Abstract
It has been considered that Ca2+ release is the causal trigger for Ca2+ entry after receptor activation. In DT40 B cells devoid of inositol 1,4,5-trisphosphate receptors (IP3R), the lack of Ca2+ entry in response to receptor activation is attributed to the absence of Ca2+ release. We reveal in this article that IP3R recognition of IP3 determines agonist-induced Ca2+ entry (ACE), independent of its Ca2+ release activity. In DT40 IP3R–/– cells, endogenous ACE can be rescued with type 1 IP3R mutants (both a ΔC-terminal truncation mutant and a D2550A pore mutant), which are defective in Ca2+ release channel activity. Thus, in response to B cell receptor activation, ACE is restored in an IP3R-dependent manner without Ca2+ store release. Conversely, ACE cannot be rescued with mutant IP3Rs lacking IP3 binding (both the Δ90–110 and R265Q IP3-binding site mutants). We conclude that an IP3-dependent conformational change in the IP3R, not endoplasmic reticulum Ca2+ pool release, triggers ACE.
Ca2+ transients elicited in response to cell surface receptor activation by neurotransmitters, hormones, and other molecular messengers are major messengers of intracellular communication (1). Stimulation of G protein-coupled receptors, tyrosine kinase receptors, and nonreceptor tyrosine kinases activate phospholipase C (PLC), catalyzing the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the second-messenger molecules: inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 mediates rapid Ca2+ store release by activating IP3 receptors (IP3Rs) in the endoplasmic reticulum (ER), whereas DAG activates protein kinase C (PKC) (2). After this initial Ca2+ release phase, external Ca2+ enters through plasma membrane channels, providing a secondary and more prolonged Ca2+ signal (1), a phenomenon designated here as agonist-induced Ca2+ entry (ACE) (3).
To date, the molecular identity of these Ca2+ entry channels as well as their coupling mechanism remain unknown, although several mechanisms have been proposed. Intracellular Ca2+ release through the IP3R could trigger ACE by means of capacitative Ca2+ entry (CCE) (4), which can be activated in a PLC-independent manner (3) by the ER Ca2+ pump blocker thapsigargin or the Ca2+ ionophore ionomycin (4, 5). In this scheme, the luminal drop in ER Ca2+ activates “store-operated” Ca2+ channels in the plasma membrane, although the basis for this coupling mechanism is entirely unknown. Although the release activity of IP3Rs may mediate Ca2+ entry, others have suggested that IP3Rs play a conformational role in the coupling process (6, 7). Also, DAG may directly initiate ACE, because DAG can activate overexpressed “canonical” transient receptor potential Ca2+ entry channels (TRPC) (8).
We recently demonstrated a functional distinction between ACE and CCE based on the requirement of the former for PLC-γ, in a lipase-independent manner (3). However, it is unclear whether endogenous ACE is an integrated process accounting for both receptor and store-operated channel activation. At the molecular level, it is unclear whether IP3R-mediated Ca2+ release triggers ACE and/or whether a conformational alteration of the IP3R is sufficient for ACE. Evidence for the activation by Ca2+ pool emptying alone (and the dispensability of the IP3R for Ca2+ entry) includes the ability of thapsigargin or ionomycin, but not PLC activation, to stimulate Ca2+ entry in the DT40 triple IP3R knockout cell line, a form of B lymphocytes devoid of any IP3R (9). These interpretations assume that ACE and CCE are functionally overlapping mechanisms. In the present study, we demonstrate that IP3 recognition by the IP3R but not the receptor's Ca2+ channel activity is required for activation of endogenous ACE.
Materials and Methods
Culture of Cells. Rat PC12 cells (passage numbers 6–15), human embryonic kidney (HEK)293 cells, rat aortic smooth muscle A7r5 cells (passage numbers 10–25), and DT40 chicken B lymphocyte IP3R–/– cells were cultured as described (3, 9, 10).
Expression Protocols. DT40, HEK293, PC12, and A7r5 were transfected as described (3). For experiments shown in Figs. 3 and 4, 5 μg of yellow fluorescent protein (YFP) cDNA ± 20 μg of IP3R cDNA was used for transfection.
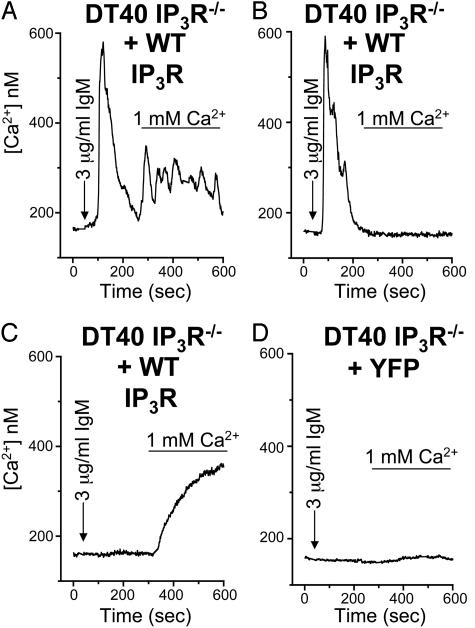
Fig. 3.
Differential rescue by type 1 WT IP3R of Ca2+ release and entry in DT40 IP3R–/– B cells. Free Ca2+ measurements were made in DT40 IP3R–/– knockout cells transfected with either YFP plus WT IP3R (WT; A–C) or YFP alone (D). Ca2+ pools were released in cells by 3 μg/ml anti-IgM (arrow) in nominally Ca2+-free medium followed by replacement with anti-IgM and normal 1 mM Ca2+ medium (bar). Representative traces are individual single-cell responses.
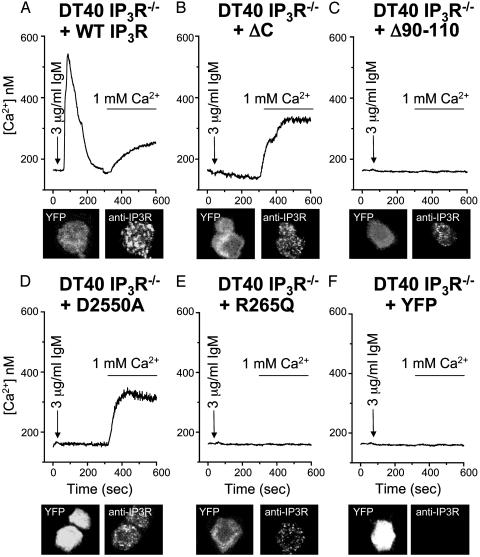
Fig. 4.
Channel-deficient type 1 IP3Rs rescue Ca2+ entry in DT40 IP3R–/– B cells, whereas IP3-binding-deficient type 1 IP3Rs do not. Free Ca2+ measurements were made in DT40 IP3R–/– knockout cells transfected with either YFP plus WT IP3R (WT; A), YFP plus ΔC-terminal IP3R mutant (ΔC; B), YFP plus Δ90–110 IP3R mutant (Δ90–110; C), YFP plus D2550A IP3R mutant (D2550A; D), YFP plus R265Q IP3R mutant (R265Q; E), or YFP alone (F). Ca2+ pools were released in cells by 3 μg/ml anti-IgM (arrow) in nominally Ca2+-free medium followed by replacement with anti-IgM and normal 1 mM Ca2+ medium (bar). DT40 IP3R–/– knockout cells, transfected as above, were prepared for immunocytochemistry and visualized by confocal microscopy, and they appear directly below their respective Ca2+ trace. (A–F) The Left images corresponds to YFP expression, and the Right images corresponds to IP3R staining. All traces are averages of ≈15 cells because the mutants Δ90–110 or R265Q IP3Rs do not respond and ΔC or D2550A only displays the NRE response.
Ca2+ Imaging. Ca2+ measurements were as described (11). Fura-2/acetoxymethyl ester loading was for 25 min at 20°C for DT40 cells, 1 h at 20°C for PC12 cells, 25 min at 20°C for HEK293 cells, and 30 min at 20°C for A7r5 cells. Transfected enhanced YFP served as the transfection marker and was detected at excitation wavelength 485 nm. Resting Ca2+ levels in cell lines were similar, 100–200 nM, and cells with higher basal levels were excluded from data collection because these cells tend to have constitutive Ca2+ entry. All measurements shown are representative of a minimum of three and, in most cases, a larger number of independent experiments. For the population studies in Fig. 1, YFP-transfected cells were used to control for experiments in HEK293 cells transfected with YFP plus the sarcoplasmic ER Ca2+ ATPase (SERCA)-2b. Functional rescue of Ca2+ responses in the DT40 IP3R–/– cells was totaled from five separate experiments with six conditions each: (i) YFP alone, 0 out of 241 cells (0% rescue, 0.0 SEM); (ii) WT IP3R, 49 out of 261 cells (18.7% rescue, 0.36 SEM); (iii) ΔCIP3R, 58 out of 230 cells (25% rescue, 1.62 SEM); (iv) Δ90–110 IP3R, 1 out of 239 cells (0.4% rescue, 0.19 SEM); (v) D2550A IP3R, 67 out of 258 cells (25.9% rescue, 0.64 SEM); and (vi) R265Q IP3R, 0 out of 255 cells (0% rescue, 0.0 SEM).
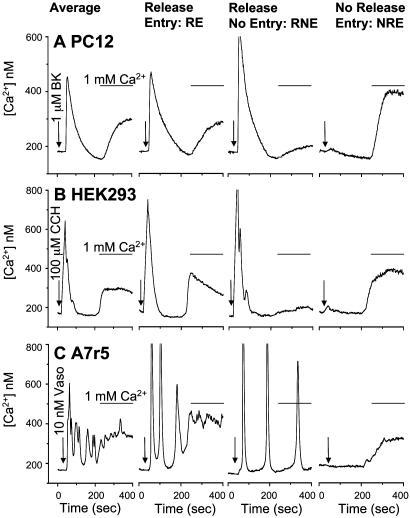
Fig. 1.
Ca2+ entry in the absence of IP3R-mediated Ca2+ release. Free Ca2+ measurements were made in YFP-transfected PC12, HEK293, and A7r5 cells. (A) Ca2+ pools were released by bradykinin (BK, 1 μM; arrow) in nominally Ca2+-free medium followed by replacement with bradykinin and normal 1 mM Ca2+ medium (bar). (B) Ca2+ pools were released by CCH (100 μM; arrow) in nominally Ca2+-free medium followed by replacement with CCH and normal 1 mM Ca2+ medium (bar). (C) Ca2+ pools were released by vasopressin (Vaso, 1 nM; arrow) in nominally Ca2+-free medium followed by replacement with vasopressin and normal 1 mM Ca2+ medium (bar). Average traces are representative of 30–50 cells, and the subpopulation traces are representative single cells extracted from that average.
Immunohistochemistry. Immunohistochemistry was as described in ref. 3.
IP3R Mutagenesis. Δ90–110 IP3R was constructed with two rounds of PCR by using overlapping 40-mers in which the bases 270–330 were omitted.
Antibodies and Reagents. Plasmids were obtained from the following sources: enhanced YFP vector cDNA was from Clontech; IP3R WT type 1, ΔC terminus, D2550A, and R265Q vectors were from Suresh Joseph (Thomas Jefferson University, Philadelphia, PA); carbachol (CCH), bradykinin, and vasopressin were from Sigma; Fura-2/acetoxymethyl ester and goat-anti rabbit Alexa-568 were from Molecular Probes; anti-chicken IgM (supernatant, M-4 clone) was from Southern Biotechnology Associates; and polyclonal anti-IP3R was from Affinity BioReagents (Golden, CO).
Results
Many studies, including our own, have shown that Ca2+ entry is coincident with IP3R-mediated Ca2+ release (3, 10, 12, 13). Such studies are almost exclusively based on average responses within large numbers of cells, despite the risks of interpreting Ca2+ signals from populations (14). In the present study, we have compared Ca2+ release and Ca2+ entry in individual cells. We measured single-cell receptor-mediated Ca2+ responses to bradykinin receptors in PC12 cells (neuronal origin), muscarinic receptors in HEK293 cells (kidney origin), and vasopressin receptors in A7r5 cells (vascular smooth muscle-derived). Ca2+ release was induced by the addition of agonist in nominally Ca2+ free media (Fig. 1, arrows). Subsequent addition of agonist with 1 mM extracellular Ca2+ induces an increase in cytosolic Ca2+, resulting from ACE (Fig. 1, bars). In averaged responses from multiple (30–50) cells, Ca2+ release coincides with ACE in each of the three cell types examined (Fig. 1, leftmost traces). However, analysis of the single-cell responses comprising these averages reveals three subpopulations of Ca2+ signals. Overall, among the three cell types, 62–72% of individual cells display both Ca2+ release and Ca2+ entry (RE), 17–20% of cells exhibit Ca2+ release with little to no Ca2+ entry (RNE), and 10–17% of cells manifest little to no Ca2+ release yet substantial Ca2+ entry (NRE) (Fig. 1 and Table 1). In all three cell types, constitutive Ca2+ entry is a rare event (<1% of cells), and a similarly small population of cells do not show any Ca2+ response (these cells were excluded from the population analyzed).
Table 1. Frequency of various Ca2+ entry responses to agonist stimulation.
| Cell/agonist | n | RE, % | RNE, % | NRE, % |
|---|---|---|---|---|
| PC12/1 μM bradykinin | 427 | 72.60 | 17.56 | 9.84 |
| A7r5/10 nM vasopressin | 208 | 62.98 | 20.19 | 16.83 |
| HEK293/100 μM CCH | 411 | 72.70 | 17.03 | 10.21 |
Ca2+ transients were collected for YFP-transfected PC12, A7r5, and HEK293 cells over five standardized experiments. These responses were categorized into their respective groups by a semiquantitative approach in which Ca2+ - release events <20 nM were considered little to no release and Ca2+ entry events <20 nM were considered little to no entry.
We wondered whether these frequencies could be manipulated by overexpressing SERCA-2b, which is known to increase IP3-releasable Ca2+ stores in transfected cells (15, 16). Compared with control-transfected HEK293 cells (Table 1), the proportion of cells manifesting RE is reduced in SERCA-2b-transfected HEK293 cells (control, 72.7% vs. 59.82%; n = 131/219 cells) (data not shown). By contrast, the proportion of cells with RNE is markedly augmented (control, 17.03% vs. 39.73%; n = 87/219), and virtually no cells are detected in the NRE phenotype (control, 10.21% vs. 0.457%; n = 1/219). This result is inconsistent with the Ca2+ entry process being triggered by Ca2+ release, because SERCA-2b expression would have augmented ACE.
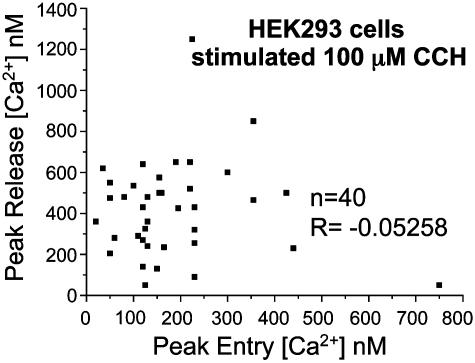
We characterized the relationship between Ca2+ release and entry in HEK293 cells by performing scatter analysis of the two parameters in response to the muscarinic receptor agonist CCH (100 μM) (Fig. 2). There is no correlation between extent of peak Ca2+ pool release and peak Ca2+ entry (n = 40; R = –0.05258). Thus, ACE in cells is not graded with respect to the magnitude of IP3R-mediated Ca2+ release. This result agrees with the nonlinear activation of the store-operated Ca2+ current (Icrac) by IP3 (14).
Fig. 2.
Agonist-induced Ca2+ release and entry are uncorrelated. Scatter plot of peak release size vs. peak entry magnitude from YFP-transfected HEK293 cells assayed by Ca2+ imaging. These single-cell traces were randomly chosen from experiments on 4 different days (10 per experiment), and absolute numbers reflect subtraction from baseline (n = 40, R = –0.05258).
Because our imaging assay reflects only whole-cell Ca2+ transients, we cannot exclude the possibility of subthreshold/local IP3R-mediated Ca2+ release within the subpopulations of cells exhibiting NRE. Undetectable release from possibly specific “coupling pools” could trigger ACE and subsequent Ca2+-induced Ca2+ release through Ca2+ release channels. Under this paradigm, NRE (Fig. 1, rightmost traces) might be a mixture of Ca2+ release and Ca2+ entry. To overcome the difficulty in analyzing microdomains of local IP3R-mediated Ca2+ release, we used the mutant DT40 chicken B lymphocyte cell line, which is deficient in all three genes for the IP3R (IP3R–/–) (17). Stimulation of DT40 cells with the B cell receptor agonist anti-IgM (IgM) leads to a nonreceptor tyrosine kinase-linked activation of PLC-γ2 and subsequent production of IP3 and DAG (3, 18). Introduction of Ca2+ reveals corresponding ACE as described (3, 9). Importantly, neither IP3/DAG production nor thapsigargin- or ionomycin-induced activation of CCE differs between the WT DT40 and mutant IP3R–/– cells (9, 12). In rescue experiments, transfection of DT40 IP3R–/– cells with YFP and WT type 1 IP3R (WT) (Fig. 3 A–C) restores PLC-dependent activation of ACE. Similar subpopulations of Ca2+ responses are seen in these rescued cells as in WT DT40 cells (data not shown). Moreover, even though mediated by PLC-γ, these subpopulations occur with comparable frequency as the PLC-β-stimulated responses in PC12, HEK293, and A7r5 cells (Fig. 1). Because YFP control DT40 IP3R–/– cells lack any Ca2+ release or ACE (Fig. 3D), we conclude that each of the three Ca2+ response subtypes requires the IP3R. Thus, even the NRE response (Fig. 3C) is restored by IP3R expression.
The DT40 IP3R–/– cells allowed us to assess directly the role of functionally modified IP3Rs on ACE. We transfected the cells with two different IP3R mutants: (i) C-terminal truncation mutant (ΔC), which binds IP3 but cannot release Ca2+ because of defects in Ca2+ channel gating; or (ii) a mutant IP3R lacking N-terminal amino acids 90–110 (Δ90–110), producing a channel with a ≈100-fold decrease in IP3 binding (19). Compared with WT IP3R rescue (Fig. 4A), expression of ΔC restores ACE without any Ca2+ release (Fig. 4B) (see Materials and Methods for the percentage of cells rescued), because the only response seen is the NRE response. In contrast, neither agonist-induced Ca2+ release nor Ca2+ entry is evident in cells transfected with Δ90–110 IP3R, which cannot bind IP3 (Fig. 4C). Because neither mutant channel can elicit intracellular Ca2+ release, the functional requirement for coupling to ACE appears to be IP3 recognition by the IP3R.
As the ΔC deletion involves removal of ≈300 amino acids, other receptor functions may have been altered. Additionally, the Δ90–110 IP3R mutant still can bind IP3, albeit with ≈100-fold lower affinity than WT. We addressed these issues in rescue experiments by using single point-mutant IP3R constructs: (i) an IP3R pore mutant (D2550A) lacking a critical aspartate-2550, mutation of which to alanine abolishes Ca2+ channel activity (20), or (ii) an IP3R ligand-binding mutant (R265Q) lacking a critical arginine-265, mutation of which to glutamine abolishes IP3 binding (21). The pore mutant (D2550A), like ΔC inactive channel preparation, restores Ca2+ entry but not release (NRE) (Fig. 4D), establishing that intracellular Ca2+ release is not required for entry. The deficient IP3 binding mutant (R265Q), like the Δ90–110 mutant that cannot bind IP3, displays neither Ca2+ release nor ACE (Fig. 4E).
D2550A rescue fails to support agonist-induced Ba2+ (1 mM) entry, fitting with the ion selectivity of IgM-activated WT DT40 cells (ref. 9 and data not shown). Vazquez et al. (12) reported that a pore-deficient mutant of type 3 IP3R did not rescue agonist-induced Ba2+ (10 mM) entry in the DT40 IP3R–/– cells. We used type 1 IP3R constructs and measured ACE. If IP3R specificity (type 1 vs. type 3) does not underlie this difference, then we would conclude that the channel activity rescued by D2550A expression is Ca2+-specific.
In control experiments, we assessed the expression of DT40 IP3R–/– cells transfected with YFP alone, YFP plus WT IP3R, or YFP plus mutant IP3Rs by using a rabbit polyclonal antibody against an N-terminal IP3R epitope and YFP as a marker for transfected cells. Confocal immunocytochemistry with goat anti-rabbit Alexa-568 secondary antibodies confirms the expression of the IP3R constructs in transfected DT40 IP3R–/– cells [Fig. 4 A–E, YFP (Left) and anti-IP3R (Right)]. In summary, our IP3R mutational analysis establishes that ACE requires functionally active IP3Rs and depends on IP3 recognition but is independent of Ca2+ release activity.
Discussion
The present study shows that, unlike CCE, ACE activation does not require ER Ca2+ release. Rather, ACE requires recognition of IP3 by the IP3R, irrespective of any Ca2+ release activity. These conclusions are supported by several key findings. First, single-cell Ca2+ measurements reveal subpopulations of Ca2+ responses (RE, RNE, and NRE), and the magnitude of Ca2+ release does not correlate with the magnitude of ACE. Second, in DT40 IP3R–/– cells devoid of ACE, restoration of WT IP3R expression rescues ACE in the three modes described, illustrating the requirement of the IP3R for each response type. Third, ACE is rescued in the DT40 IP3R–/– cells by two distinct Ca2+ release-deficient IP3R mutants but not by two different ligand-binding mutants. Thus, the IP3R is coupled to ACE independently of its Ca2+ release activity, supporting the notion that conformational changes in the IP3R, subsequent to IP3 binding, gate endogenous Ca2+ entry channels.
Our findings on the requirement of IP3 for ACE are consistent with studies showing that the IP3R binds TRPC3 in HEK293 cells (22, 23) and that an N-terminal IP3R fragment bound to IP3 is sufficient to gate TRPC3 in reconstituted vesicles (22, 24). Moreover, the IP3-dependence on ACE accords with experiments in the DT40 PLC-γ–/– cells, in which a lipase-inactive PLC-γ mutant rescues ACE only in the presence of receptor-generated IP3 (3). Our findings also complement observations of Penner and colleagues (25) that repetitive subthreshold CCH application stimulates ACE independently of Ca2+ release in RBL-2H3-M1 cells.
DAG, The other product of phosphatidylinositol 4,5-bisphosphate (PIP2) degradation, also has been proposed as a physiological activator of ACE, given that it can stimulate overexpressed TRPC3, -6, and -7 channels, even in the absence of IP3Rs (8, 9). Conversely, recent work reveals DAG-induced PKC activation as an inhibitor of endogenous ACE as well as overexpressed TRPC channels (26). In our experiments, endogenous ACE is not demonstrable in DT40 IP3R–/– cells, even though these cells contain active PLC and can produce DAG (9, 26). Thus, if DAG is an activator of Ca2+ entry, it is unlikely to function alone. Alternatively, IP3 and DAG may function coordinately in an activation–deactivation loop. Recently, Delmas et al. (27) demonstrated that G protein-coupled receptors signal differently to specific microdomains of IP3Rs in rat sympathetic neurons. By using overexpressed TRPC1 and TRPC6, these authors correlated Ca2+ release with TRPC1 activation and lack of Ca2+ release with TRPC6 (i.e., DAG activation). Our findings suggest that, although both TRPC1 and TRPC6 respond differentially to DAG, both still may be regulated by the IP3R, with the activation of TRPC1 merely being coincident with Ca2+ release.
Our findings support a model whereby Ca2+ entry is elicited by an IP3R pool in which no release occurs but not by an IP3R pool in which only release occurs. This interpretation is supported by overexpression experiments of SERCA-2b in HEK293 cells that decrease RE and NRE but augment the RNE phenotype. Accordingly, the preferred coupling mode is presumably a functional IP3-bound IP3R in a nonfunctional ER Ca2+ pool. Alternatively, but less likely, overactive expressed SERCA-2b might scavenge Ca2+ as it enters the cell, thereby obscuring our measurements. Compatible with the former hypothesis, expression of ΔC or D2550A IP3R, that can bind IP3 but not release, augments ACE. Our model fits with suggestions of Parekh et al. (14), that distinct IP3-sensitive ER stores function in release and entry modes, and Delmas et al. (27), who demonstrated IP3R-signaling microdomains in neurons. As discrete processes, Ca2+ release and Ca2+ entry may be dynamically regulated through changes in the movement of IP3Rs into or out of functional ER pools, generating microdomains that could respond differentially to various receptor stimuli and Ca2+-signaling functions.
Expression of WT IP3Rs in the DT40 IP3R–/– rescues the NRE response, as does the pore mutant. If coupling occurs within a nonfunctional ER pool, then Ca2+ release by WT IP3Rs would need to be rendered nonfunctional, at least in zones of Ca2+ entry. Modification of ER Ca2+ store content could be accomplished by proteins such as SERCA or by ER Ca2+ buffers (e.g., calsequestrin). Alternatively, but not mutually exclusive, a soluble truncated form of the IP3R, containing the N-terminal IP3 binding domain alone could couple IP3 recognition to Ca2+ entry. Cells contain transcripts for IP3Rs lacking Ca2+ channels (28), although it is not known whether these transcripts are translated into functional proteins. We suggest that such a protein may provide physiological signals for ACE, binding IP3 and TRPCs but unable to release Ca2+. Overall, this mechanism resembles proposals of Irvine (6) and Berridge (7) over a decade ago for IP3R-mediated conformational coupling.
Acknowledgments
We thank Drs. Joseph P. Kao, Robert E. Rothe, Gabriela Caraveo, and Klick Klerpa for useful discussion, Dr. Suresh Joseph for the kind gift of mutant IP3R constructs, and Dr. Tomohiro Kurosaki (Kansai Medical University, Moriguchi, Japan) for the gift of the DT40 IP3R–/– cell line. This research was supported by U.S. Public Health Service Grants MH-18501 and DA-000266 and Research Scientist Award DA-00074 (to S.H.S.), National Institutes of Health Grant HL55426 (to D.L.G.), American Heart Association Grant 0130268N (to K.K.), and National Research Service Awards NH65090 (to R.L.P.) and NS-043850 (to D.B.).
Abbreviations: IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; ACE, agonist-induced Ca2+ entry; PLC, phospholipase C; DAG, diacylglycerol; ER, endoplasmic reticulum; CCE, capacitative Ca2+ entry; TRPC, transient receptor potential Ca2+ entry channels; YFP, yellow fluorescent protein; SERCA, sarcoplasmic ER Ca2+ ATPase; RE, Ca2+ release and Ca2+ entry; RNE, Ca2+ release with little to no Ca2+ entry; NRE, little to no Ca2+ release yet substantial Ca2+ entry; CCH, carbachol; HEK, human embryonic kidney.
References
- 1.Berridge, M. J., Lipp, P. & Bootman, M. D. (2000) Nat. Rev. Mol. Cell. Biol. 1, 11–21. [DOI] [PubMed] [Google Scholar]
- 2.Sigano, D. M., Peach, M. L., Nacro, K., Choi, Y., Lewin, N. E., Nicklaus, M. C., Blumberg, P. M. & Marquez, V. E. (2003) J. Med. Chem. 46, 1571–1579. [DOI] [PubMed] [Google Scholar]
- 3.Patterson, R. L., van Rossum, D. B., Ford, D. L., Hurt, K. J., Bae, S. S., Suh, P. G., Kurosaki, T., Snyder, S. H. & Gill, D. L. (2002) Cell 111, 529–541. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam, K., van Rossum, D. B., Patterson, R. L., Ma, H. T. & Gill, D. L. (2002) Nat. Cell Biol. 4, E263–E272. [DOI] [PubMed] [Google Scholar]
- 5.Putney, J. W. (1997) Capacitative Calcium Entry (Springer, New York).
- 6.Irvine, R. F. (1990) FEBS Lett. 263, 5–9. [DOI] [PubMed] [Google Scholar]
- 7.Berridge, M. J. (1995) Biochem. J. 312, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T. & Schultz, G. (1999) Nature 397, 259–263. [DOI] [PubMed] [Google Scholar]
- 9.Venkatachalam, K., Ma, H. T., Ford, D. L. & Gill, D. L. (2001) J. Biol. Chem. 276, 33980–33985. [DOI] [PubMed] [Google Scholar]
- 10.Patterson, R. L., van Rossum, D. B. & Gill, D. L. (1999) Cell 98, 487–499. [DOI] [PubMed] [Google Scholar]
- 11.Ufret-Vincenty, C. A., Short, A. D., Alfonso, A. & Gill, D. L. (1995) J. Biol. Chem. 270, 26790–26793. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez, G., Wedel, B. J., Bird, G. S., Joseph, S. K. & Putney, J. W. (2002) EMBO J. 21, 4531–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishida, M., Sugimoto, K., Hara, Y., Mori, E., Morii, T., Kurosaki, T. & Mori, Y. (2003) EMBO J. 22, 4677–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh, A. B., Fleig, A. & Penner, R. (1997) Cell 89, 973–980. [DOI] [PubMed] [Google Scholar]
- 15.Boehning, D. & Joseph, S. K. (2000) J. Biol. Chem. 275, 21492–21499. [DOI] [PubMed] [Google Scholar]
- 16.Lechleiter, J. D., John, L. M. & Camacho, P. (1998) Biophys. Chem. 72, 123–129. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara, H., Kurosaki, M., Takata, M. & Kurosaki, T. (1997) EMBO J. 16, 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T. & Snyder, S. H. (2003) Nat. Cell Biol. 5, 1051–1061. [DOI] [PubMed] [Google Scholar]
- 19.Patterson, R. L., van Rossum, D. B., Barrow, R. K. & Snyder, S. H. (2004) Proc. Natl. Acad. Sci. USA 101, 2328–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehning, D., Mak, D. O., Foskett, J. K. & Joseph, S. K. (2001) J. Biol. Chem. 276, 13509–13512. [DOI] [PubMed] [Google Scholar]
- 21.Boehning, D. & Joseph, S. K. (2000) EMBO J. 19, 5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiselyov, K. I., Xu, X., Mohayeva, G., Kuo, T., Pessah, I. N., Mignery, G. A., Zhu, X., Birnbaumer, L. & Muallem, S. (1998) Nature 396, 478–482. [DOI] [PubMed] [Google Scholar]
- 23.Boulay, G., Brown, D. M., Qin, N., Jiang, M., Dietrich, A., Zhu, M. X., Chen, Z., Birnbaumer, M., Mikoshiba, K. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiselyov, K. I., Mignery, G. A., Zhu, M. X. & Muallem, S. (1999) Mol. Cell 4, 423–429. [DOI] [PubMed] [Google Scholar]
- 25.Hermosura, M. C., Takeuchi, H., Fleig, A., Riley, A. M., Potter, B. V., Hirata, M. & Penner, R. (2000) Nature 408, 735–740. [DOI] [PubMed] [Google Scholar]
- 26.Venkatachalam, K., Zheng, F. & Gill, D. L. (2003) J. Biol. Chem. 278, 29031–29040. [DOI] [PubMed] [Google Scholar]
- 27.Delmas, P., Wanaverbecq, N., Abogadie, F. C., Mistry, M. & Brown, D. A. (2002) Neuron 34, 209–220. [DOI] [PubMed] [Google Scholar]
- 28.Patel, S., Joseph, S. K. & Thomas, A. P. (1999) Cell Calcium 25, 247–264. [DOI] [PubMed] [Google Scholar]