offers for the first time an estimate of human corneal diffusion coefficients, which may be utilized to more accurately model the cornea for various applications.
Acknowledgments
This research was partially funded by the National Institutes of Health, Bethesda, Maryland by NIH Grant 1 R01 EY016987.
References
- 1.Chew HF, Ayres BD, Hammersmith BM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28(9):989–96. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 2.Kleinmann G, Larson S, Neuhann IM, et al. Intraocular concentrations of gatifloxacin and moxifloxacin in the anterior chamber via diffusion through the cornea using collagen shields. Cornea. 2006;25(2):209–213. doi: 10.1097/01.ico.0000170689.75207.5e. [DOI] [PubMed] [Google Scholar]
- 3.Allansmith M, de Ramus A, Maurice DM. The dynamics of IgG in the cornea. Invest Ophthalmol Vis Sci. 1979;18:947–955. [PubMed] [Google Scholar]
- 4.Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J Pharm Sci. 1987;76:583–586. doi: 10.1002/jps.2600760802. [DOI] [PubMed] [Google Scholar]
- 5.Araie M, Maurice D. The rate of diffusion of fluorophores through the corneal epithelium and stroma. Exp Eye Res. 1987;44:73–87. doi: 10.1016/s0014-4835(87)80027-1. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JR, Grass GM. Mechanisms of corneal drug penetration I: in vivo and in vitro kinetics. J Pharm Sci. 1988;77:3–14. doi: 10.1002/jps.2600770103. [DOI] [PubMed] [Google Scholar]
- 7.Myung D, Derr K, Huie P, Noolandi J, Ta KP, Ta CN. Glucose permeability of human, bovine, and porcine corneas in vitro. Ophthalmic Res. 2006;38:158–163. doi: 10.1159/000090726. [DOI] [PubMed] [Google Scholar]
- 8.Ottinger M, Thiel MA, Feige U, Lichtlen P, Urech DM. Efficient intraocular penatration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest Opthalmol Vis Sci. 2009;50:779–786. doi: 10.1167/iovs.08-2372. [DOI] [PubMed] [Google Scholar]
- 9.Lee CJ, Vroom JA, Fishman HA, Bent SF. Determination of human lens capsule permeability and its feasibility as a replacement for Bruch’s membrane. Biomaterials. 2006;27:1670–1678. doi: 10.1016/j.biomaterials.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Karring H, Thøgersen IB, Klintworth GK, Møller-Pedersen T, Enghild JJ. A dataset of human cornea proteins identified by peptide mass fingerprinting and tandem mass spectrometry. Mol Cell Proteomics. 2005;4:1406–1408. doi: 10.1074/mcp.D500003-MCP200. [DOI] [PubMed] [Google Scholar]


