Abstract
Traumatic brain injury (TBI) is a leading cause of death in the elderly and the incidence of mortality and morbidity increases with age. This study tested the hypothesis that, after TBI followed by hemorrhagic hypotension (HH) and resuscitation, cerebral blood flow (CBF) would decrease more in aged compared with young rats. Young adult (4–6 months) and aged (20–24 months) male Sprague-Dawley rats were anesthetized with isoflurane, prepared for parasagittal fluid percussion injury (FPI) and randomly assigned to receive either moderate FPI (2.0 atm) only, moderate FPI + severe HH (40 mm Hg for 45 minutes) followed by return of shed blood, or sham FPI. Intracranial pressure (ICP), CBF, and mean arterial pressure (MAP) were measured and, after twenty-four hours survival, the rats were euthanized and their brains were sectioned and stained with Fluoro-Jade (FJ), a dye that stains injured neurons. After moderate FPI, severe HH and reinfusion of shed blood, MAP and CBF were significantly reduced in the aged group, compared to the young group. Both FPI and FPI + HH groups significantly increased the numbers of FJ-positive neurons in hippocampal cell layers CA1, CA2 and CA3 (p < 0.05 vs Sham) in young and aged rats. Despite differences in post-resuscitation MAP and CBF, there were no differences in the numbers of FJ-positive neurons in aged compared to young rats after FPI, HH and blood resuscitation. Although cerebral hypoperfusion in the aged rats was not associated with increased hippocampal cell injury, the trauma-induced reductions in CBF and post resuscitation blood pressure may have resulted in damage to brain regions that were not examined or neurological or behavioral impairments that were not assessed in this study. Therefore, the maintenance of normal blood pressure and cerebral perfusion would be advisable in the treatment of elderly patients after TBI.
Keywords: age, cerebral blood flow, hemorrhage, neuron cell death, traumatic brain injury, fluid percussion injury
1.0 Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality of Americans both in the 15–25 age range and in the elderly population (Thurman et al., 1999). Experimental TBI also was associated with age-related increases in mortality (Hamm et al., 1991). Post-traumatic cerebral hypoperfusion appears to correlate with the severity of clinical TBI. Although in most patients, cerebral blood flow (CBF) is adequate for the reduced metabolic demands after TBI, there are patients in whom CBF is significantly reduced. Other groups have reported CBF values of less than 25 ml/min/100g in patients in the first several hours after severe TBI (Bouma et al., 1991) and a subpopulation of very severely injured patients exhibited CBF levels of 15 ± 9 ml/min/100g (Bouma et al., 1992). Martin et al. observed cerebral hypoperfusion within the first 24 hrs, followed by hyperemia during days 1–3 and then vasospasm accompanied by hypoperfusion days 4–15 after severe head injury (Martin et al., 1997). Imaging studies using PET and MRI in TBI patients revealed significant increases in the numbers of ischemic brain regions in TBI patients (Coles et al., 2004). These results suggest that cerebral hypoperfusion may contribute to focal cerebral ischemia in at least some severely injured patients, a hypothesis supported by histopathologic evidence of ischemic neuronal injury in patients dying from severe TBI (Graham and Adams, 1971; Graham et al., 1989). Posttraumatic cerebral hypoperfusion correlated with worsened outcome in severe TBI patients (Hlatky et al., 2004), suggesting that posttraumatic hypoperfusion contributes to their impaired cognitive function. In addition, posttraumatic cerebral hypoperfusion may be exacerbated by reductions in systemic arterial pressure. Even mild arterial hypotension (systolic blood pressure 10–29 mm Hg below normal) was associated with significantly increased mortality after TBI (Chesnut et al., 1993; Miller, 1985).
Although CBF has been measured following experimental TBI (DeWitt et al., 1986; Lewelt et al., 1980) and hemorrhagic hypotension (HH) (Armstead, 2002; Matsushita et al., 2001; Prough et al., 2006), there are no comparable studies in aged rats. To address this gap, we measured CBF, intracranial pressure (ICP), blood gases and plasma glucose and examined neuronal injury in young adult and aged rats following fluid percussion injury (FPI) with or without HH and reinfusion of shed blood. This study was designed to test the hypothesis that, after FPI followed by HH and resuscitation, CBF would be significantly lower in aged compared with young rats.
2.0 Results
2.1 Mean Arterial Pressure Following Fluid Percussion Injury and Hemorrhage
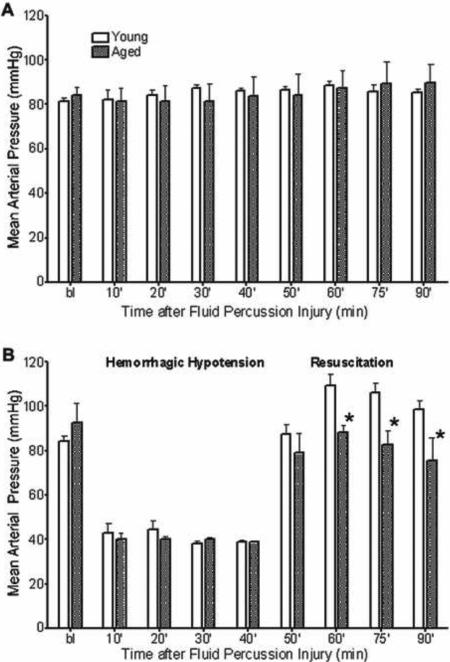
Mean arterial pressure at baseline did not differ among treatment groups. There were no age-related differences in MAP between rats subjected to either sham injury or moderate FPI only (Figure 2A) for the duration of 90 minutes post-FPI. However, after moderate FPI + severe HH and resuscitation phase, MAP was significantly lower in aged (p < 0.05) than in young rats (Figure 2B).
Figure 2. Mean Arterial Pressure in Young and Aged Rats Subjected to Moderate FPI and Severe Hemorrhage.
Mean arterial pressure (MAP) in young and aged rats after moderate FPI for 90 minutes post-FPI (A) and moderate FPI + severe HH for 45 minute post-FPI followed by a 45-minute resuscitation period (B). Data are expressed as mean ± SEM and significance testing (* = p< 0.05, compared to young) was performed using a t-test for the individual time points during the resuscitation phase.
2.2 Blood Gases, Intracranial Pressure and Temperature
Blood gases (PaCO2 shown in Table 1, pH and PaO2, which always exceeded 100 mm Hg, not shown) were not significantly different in any group at any of the time points tested. There were no significant differences in ICP between aged and young rats after sham injury, moderate FPI only or FPI + severe hemorrhage and resuscitation. Both rectal and temporalis temperatures remained within normal ranges and there were no statistically significant differences between any of the treatment groups at any of the time points tested.
Table 1.
Physiological measurements
| PaCO2 | |||
| Baseline | 60 min Post FPI | 90 min Post FPI | |
| Young Sham | 36.5 ± 1.8 | 36.1 ± 4.8 | 35.3 ± 4.2 |
| Aged Sham | 36.4 ± 2.3 | 37.3 ± 2.5 | 39.3 ± 4.4 |
| Young FPI | 36.6 ± 1.3 | 33.8 ± 6.3 | 31.2 ± 4.3 |
| Aged FPI | 36.9 ± 3.1 | 39.0 ± 8.7 | 37.0 ± 2.5 |
| Young FPI + Hem | 33.8 ± 3.3 | 37.1 ± 5.2 | 30.5 ± 3.6 |
| Aged FPI + Hem | 37.4 ± 3.2 | 42.4 ± 6.4 | 32.8 ± 5.1 |
| ICP | |||
| Baseline | 60 min Post FPI | 90 min Post FPI | |
| Young Sham | 7.6 ± 3.11 | 5.2 ± 5.11 | 6.1 ± 4.71 |
| Aged Sham | 11.3 ± 4.72 | 8.7 ± 2.28 | 9.0 ± 2.60 |
| Young FPI | 8.7 ± 3.70 | 9.7 ± 3.87 | 9.2 ± 3.83 |
| Aged FPI | 11.5 ± 3.22 | 8.7 ± 3.49 | 8.4 ± 2.00 |
| Young FPI + Hem | 9.1 ± 2.81 | 14.8 ± 11.63 | 13.5 ± 9.37 |
| Aged FPI + Hem | 9.3 ± 2.33 | 8.6 ± 4.16 | 6.6 ± 3.56 |
| Rectal Temp | |||
| Baseline | 60 min Post FPI | 90 min Post FPI | |
| Young Sham | 37.5 ± 0.51 | 37.5 ± 0.30 | 37.6 ± 0.14 |
| Aged Sham | 37.7 ± 0.39 | 37.7 ± 0.20 | 37.8 ± 0.18 |
| Young FPI | 37.5 ± 0.28 | 37.5 ± 0.18 | 37.6 ± 0.27 |
| Aged FPI | 37.6 ± 0.25 | 37.7 ± 0.25 | 37.7 ± 0.37 |
| Young FPI + Hem | 37.5 ± 0.16 | 37.7 ± 0.26 | 37.6 ± 0.22 |
| Aged FPI + Hem | 37.6 ± 0.35 | 37.6 ± 0.29 | 37.7 ± 0.21 |
| Temporalis Temp | |||
| Baseline | 60 min Post FPI | 90 min Post FPI | |
| Young Sham | 36.6 ± 0.75 | 36.6 ± 0.78 | 37.0 ± 0.64 |
| Aged Sham | 36.8 ± 0.12 | 36.6 ± 0.72 | 36.6 ± 0.69 |
| Young FPI | 35.9 ± 1.55 | 36.3 ± 0.85 | 36.2 ± 0.73 |
| Aged FPI | 36.5 ± 0.47 | 36.4 ± 0.55 | 36.1 ± 0.41 |
| Young FPI + Hem | 36.7 ± 0.55 | 37.0 ± 0.12 | 36.9 ± 0.33 |
| Aged FPI + Hem | 36.4 ± 1.3 | 36.8 ± 0.32 | 36.1 ± 0.78 |
2.3 Effects of Fluid Percussion Injury and HH on Cerebral Blood Flow
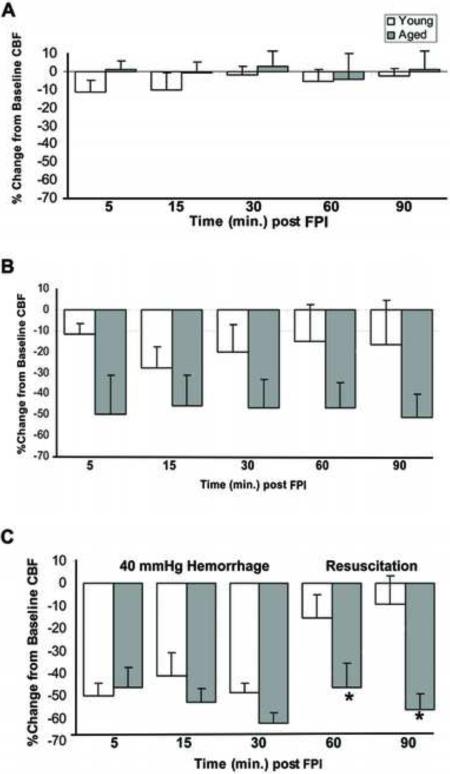
Cerebral blood flow did not change significantly from baseline in either the young or aged rats that underwent sham injury (Figure 3A). There were no statistically significant differences in CBF after FPI without HH between the aged and young rats (Figure 3B). During the severe HH period following moderate FPI, both young and aged rats experienced similar decreases in CBF (Figure 3C). In contrast, during the reinfusion phase, CBF in young rats returned nearly to pre-trauma baseline levels while CBF in the aged rats remained significant below baseline (p < 0.05 vs baseline).
Figure 3. Changes in Cerebral Blood Flow in Young and Aged Rats.
Laser Doppler cerebral blood flow (measured as % change from baseline) in young and aged rats after either sham injury (A), moderate FPI (B), or moderate FPI + severe HH (C). Data are expressed as mean ± SEM and significance testing (* = p< 0.05, compared to young) was performed using a t-test for the individual time points during the resuscitation phase.
2.4 Effects of FPI and HH on Autoregulatory Index values of Young and Aged Rats
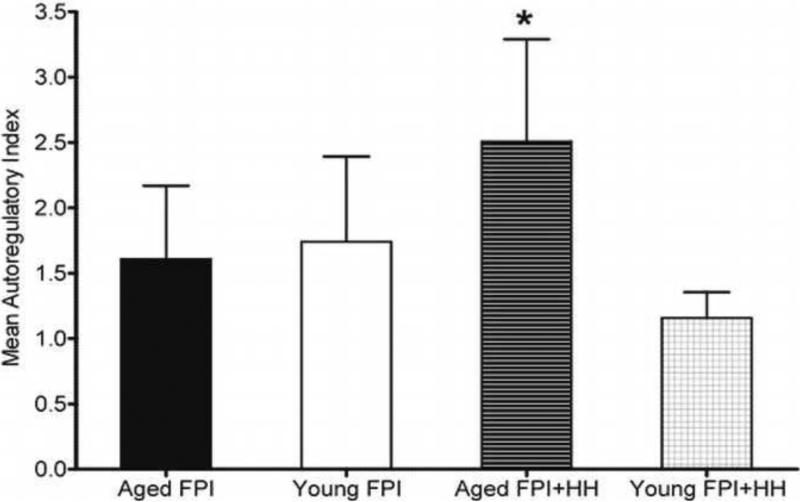
The autoregulatory index (ARI) remained between 2 and −2 in young rats after moderate FPI only or moderate FPI + severe HH but fell outside the normal range in the aged moderate FPI + severe HH animals for some time points post-injury (Figure 4). ARI values for each group of animals were analyzed for each time point using ANOVA. Mean ARI was significantly higher in the aged than young TBI + HH groups (p < 0.05), suggesting autoregulatory impairment in aged rats (Figure 4).
Figure 4. Autoregulation in Young and Aged Rats.
Calculated autoregulatory index values in young and aged rats after undergoing either sham injury, moderate FPI, or moderate FPI + severe HH. Calculations were based on each individual animal's measurements that were pooled to yield one mean value per experimental animal group was performed. Data are expressed as mean ± SEM and significance testing (* = p< 0.05, compared to young) was performed using a one-tailed t-test.
2.5 Hippocampal Neuronal Injury
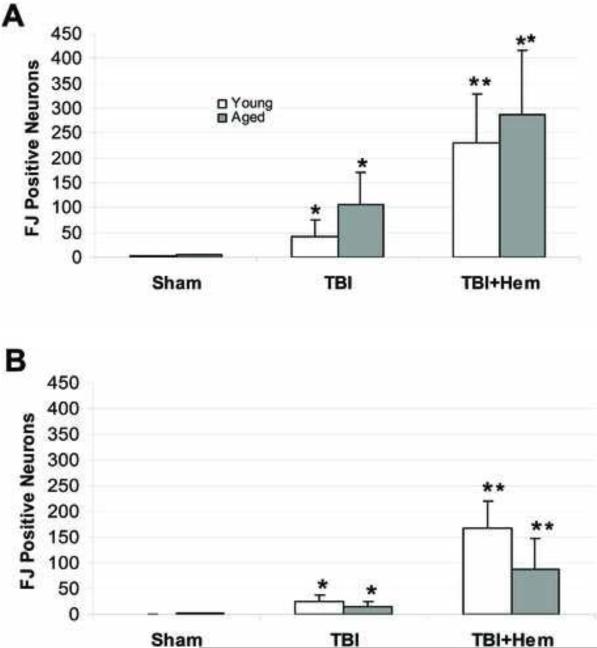
In young rats, there were significantly greater numbers of FJ-positive neurons in CA1&2 (Figure 5A) after FPI + HH when compared to sham (p < 0.01) and FPI (p < 0.05) animals. In CA3 (Figure 5B), we observed significantly greater numbers of FJ-positive neurons after FPI + HH when compared to sham (p < 0.01) and FPI (p < 0.05) animals.
Figure 5. Hippocampal Neuronal Injury in Young and Aged Rats.
Fluoro-Jade-positive neurons in the hippocampal CA1/2 (A) and CA3 (B) regions following sham (n = 6 young; n = 5 aged), moderate FPI (n = 6 young; n = 5 aged) or moderate FPI + severe HH (n = 5 young; n = 5 aged) in aged and young rats that were survived for 24 hours post-injury. Data are expressed as mean ± SEM and significance testing (* = p < 0.01, # = p < 0.05) was performed using ANOVA.
In aged rats, there were significantly greater numbers of FJ-positive neurons in CA1&2 (Figure 5A) after FPI + HH when compared to sham animals (p < 0.01). In CA3 (Figure 5B), we also observed significantly greater numbers of FJ-positive neurons after FPI + HH when compared to sham animals (p < 0.01). However, there were no statistically significant differences between the numbers of FJ-positive neurons between aged and young rats. There were no FJ-positive neurons in the contralateral (uninjured) hippocampus of any animal in any group.
3.0 Discussion
Our results show a prolonged posttraumatic hypoperfusion during the post-resuscitation phase after FPI + HH in aged compared to young adult rats. Although determined using a different method, the decreases in CBF following FPI and HH in rats in this study are consistent with those reported by other groups (Matsushita et al., 2001) and the reduced laser Doppler perfusion observed in our previous study of the effects of hypertonic arginine resuscitation after FPI and HH (Prough et al., 2006). These experimental results reflect the correlation between decreased CBF post-TBI and worse outcome seen clinically (Marion et al., 1991).
Changes in cerebrovascular reactivity occur during the normal aging process. Endothelium-dependent vasodilatory responses are impaired in large extracranial arteries in aged rats (Hongo et al., 1988) as well as endothelium-dependent relaxation to acetylcholine in rat arterioles (Mayhan et al., 1990). Age-related decreases in the synthesis of vascular proteins have also been demonstrated (Gozes et al., 1981). Although cerebrovascular reactivity is impaired by experimental TBI (Bramlett et al., 1999; Cherian et al., 1996; DeWitt and Prough, 2003) or hemorrhagic stroke (Smeda, 1992) in young adult rats, the effects of cerebral traumatic or ischemic injury on cerebral vascular function in aged rats needs thorough characterization.
Elevated blood pressure in response to neurotrauma has recently been shown to have a less deleterious effect on overall outcomes in elderly patients as compared to younger ones (Ley et al., 2012). This suggests that blood pressure maintenance, especially within the elderly population, is critical to positive outcomes following traumatic brain injury. More research is needed to determine optimal ranges for patients depending on their age and other complications or injuries sustained.
3.1 Autoregulation
Autoregulation, the ability of the cerebral vasculature to maintain constant CBF during reductions in systemic blood pressure, is significantly reduced locally (Lewelt et al., 1980), regionally (DeWitt et al., 1992a), and globally (DeWitt et al., 1992b) after FPI in cats. In rats, impact acceleration (Prat et al., 1997; Engelborghs et al., 2000) and weight drop (Nawashiro et al., 1995) TBI significantly reduced the ability of the cerebral vasculature to alter cerebral vascular resistance in order to maintain constant levels of CBF when arterial blood pressure changed. Both age and hypotension may impair the ability of the cerebrovasculature to respond to reduced systemic arterial blood pressure in spontaneously hypertensive and normal control rats after drug-induced hypotension (Hoffman et al., 1981).
In the present study, ARI remained between the normal values of 2 and −2 in young rats after FPI only and FPI + HH. In contrast, ARI values were less than −2, 30 and 60 min after FPI only in aged rats. However, at other time points, the ARI values did not correlate well with CBF. For example, ARI values were within normal limits in young rats after FPI + HH while CBF was reduced 40 – 50% below baseline. This apparent contradiction may have been related to the severity of HH used in this study. Since mean arterial blood pressure levels of 40 mm Hg are below the lower limit of autoregulation in normal rats (Engelborghs et al., 2000) even “normal” autoregulatory responses would be inadequate to maintain CBF. Another potential explanation for the observed differences is that the calculation we used for ARI was originally defined using CBF measured using radiolabeled microspheres (Muizelaar et al., 1984) whereas, CBF in our study was measured using laser Doppler flowmetry, a method that measures relative perfusion as a percent of baseline rather than absolute CBF.
3.2 Post-Resuscitation Hyperperfusion
Blood pressure changes (reductions as small as 20mmHg) have been shown to correlate with increased ischemic brain damage in a middle cerebral artery occlusion model (Zhu and Auer, 1995). Age-associated increases in mortality following FPI with HH may be due, in part, to delayed reperfusion in the resuscitation period following FPI and severe hemorrhage. In our study, aged rats' lower CBF post-resuscitation was associated with less robust restoration of MAP than in younger rats. This may be due to reduced ability of the aged rats to respond hemodynamically to the FPI, HH and resuscitation sequence.
3.3 Neuronal Injury
Fluoro-Jade is a well-studied fluorescent histological stain utilized as an indicator of neuronal health status. We acknowledge that FJ's use as an indicator of neuronal death or degeneration (with the potential to recover) remains a subject of controversy. Since FJ's mechanism of action is currently unknown and we do not know what protein or cell signaling molecule it binds to, it cannot be determined. However, in fixed tissue, it appears to stain dead cells, but in living cell culture systems the FJ positive cells may recover from injury. In our experimental studies, we used FJ to detect cell death in fixed brain sections. Fluid percussion TBI + HH was associated with FJ-stained neurons in hippocampal cell layers CA1, CA2 and CA3 (Figure 5A & B). Interestingly, the numbers of FJ-positive neurons was significantly higher after FPI + HH than FPI alone in young but not aged rats. We saw some FJ positive neurons in our sham-injured group and acknowledge that it is not uncommon to see a few FJ positive cells in a sham-injured animal as they do undergo similar surgical procedures as the TBI animals: isoflurane anesthesia, intubation and ventilation, jugular vein and tail artery cannulations, craniotomy to remove the skull piece and the placement of the ICP probe, which could cause cell death detectable by FJ.
Typically, brain injury models have demonstrated increased injury in aged animals compared to younger ones that have undergone similar injury (Davis et al., 1995). However, in our study, there were no significant differences in the numbers of FJ stained hippocampal neurons between young and aged rats in any injury group. This is similar to the fewer numbers of injured neurons in the CA1 in aged (compared to young) rats after forebrain ischemia (Sutherland et al., 1996). Our findings also support those of Wasserman et al. who reported no differences in the numbers of FJ-positive injured neurons between aged and young male Sprague-Dawley rats twenty-four hours after intracerebral hemorrhage; however they found a greater number of FJ-positive neurons in the brains of aged rats three days post-injury (Wasserman et al., 2008). With longer survival times, we may have observed a similar increase in the number of injured neurons in aged rats. There was a trend towards higher numbers of FJ stained neurons after FPI and FPI + HH in CA1 and CA2 (but not CA3) but a post-hoc power analysis of our data revealed that we would need 61 and 153 rats, respectively, to detect a difference between aged and young rats at the moderate FPI and moderate FPI + HH injury groups surviving 24-hours post-injury.
Injury to brain cells other than neurons may account for the age-related morbidity following TBI. Excitotoxic damage in aged rats resulted in delayed neurodegeneration and early astrogliosis which led to a larger glial scar compared to the young adult rats (Castillo-Ruiz et al., 2007). Moreover, decreased production of neurotrophins in the aged hippocampus following kainic acid-induced injury was found to result in a normal astrocytic response but significantly diminished microglial reaction (Shetty et al., 2004) whereas accelerated glial reactivity in aged rats produced a reduction in functional recovery following stroke (Badan et al., 2003). Differences in debris clearance rates may contribute to age-related functional impairment following TBI.
3.4 Conclusions
Age-associated increases in mortality following TBI with hemorrhage may be due, in part, to delayed reperfusion after HH in aged animals, which in part may result from poorer restoration of MAP by reinfusion of shed blood in aged rats. Further research is necessary to determine the precise mechanisms that contribute to trauma-related CBF impairment after resuscitation in aged rats. Our results showing reduced CBF and potentially impaired autoregulation in aged but not young rats after TBI + HH suggest that maintenance of adequate cerebral perfusion pressure may be more important in aged than in young TBI patients.
4.0 Materials and Methods
4.1 Animals
This study was conducted in a facility approved by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and all experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Young adult (4–6 months, 350–500 g) and aged (22–24 months, 800–1000 g) male Sprague-Dawley rats were obtained from Charles Rivers Laboratories, Inc. (West Chester, Ohio), housed with food and water ad libitum, and maintained at a constant temperature (21°C to 23°C) and humidity (45%–50%) with lights on from 0700 to 1900 hours. All animals were housed separately following surgery.
4.2 Surgical Procedures
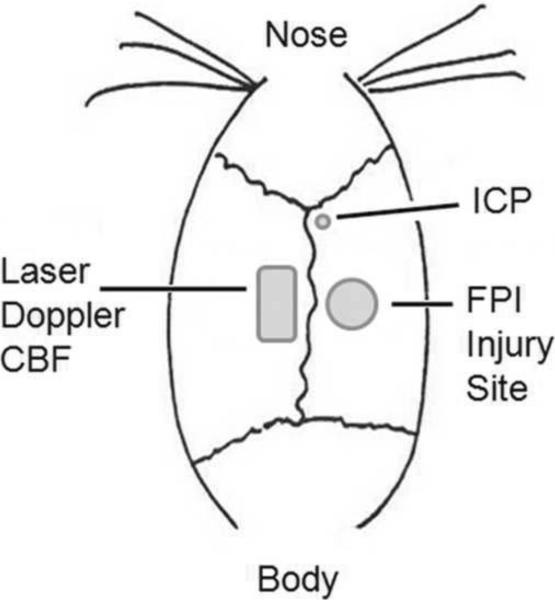
Animals were anesthetized (4% isoflurane in an anesthetic chamber), intubated and mechanically ventilated (1.5% isoflurane in O2:air (20:80)) for the duration of the surgical procedures and physiological experiments. Right common jugular veins were cannulated with silastic tubing for intravenous fluid administration and hemorrhage. Tail arteries were cannulated with polyethylene tubing (PE 50) for fluid administration, arterial blood gas and mean arterial pressure (MAP) monitoring. To prepare rats for FPI (Dixon et al., 1987), a craniotomy was performed lateral right to the sagittal suture, midway between the bregma and lambda sutures (see Figure 1). Intracranial pressure was monitored using a modified 22-Gauge spinal needle (placed 1.5 mm caudal from bregma, 1.2 mm lateral from sagittal suture and 3 mm insertion where a waveform confirmed the location of the ventricular space) and CBF was measured using a laser Doppler probe placed contralateral to the injury site (due to the size of the probe in comparison to the size of the animal's skull and to allow for measurements to be collected during the time of injury) and shielded by a black rubber tube glued to an area of thinned skull. An adapter (machined needle hub) was placed in the craniotomy site and held in place by hygienic dental acrylic. Baseline MAP values of at least 80 mm Hg and PaCO2 (35–41 mm Hg) were recorded before injury and maintained constant throughout the procedure using ventilatory rate and tidal volume adjustments. Animals were randomly assigned to one of the following groups: 1) moderate FPI + severe HH (n = 7 young, 6 aged); 2) moderate FPI only (n = 6 young, 4 aged); or 3) sham FPI only (n = 6 young, 5 aged).
Figure 1.
Drawing of injury site and probe placement.
Hemorrhage was initiated within 5 minutes of moderate FPI and maintained for a total of 45 minutes by withdrawing blood through the jugular catheter into a syringe to achieve MAP values of 40 mm Hg. To reduce clotting, heparin (100 units/kg) diluted in saline was administered to the rats in both the sham and the severe HH groups via the jugular vein catheter. Shed blood was reinfused at a rate of two mL per minute after 45 minutes. The animals were monitored for 90 minutes post-FPI, in the group receiving HH; this amounted to 45 minutes during hemorrhage followed by 45 minutes during the reinfusion of shed blood. Temporalis muscle and rectal temperatures, MAP, ICP, laser Doppler perfusion, and shed blood volumes were recorded every five minutes. All animals received 120 mg/kg acetaminophen (suppository) before emerging from anesthesia. Wound sites were sutured and cleaned with hydrogen peroxide. Twenty-four hours later, rats were reanesthestized with 4% isoflurane, decapitated and the brains were rapidly removed, snap frozen, and stored at −80°C until sectioned.
4.3 Autoregulatory Index Calculation
We calculated the autoregulatory index (ARI) by dividing the percent change in cerebral perfusion pressure (CPP) by the percent change in cerebrovascular resistance (CVR) (Muizelaar et al., 1984). CPP was calculated as MAP - ICP for each measurement interval (pre-injury baseline to 90 minutes post-injury). CVR was calculated by dividing CBF by CPP for each measurement interval (pre-injury baseline to 90 minutes post-injury). Based on our previous report and that of other groups, ARI values between negative 2 and 2 represent intact autoregulation (DeWitt et al., 1992b; Muizelaar et al., 1984).
4.4 Fluoro-Jade (FJ) Staining and Neuronal Counting
Brains were embedded in OCT compound and 10 μm frozen coronal sections (every 15th) containing both the ipsilateral and contralateral hippocampi were cut from each brain (3.6 to 4.5 mm posterior to bregma; 10 sections per brain) using a cryostat (Leica Microsystems, Inc., Bannockburn, IL). Sections were collected and mounted on precleaned microscope slides. Slides were then immersed in 75% ethanol (fixation), stained briefly (15 seconds) with 1% cresyl violet and with 0.001% FJ (Histo-Chem, Inc., Jefferson, AR) (Schmued et al., 1997) for 4 minutes (Hawkins et al., 2012; Hellmich et al., 2005; Hellmich et al., 2006). Fluoro-Jade-positive neurons were counted by investigators blinded to the treatment groups using a FITC filter on a PixCell IIe (Arcturus Engineering, Mountain View, CA) imaging system monitor. Numbers of FJ-positive neurons were recorded for CA1/2 and CA3 hippocampal regions in each of the ten sections per rat.
4.5 Statistical Analysis
MAP, ICP and CBF
Mean arterial pressure, ICP and laser Doppler CBF were analyzed using analysis of variance (ANOVA) with repeated measures of time using StatView 5.0. To adjust for multiple comparisons, differences in these groups were analyzed using Fisher's protected least significant difference (PLSD) test with p < 0.05 considered significant. Student's t-test was used to evaluate age-related differences at individual time points during the resuscitation phase in the trauma + severe HH group, using Microsoft Excel. A 95% confidence interval was used, with p < 0.05 considered a significant difference between compared groups.
Autoregulatory Index
Student's t-test using GraphPad Prism software version 5.03 was used to compare mean autoregulatory indicies between aged and young rats after FPI and FPI + HH (GraphPad Software, Inc., La Jolla, CA). Differences in these groups were analyzed using Fisher's protected least significant difference (PLSD) test with p < 0.05 considered significant.
Neuronal Cell Death
Numbers of Fluoro-Jade-positive neurons were analyzed by ANOVA using StatView 5.0 (SAS Institute, Cary, NC). Differences in these groups were analyzed using Fisher's protected least significant difference (PLSD) test with p < 0.05 considered significant.
Highlights
Effects of trauma and hemorrhage were studied.
Decreases in cerebral blood flow for aged animals were observed.
No extra increase in neuronal cell death with aged animals.
Autoregulation impairment found in aged animals post trauma and hemorrhage.
BP maintenance is critical for treatment of elderly patients post trauma.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (NS19355 to DSD), UTMB Sealy Center on Aging (DSP) and Moody Center for Traumatic Brain and Spinal Cord Injury Research/Mission Connect (to DSD). Bridget E. Hawkins' work was supported by a predoctoral fellowship under NIEHS grant no. 5T32ES007254-17 (“Molecular Mechanisms for Environmental Injury,” Dr. Mary Moslen, PI). We thank Tatsuo Uchida for his help performing the post-hoc power analysis.
Abbreviations
- ARI
autoregulatory index
- BP
blood pressure
- CA1, 2 and 3
Cornu Ammonis regions 1, 2 and 3
- CBF
cerebral blood flow
- CPP
cerebral perfusion pressure
- CVR
cerebrovascular resistance
- FJ
fluoro-jade
- FPI
fluid percussion injury
- HH
hemorrhagic hypotension
- ICP
intracranial pressure
- MAP
mean arterial pressure
- MRI
magnetic resonance imaging
- OCT
optimum cutting temperature (for embedding)
- PaCO2
partial pressure of carbon dioxide in arterial blood
- PaO2
partial pressure of oxygen in arterial blood
- PET
positron emission tomography
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
The authors state no conflict of interest.
Reference List
- 1.Armstead WM. (Age-dependent NOC/oFQ contribution to impaired hypotensive cerebral hemodynamics after brain injury. J Neurotrauma. 2002;19:1193–1202. doi: 10.1089/08977150260337994. [DOI] [PubMed] [Google Scholar]
- 2.Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. (Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- 3.Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. (Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- 4.Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. (Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- 5.Bramlett HM, Dietrich WD, Green EJ. (Secondary hypoxia following moderate fluid percussion brain injury in rats exacerbates sensorimotor and cognitive deficits. J Neurotrauma. 1999;16:1035–1047. doi: 10.1089/neu.1999.16.1035. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Ruiz MM, Campuzano O, Acarin L, Castellano B, Gonzalez B. (Delayed neurodegeneration and early astrogliosis after excitotoxicity to the aged brain. Exp Gerontol. 2007;42:343–354. doi: 10.1016/j.exger.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Cherian L, Robertson CS, Goodman JC. (Secondary insults increase injury after controlled cortical impact in rats. J Neurotrauma. 1996;13:371–383. doi: 10.1089/neu.1996.13.371. [DOI] [PubMed] [Google Scholar]
- 8.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the traumatic coma data bank. Acta Neurochir. 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 9.Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, Downey SP, Williams GB, Aigbirhio F, Hutchinson PJ, Rice K, Carpenter TA, Clark JC, Pickard JD, Menon DK. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- 10.Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- 11.DeWitt DS, Jenkins LW, Wei EP, Lutz H, Becker DP, Kontos HA. Effects of fluid percussion brain injury on regional cerebral blood flow and pial arteriolar diameter. J Neurosurg. 1986;64:787–794. doi: 10.3171/jns.1986.64.5.0787. [DOI] [PubMed] [Google Scholar]
- 12.DeWitt DS, Prough DS, Taylor CL, Whitley JM, Deal DD, Vines SM. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am J Physiol. 1992;263(4 Pt2):H1276–84. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt DS, Prough DS, Taylor CL, Whitley JM. Reduced cerebral blood flow, oxygen delivery, and electroencephalographic activity after traumatic brain injury and mild hemorrhage in cats. J Neurosurg. 1992b;76:812–821. doi: 10.3171/jns.1992.76.5.0812. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt DS, Prough DS. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 15.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 16.Engelborghs K, Haseldonckx M, Van RJ, Van RK, Wouters L, Borgers M, Verlooy J. Impaired autoregulation of cerebral blood flow in an experimental model of traumatic brain injury. J Neurotrauma. 2000;17:667–677. doi: 10.1089/089771500415418. [DOI] [PubMed] [Google Scholar]
- 17.Gozes I, Cronin BL, Moskowitz MA. Protein synthesis in rat brain microvessels decreases with aging. J Neurochem. 1981;36:1311–1315. doi: 10.1111/j.1471-4159.1981.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 18.Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;1:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- 19.Graham DI, Ford I, Adams JH, Doyle D, Teasdale GM, Lawrence AE, McLellan DR. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamm RJ, Jenkins LW, Lyeth BG, White-Gbadebo DM, Hayes RL. The effect of age on outcome following traumatic brain injury in rats. J Neurosurg. 1991;75:916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BE, Frederickson CJ, DeWitt DS, Prough DS. Fluorophilia: Fluorophore-Containing Compounds Adhere Non-Specifically to Injured Neurons. Brain Res. 2012;1432:28–35. doi: 10.1016/j.brainres.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellmich HL, Capra B, Eidson K, Garcia J, Kennedy D, Uchida T, Parsley M, Cowart J, DeWitt DS, Prough DS. Dose-dependent neuronal injury after traumatic brain injury. Brain Res. 2005;1044:144–154. doi: 10.1016/j.brainres.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Hellmich HL, Eidson KA, Capra BA, Garcia JM, Boone DR, Hawkins BE, Uchida T, DeWitt DS, Prough DS. Injured Fluoro-Jade-positive hippocampal neurons contain high levels of zinc after traumatic brain injury. Brain Res. 2006;1127:119–126. doi: 10.1016/j.brainres.2006.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hlatky R, Contant CF, Diaz-Marchan P, Valadka AB, Robertson CS. Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit Care. 2004;1:69–83. doi: 10.1385/NCC:1:1:69. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman WE, Albrecht RF, Miletich DJ. The influence of aging and hypertension on cerebral autoregulation. Brain Res. 1981;214:196–199. doi: 10.1016/0006-8993(81)90454-6. [DOI] [PubMed] [Google Scholar]
- 26.Hongo K, Nakagomi T, Kassell NF, Sasaki T, Lehman M, Vollmer DG, Tsukahara T, Ogawa H, Torner J. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- 27.Lewelt W, Jenkins LW, Miller JD. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J Neurosurg. 1980;53:500–511. doi: 10.3171/jns.1980.53.4.0500. [DOI] [PubMed] [Google Scholar]
- 28.Ley EJ, Singer MB, Gangi A, Clond MA, Bukur M, Chung R, Margulies DR, Salim A. Elevated systolic blood pressure after trauma: tolerated in the elderly. J Surgical Research. 2012;177:326–329. doi: 10.1016/j.jss.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 1991;74:407–14. doi: 10.3171/jns.1991.74.3.0407. [DOI] [PubMed] [Google Scholar]
- 30.Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, Hovda DA, Becker DP. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita Y, Bramlett HM, Kuluz JW, Alonso O, Dietrich WD. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J Cereb Blood Flow Metab. 2001;21:847–856. doi: 10.1097/00004647-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Mayhan WG, Faraci FM, Baumbach GL, Heistad DD. Effects of aging on responses of cerebral arterioles. Am J Physiol. 1990;258:H1138–H1143. doi: 10.1152/ajpheart.1990.258.4.H1138. [DOI] [PubMed] [Google Scholar]
- 33.Miller JD. Head injury and brain ischaemia - implications for therapy. Br J Anaesth. 1985;57:120–130. doi: 10.1093/bja/57.1.120. [DOI] [PubMed] [Google Scholar]
- 34.Muizelaar JP, Lutz HA, III, Becker DP. Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J Neurosurg. 1984;61:700–706. doi: 10.3171/jns.1984.61.4.0700. [DOI] [PubMed] [Google Scholar]
- 35.Nawashiro H, Shima K, Chigasaki H. Immediate cerebrovascular responses to closed head injury in the rat. J Neurotrauma. 1995;12:189–197. doi: 10.1089/neu.1995.12.189. [DOI] [PubMed] [Google Scholar]
- 36.Prat R, Markiv V, Dujovny M, Misra M. Evaluation of cerebral autoregulation following diffuse brain injury in rats. Neurol Res. 1997;19:393–402. doi: 10.1080/01616412.1997.11740832. [DOI] [PubMed] [Google Scholar]
- 37.Prough DS, Kramer GC, Uchida T, Stephenson RT, Hellmich HL, DeWitt DS. Effects of hypertonic arginine on cerebral blood flow and intracranial pressure after traumatic brain injury combined with hemorrhagic hypotension. Shock. 2006;26:290–295. doi: 10.1097/01.shk.0000225405.66693.49. [DOI] [PubMed] [Google Scholar]
- 38.Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1668. doi: 10.1161/01.str.27.9.1663. [DOI] [PubMed] [Google Scholar]
- 40.Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- 41.Smeda JS. Cerebral vascular changes associated with hemorrhagic stroke in hypertension. Can J Physiol Pharmacol. 1992;70:552–564. doi: 10.1139/y92-070. [DOI] [PubMed] [Google Scholar]
- 42.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci. 2008;28:1316–1328. doi: 10.1111/j.1460-9568.2008.06442.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhu CZ, Auer RN. Graded hypotension and MCA occlusion duration: effect in transient focal ischemia. J Cerebral Blood Flow and Metabolism. 1995;15:980–988. doi: 10.1038/jcbfm.1995.124. [DOI] [PubMed] [Google Scholar]