Abstract
From the individual perspective of the two authors who were long-time colleagues of Karl Lederis at the University of Calgary, the events and personal interactions are described, that are relevant to the discovery of Urotensin I (UI) in the Lederis laboratory, along with the concurrent discovery of Urotensin II (UII) in the Bern laboratory and corticotropin-releasing factor (CRF/CRH) in the Vale laboratory. The fortuitous sabbatical experiences that put Professors Lederis and Bern on the track of the Urotensins, along with the essential isolation paradigm that resulted in the complete sequencing and synthesis of UI and UII are summarized. The chance interaction between Drs. Vale and Lederis who, prior to the publications of the sequences of UI and CRF, realized the sequence commonalities of these peptides with the vasoactive frog peptide, sauvagine, is outlined. Further, the relationship between the pharmacological studies done with UI in the Calgary laboratory and the more recent understanding of the biology and receptor pharmacology for the entire Urotensin I–CRF–Urocortin peptide family is dealt with. The value of a comparative endocrinology approach to understanding hormone action is emphasized, along with a projection to the future, based on new hypotheses that can be generated by unexplained data already in the literature. Based on the previously described pharmacology of the UI–CRF–Urocortin peptides in a number of target tissues, it is suggested that the use of current molecular approaches can be integrated with a ‘classical’ pharmacological approach to generate new insights about the UI–CRF–Urocortin hormone family.
Keywords: Corticotropin-releasing factor (CRF), CRF1, CRF2, Oxytocin, Urocortin, Urophysis, Urotensin, Vasopressin, Fish, Teleosts
1. Overview
The discovery of a new hormone family is always an exciting event. That said, the path to such a discovery is often long, circuitous and serendipitous. Such was the case for the discovery and elucidation of the Urotensin I–Corticotropin-Releasing Factor–Urocortin family of peptide hormones, now known to regulate the function of a diverse set of target tissues in mammals, ranging from the cardiovascular to central nervous systems (Davidson et al., 2009; Oki and Sasano, 2004). What is often absent in the literature is the kind of personal and scientific interchanges that lead to such discoveries. This kind of information, if present at all in peer-reviewed publications, is there usually only in a cryptic form and is often lost as individuals pass onto other interests or retire. As luck would have it, the two authors of this article were colleagues in the same Department at the University of Calgary with Karl Lederis at the time Urotensin I was first isolated; and were witness to the interchanges between the Lederis laboratory and the laboratories of Bern and Vale at the time ovine CRF1 and the teleost Urotensins (I and II) were sequenced. Further, we both had the opportunity to join Karl in collaborative projects to experience the synergistic relationship of working along with him. Those projects (Hollenberg et al., 1989; Muramatsu et al., 1988; Pittman et al., 1982; Poulin et al., 1988) provided us with insight about Karl’s approach to the pharmacology of hormones and neuropeptides in the smooth muscle and CNS systems that were central to his work with the Urotensins. Further, for the writing of this article, we had access to Karl’s archival files that contain reprints of articles no longer readily accessible (e.g. Lederis et al., 1974) and that provide a more complete detailed picture of the issues relevant to the isolation of the Urotensins. It is thus from this personal perspective that we will summarize the story of discovery that involves comparative observations involving fish, amphibians and mammals. The outcome of the work establishing a hormone family that, like the mermaid/merman, relates to the physiology of both humans and teleosts is far beyond what the original observations would have predicted. Thus, although we will present a succinct summary of the isolation, peptide chemistry, molecular pharmacology and physiology of the Urotensin I–CRF–Urocortin family and Urotensin II, we refer the reader to more comprehensive reviews of the subject for more detailed information (Davidson et al., 2009; Oki and Sasano, 2004; Hauger et al., 2003; Lu et al., 2008; Le Mével et al., 2008; Conlon, 2008 and see the May 2008 issue of Peptides, volume 29, devoted to Urotensin II topics). Further, although we will deal in brief with the co-discovery of Urotensin I and Urotensin II by the Bern and Lederis laboratories, in concert with the discovery of ovine CRF by the Vale laboratory, our focus will be primarily on Urotensin I. With due apologies to those also working on the biology and physiology of Urotensin I, this article, for historical purposes, focuses primarily on the seminal work in this area originating in the Lederis laboratory in Calgary and will not attempt to cover the expanding literature in this field. Issues relating to Urotensin II, which are summarized historically by Bern (2008), and which are described in broad detail by articles in the May 2008 volume 29 issue of Peptides, will be dealt with only briefly.
2. The mermaid factors: actions of teleost urophyseal principles in fish and mammals, their isolation and a debt to the isolation of the neurohypophyseal hormones, oxytocin and vasopressin
2.1. Parallels between mammalian and teleost neurosecretory gland ultrastructure point the way
The discovery of the Urotensins can be seen to stem from a striking morphological parallel between the ultrastructure of the mammalian neurohypophysis and the teleost caudal neurosecretory system (Bern and Takasugi, 1962; Basu et al., 1965; McCrohan et al., 2007). This homology would have been clear to Lederis, given his doctoral work on the ultrastructure of the mammalian neurohypophysis (Heller and Lederis, 1959; Lederis, 1963) and his work on the isolation of the neurohypophyseal hormone storage granules (Barer et al., 1963). In view of the expected parallels between the mammalian and teleost neurosecretory systems under discussion at the time, there is no doubt that Karl Lederis’s insight during his Bristol Pub discussions with Howard Bern led to his sabbatical in the Bern laboratory in Berkeley, working with the collected Gillichthys mirabilis urophyseal tissues that were available there. This brief sabbatical ultimately provided him with a unique research direction, distinct from that of his Doctoral thesis mentor, Hans Heller. There was thus an exchange plan proposed, with Bern having spent sabbatical time in Bristol and Lederis then planning his sabbatical in Berkeley. What Karl must have realized is that the urophyseal tissue, in contrast with the mammalian neurohypophysis, was relatively free of other ‘contaminating’ non-neural parenchymal epithelial cells, like those of the mammalian adenohypophysis that surrounds the neurohypophysis. Thus, a purification of the active principles from the caudal gland of teleosts would have appeared attractive. With Karl’s coming to Berkeley for his sabbatical in 1967–68, the stage was thus set for the discovery of the Urotensins.
2.2. Principles for the isolation of neuropeptides: oxytocin and vasopressin as a reference paradigm
In retrospect, the key elements that were ingrained in Bristol and that were to typify Karl’s work with the Urotensins, begun during his sabbatical in Berkeley, were the use of: (1) a classical method for the preservation (acetone-dried powder) and extraction of glandular tissue [mild 0.25% acetic acid or dilute HCl (0.1 N)], (2) a robust reliable bioassay for the identification and standardization of glandular extract activity (in Karl’s case, assays depending on the regulation of vascular and gut smooth muscle contractility) and (3) an anatomic approach, involving electron microscopy or immunohistochemistry to identify the site(s) of storage/synthesis of the active principles of the gland. All of these approaches were ongoing in the Heller laboratory in terms of the study of the storage and secretion of oxytocin and vasopressin from the posterior pituitary (Heller and Lederis, 1959; Barer et al., 1963). Thus, the paradigm for hormone discovery to be used for the Urotensins had already been clearly established for the identification of the vasoactive-hypertensive, galactogogic, oxytocic and antidiuretic principles present in extracts of the posterior pituitary gland, as begun by Oliver and Schäfer (1895). Paralleling the work of Oliver and Schäfer using the mammalian neurohypophysis, Sterba et al. (1965), Bern and colleagues (1967), Kobayashi and coworkers (1968) and Chan et al. (1969) noted vasoactive and other biological activities (contraction of hen oviduct, stimulation of rat uterus, toad water retention, elevation of eel blood pressure) in extracts of the fish urophysis, suggesting the presence of smooth muscle regulatory peptide factors possibly related to the vasoactive peptides, vasopressin and oxytocin. This peptide hypothesis was supported by the ability of trypsin and papain to abolish the eelpressor activity of the extracts, which mimicked the hypertensive action of angiotensin II in the eel (Chan et al., 1969). There are other marked parallels between the isolation of the Urotensins and the identification, purification and ultimate synthesis of vasopressin and oxytocin (Acher et al., 1958; Du Vigneaud et al., 1953, 1954). The parallels relate to issues of: (1) separating the two active principles by biochemical procedures, (2) obtaining sufficient amounts of pure peptides for sequencing and (3) ultimately, the challenge of synthesizing the active peptide hormones. Once synthesized, the key biological activities of the two neurohypophyseal cyclic nine-amino acid peptide hormones could be verified and their receptors identified. A similar path was followed for the isolation and characterization of the Urotensins and CRF. Fortunately, by the time the hunt was on for the Urotensins and CRF, methods for sensitive peptide sequencing and efficient solid phase peptide synthesis had improved considerably, compared with the time that Du Vigneaud and colleagues were wrestling with the posterior-pituitary peptides. Importantly, in parallel with his pursuit of the Urotensins, Karl also maintained his focus on the mammalian neurohypophysis, with an active research program directed at the anatomy and physiology of vasopressin and oxytocin in mammals. This complementary direction no doubt helped to inform his pharmacological studies of the Urotensins.
2.3. Isolating and sequencing the Urotensins in parallel with CRF
For the isolation of the Urotensins, the die was cast by Karl’s first manuscript dealing with the chromatographic separation of small amounts of vasopressin and oxytocin (Heller and Lederis, 1958). Coupled with efficient mild acid extraction procedures for acetone-dried urophyses (comparable to methods used to extract oxytocin and vasopressin from acetone-dried pituitaries), the smooth muscle/vascular activities in the fish gland extracts were used as a basis to define a ‘reference standard’ for the distinct activities in the urophyseal preparations (Bern and Lederis, 1969). The hypotensive/vasodilator activity was termed Urotensin I (UI), whilst the smooth muscle contracting activity was termed Urotensin II (UII). A separate principle in the extracts with a hydroosmotic effect, termed Urotensin IV, was ultimately identified as arginine vasotocin. An activity promoting sodium retention in fish (Maetz et al., 1964, termed Urotensin III), differing from the other teleost Urotensins, has not been identified. The chromatographic procedures that demonstrated the separation of UI from UII activity using biogel–P2 gel exclusion chromatography were monitored by the smooth muscle/vascular activities developed in the Lederis lab (Lederis, 1973; Zelnik and Lederis, 1973). That separation, linked to the previous data of Chan et al. (1969), was the first indication that UI and UII differed significantly in molecular size, with the larger UI activity eluted well before the smaller UII activity. At that time, the peptide nature of the Urotensins was established by demonstrating the reduction of the activity after digestion by trypsin. The earlier work of Chan et al. (1969) had already indicated that the eel-pressor activity in urophyseal extracts from the teleost, Mugilauratus, eluted in the excluded volume of a Sephadex G25 gel exclusion column, was trypsin–papain sensitive, but was not affected by thyoglycollate. In retrospect, the data of Chan et al. (1969) and Zelnik and Lederis (1973) indicated that UI and UII were very different peptides, both in terms of the larger molecular size of UI and in terms of their sensitivity towards reduction by thioglycollate, which does not affect UI, but abolishes the biological activity of UII by reducing its essential cysteine disulphide bond (see below). The systemic hypotensive/hindlimb vasodilatation assay was used by Zelnik and Lederis (1973) to detect UI activity in their gel filtration column fractions, whereas they used the trout rectum contraction/eel pressor assay to detect UII activity. It was thus the ‘mermaid (fish-mammal) principle’, in terms of the activity of the caudal gland extracts on both mammalian and teleost smooth muscle, that served as the essential compass for the isolation and sequencing of the Urotensins. Having established the presence of separate factors responsible for the UI/UII activity of the gland, Lederis and Bern ‘cut a deal’, agreeing that the Bern laboratory would pursue Urotensin II, whereas the Lederis laboratory would aim for the isolation of Urotensin I. As of 1973, the distinct biological actions and chemical properties of UI and UII were well understood by Lederis and Bern, and the hunt was on for the UI and UII sequences (Lederis et al., 1974).
2.3.1. Urotensin II
In terms of the challenge of isolating and sequencing the Urotensins, Bern won the toss of the coin, since Urotensin II, because of its shorter sequence (12 as opposed to 41 amino acids), turned out to be more amenable to isolation and sequencing (Pearson et al., 1980) than Urotensin I. The sequencing and synthesis of G. mirabilis UII verified that the smooth muscle activity of the urophysis extract was due to the dodecapeptide shown in Table 1, with a six amino acid cyclic structure generated by a disulphide bridge between the two key cysteines. When UII was sequenced, it was noted that there was an amino acid homology between UII and somatostatin-14 (Table 1: Pearson et al., 1980), particularly in terms of the FWK sequence present in the cyclic cystine disulphide- bonded domain of both peptides. Although the N-terminal Alanine–Glycine sequence of Gillichthys UII was the same as for human somatostatin-14, the N-terminal sequence of human UII differed, pointing to the essential nature of the FWK sequence in the cyclic 6-membered disulphide-bonded ring for the biological activity of UII. Comparative genomic studies now indicate a common ancestral gene for UII and the somatostatin/cortistatin genes (Tostivint et al., 2006). What was not commented upon at the time is the striking structural analogy between UII and the essential six amino acid cyclic conformation of both oxytocin and vasopressin (Table 1). Although very different in amino acid sequence from the cysteine-bonded ring of oxytocin and vasopressin, the size of the 6-member ring of UII was shown to be essential for its biological activity, as is the case for the cysteine-linked 6-membered ring of the neurohypophysial hormones. Thus, there was a significant biological echo between the fish hormone, UII, and the two mammalian post-pituitary peptide hormones, oxytocin and vasopressin, which are recognized in large part for their ability to regulate smooth muscle contractility, as are the Urotensins. With the availability of the synthetic peptide, it soon became apparent that UII, acting via its UT receptor (Ames et al., 1999), was responsible for triggering a wide variety of responses (Bern et al., 1985; summarized also by do Rego et al., 2008; and Le Mével et al., 2008), ranging from its recognized constriction of smooth muscle to endothelium-dependent vasorelaxation, inotropic actions in isolated cardiac tissues and behavioural effects. Given our focus on Urotensin I, as indicated above, we refer the reader to the more extensive information about UII to be found in the May 2008 volume 29 issue of Peptides, focused on Urotensin II topics (e.g. see Bern, 2008).
Table 1.
Sequences of Gillicthys mirabilis and human Urotensin II: relationships to oxytocin, vasopressin and somatostatin.a
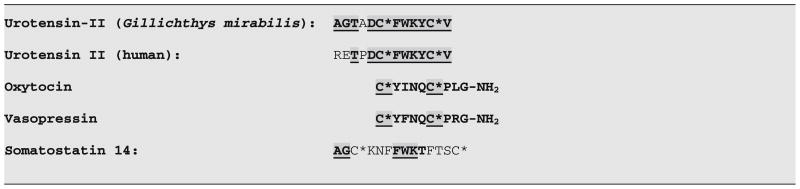
|
Sequence identities between Gillichthys mirabilis and human Urotensin II and either human somatostatin 14 or oxytocin and arginine vasopressin are indicated by the bold, grey-shaded underlined sequences. The homology between Gillichthys and human UII is denoted by amino acids represented in bold type in human UII. The cysteines (C*) are shown to be joined by a disulphide bridge (superscript asterisks), forming a 6-member ring in UII, oxytocin and vasopressin and a 12-member ring in somatostatin. Amino acids are designated by their one-letter codes (A = alanine, R = arginine, etc.).
2.3.2. Urotensin I and its relationship to sauvagine and corticotropin-releasing factor
Depending on its ability to decrease perfusion pressure in an isolated rat hind limb assay (Lederis and Medaković, 1974a), Urotensin I was isolated and sequenced from urophyseal extracts obtained from both sucker (Catostomus commersoni) and carp (Cyprinus carpio) tissues (Ichikawa et al., 1982; Lederis et al., 1982). Based on the sequence, the complete synthesis of the 4– 41 amino acid portion of UI (Lederis et al., 1983) established unequivocally that the vasorelaxant activity of urophyseal extracts (Muramatsu et al., 1981; Lederis and Medaković, 1974a) was due to UI (Tables 2 and 3). It is perhaps fitting that Lederis, always the avid fisherman, was responsible for organizing outings for the harvesting of the enormous number of urophyses (over 200,000 glands per preparation) that made the sequencing possible. These trips were usually festive ‘fishing expeditions’ (both scientifically and practically speaking), when the entire lab would spend a day or two at a time at a nearby reservoir collecting fish, isolating the urophyses and enjoying the camaraderie of ‘sport with a purpose’.
Table 2.
Sequence homologies between Urotensin I, CRF and sauvagine.a

|
Sequence identities between Catostomus Urotensin I and either human CRF or frog sauvagine are indicated by the bold, underlined shaded sequences. Additional amino acid identities amongst the three peptides are shown in bold upper case letters, shaded in grey, but not underlined. Amino acids are designated by their one-letter codes (A = alanine, R = arginine, etc.). UI and CRF are biologically active only when they are C-terminally amidated.
Table 3.
Sequence homologies between Urotensin I, CRF and the Urocortins.a

|
Sequence identities between Catostomus Urotensin I and one or more of the human CRF–Urocortin family are indicated by the bold, shaded underlined sequences. Additional amino acid identities between the CRF–Urocortin family peptides, not present in Urocortin I, are shown in bold upper case letters. Amino acids are designated by their one-letter codes (A = alanine, R = Arginine, etc.). Like UI and CRF, the Urocortins are active only when they are C-terminally amidated. Amongst the UI–CRF–Urocortin family, only four amino acids are completely conserved (S, D, A, N), as indicated by the four more heavily shaded underlined amino acids in the sequence of Urocortin 3.
What became apparent when the partial sequence of UI was in process was its striking relationship to the frog peptide, sauvagine (Table 2), that had already been sequenced as a ‘curiosity’ isolated from frog skin prior to the sequencing of either UI or UII (Montecucchi et al., 1979). It was in discussions between Karl Lederis and Wylie Vale immediately after a seminar at the University of Calgary in 1981, that the subject of the unusual smooth muscle activity of sauvagine came up. In an instant, when looking at the sauvagine sequence that they recognized in common, both individuals realized that they were in the process of sequencing a comparable peptide, one from fish and the other from sheep. Vale’s sequence of ovine CRF soon appeared (Spiess et al., 1981; Vale et al., 1981), followed shortly thereafter by the sequences of carp and sucker UI (Ichikawa et al., 1982; Lederis et al., 1982). By that time, use of the synthetic peptides had established that in common, UI has ACTH-releasing activity (Rivier et al., 1983); whilst CRF like UI exhibits vasoactive activity (MacCannell et al., 1982). More than a decade after the isolation and sequencing of UI and CRF, three other members of the CRF–Urocortin family have been identified: Urocortins 1, 2 and 3 (Table 3: Vaughan et al., 1995; Reyes et al., 2001; Lewis et al., 2001). All of the CRF–Urocortin family members can be seen to have sequence homology with UI (Table 3), but with only four amino acids in common amongst all of the sequences. UI can be seen to be most closely related to CRF and Urocortin 1, and most distantly related to Urocortin 3. To date, many actions of the CRF–Urocortin family have been observed in vivo and in vitro in a variety of test systems. In retrospect, the pharmacology of the UI peptide worked on in the Lederis laboratory can be seen to herald many of the more recent observations documented for the Urocortins (Oki and Sasano, 2004; Davidson et al., 2009).
3. Exploring the pharmacology of urotensin I in mammals and fish
3.1. Studies with purified UI
Even prior to the characterization of the structure of UI, gel filtration- isolated preparations of the peptide were of sufficient purity such that the pharmacological characterization of some of its activities in vivo and in vitro had commenced in mammals. In the mid 1970s, the Lederis lab reported further on the hypotensive action of UI by characterizing its actions on regional vascular beds and on heart rate in the rat (Lederis and Medaković, 1974a,b; Medaković and Lederis, 1975; Medaković et al., 1975a,b). A potential therapeutic value was noted, in that UI reduced the blood pressure of spontaneously hypertensive rats (Medaković and Lederis, 1975). In collaboration with the cardiovascular–gastrointestinal system pharmacologist and departmental colleague, Keith Mac- Cannell, Karl explored the effects of UI administered in vivo in dogs. The surprisingly vessel-selective and direct vasodilatation of the mesenteric artery bed, along with minimal cardiac activity, prompted them to suggest a possible therapeutic utility of UI in conditions where reductions in cardiac afterload are desired (MacCannell and Lederis, 1977; MacCannell et al., 1980). Initial attempts to characterize the cellular ‘second messengers’ for UI action were also initiated in the late 1970s, with the identification of elevated cyclic AMP as the likely mediator of the vasorelaxant activity of UI in the rat tail artery (Gerritsen and Lederis, 1979a,b). This result was obtained well in advance of the demonstration of the coupling of CRH receptors to Gs, thereby activating adenylyl cyclase (see below).
3.2. Studies with synthetic peptides
As outlined above, the sequencing of UI revealed its structural similarities to both frog sauvagine and mammalian CRH (Table 2). Subsequently, pharmacological studies in collaboration with the Salk investigators, who provided ample quantities of synthetic UI and CRF, focused on a comparative evaluation of the activities of each of these two related peptides. It became apparent that both mammalian CRF and teleost UI possessed similar vasodilatory properties, but with very different potencies (MacCannell et al., 1982, 1984). In retrospect, these findings can now be rationalized in terms of the distinct interactions of the peptides with the different CRF1 and CRF2 receptors (Hauger et al., 2003 and see below) now known to account for the biological effects. Correctly, the work suggested: (a) the existence of a UI-related peptide in the intestine (very likely one of the Urocortins) that acts to regulate intestinal blood flow and (b) the potential therapeutic utility of a UI-like peptide in treating non-occlusive ischaemia in human gastrointestinal disease (MacCannell et al., 1984).
3.3. Focusing on the central nervous system
Interestingly, in addition to the vascular actions that first characterized the actions of UI and sauvagine, these two peptides, like CRF, also show ACTH-releasing activity in rats, in accord with the structural homology between CRF and UI (Rivier et al., 1983: Table 2). This result prompted further studies that revealed a similar hypophysiotropic action of UI on the fish pituitary, suggesting that UI may serve as the corticotropin releasing hormone of fish (Fryer et al., 1983). This study of the action of UI on the teleost pituitary was followed by a continuing focus on the potential role of UI in the CNS. Subsequent to the development of a radioimmunoassay for UI (Suess et al., 1986), it was possible to demonstrate its presence not only in fish plasma, but also throughout the central nervous system in fish and mammals (Kobayashi et al., 1986). Thus, UI shared features that were beginning to be accepted as characteristic of most neurohypophyseal peptides in that they can act both as systemic circulating hormones and as localized neuromodulators. Drawing on his early experience as a neuroanatomist (Barer et al., 1963; Lederis, 1963), and his interactions with the comparative endocrinologist, Howard Bern, Karl turned to immunohistochemistry to localize UI, sauvagine and CRF in the nervous system of fish (Sakanaka et al., 1987a), amphibians (Gonzalez and Lederis, 1988), mammals (Sakanaka et al., 1987b) and even invertebrates such as Aplysia californica (Gonzalez et al., 1991). The work with Aplysia was due to the nearby location of a molluscophile colleague, Ken Lukowiak, who worked on the neural pathways in this slug involved in neuronal mechanisms of learning, memory formation and forgetting. Whether UI plays any role in such processes is an open question. Clearly in the fish brain, UI is synthesized, UI receptors are present and when administered centrally in vivo (intracerebroventricularly), UI can cause systemic effects such as increased ventilation (frequency and amplitude) and hypertension (Le Mével et al., 2009). The ubiquity of the UI/CRF/Urocortin family and the actions of the peptides in many phyla throughout evolutionary history, presumably via homologous receptors, are features shared by many of the peptides of the hypothalamo- neurohypophysial system.
4. Receptors for the UI/CRF/Urocorin family
4.1. CRF/UII receptors: CRF1, CRF2 and the actions of UI
Although the receptors responsible for the actions of UI and CRF were not known at the time the peptides were sequenced, it is now clear that the multiple activities of UI that had already been observed in a number of mammalian preparations (Lederis et al., 1985) are due to the activation of two homologous G-protein- coupled receptors, termed CRF1 and CRF2 (Hauger et al., 2003). There are three isoforms of CRF2 (CRF2a,2b,2c) that differ in their N-terminal extracellular sequences, but not in their extracellular loops, transmembrane domains and intracellular sequences that are responsible for ligand binding and signal transduction, respectively. The ability of CRF to release ACTH from pituitary corticotropes is due to the activation of CRF1, that is coupled primarily to Gs, resulting in an elevation of cyclic AMP. Other actions of CRF and UI can be due to interactions with CRF2, which is also a Gs-coupled receptor present in cardiovascular tissues and elsewhere. As mentioned above, the early work by Gerritsen and Lederis (1979b) with the rat tail artery in the Lederis lab pointed to a Gs-coupled receptor in vascular tissue that may well be CRF2. As is the case for most members of the 7-transmembrane G-protein-coupled receptor (GPCR) superfamily, CRF1 and CRF2 are now known to trigger multiple signalling pathways in addition to those activated by cyclic AMP. Thus, stimulation of CRF1 and CRF2 can activate p42/44 MAPkinase (ERK 1/2), possibly via an arrestin-mediated process. This activation of MAPkinase, as for other GPCRs, involves transactivation of the EGF receptor by a metalloproteinase-mediated mechanism (Markovic et al., 2008; Punn et al., 2006; Daub et al., 1996; Zwick et al., 1999). Thus, the signal pathways activated via the two CRF receptors can in principle differ, depending on the so-called signal trafficking that may result from the interactions of different agonists with the same CRF receptor (Kenakin, 2007). The diverse actions of UI, compared with other members of the CRF/Urocortin family, may now be rationalized by its ability to cause agonist-biased signalling (Kenakin, 2007) that is known to involve Gs-coupled GPCRs, like the beta1-adrenergic receptor (Galandrin et al., 2008) and may also apply to the Gs-coupled CRF receptors. In a sense, the distinct sequence of UI, compared with the other CRF-related peptides (Table 3) may confer on this fish peptide a unique ability to trigger either CRF1 or CRF2 in a way that produces a different signalling matrix in the target cells compared with the other peptides. Thus, in retrospect, the pharmacology of the UI peptide explored in the Lederis laboratory may harbour clues for the development of signal-selective ‘biased’ agonists for CRF1 and CRF2 that may be of future therapeutic utility.
4.2. Teleost neuropeptide receptors and their ligands: CRF1/CRF2 homologues and more
Although the molecular pharmacology of the mammalian CRF receptors is beginning to be understood, work with the teleost receptors is in its infancy. Here too, the Lederis laboratory, in collaboration with the Richter laboratory, has provided a basis for further work. Beginning in the 1980s, the Lederis-Richter team (Karl doing the fishing using nets, with the help of his long-time Calgary colleague, Henk Zwiers; Dietmar fishing with molecular probes) was able to clone and sequence a variety of peptide receptor and neuropeptide genes from teleosts, focusing not only on the teleost homologues of CRF1 and CRF2 (Pohl et al., 2001), but also on receptors for vasotocin (Mahlmann et al., 1994), isotocin (Hausmann et al., 1995), TRH (Harder et al., 2001) and opiates (Darlison et al., 1997). The cloning of the receptors from the teleost source took its place beside the parallel cloning of a number of neuropeptides (isotocin, vasotocin, CRF) (Okawara et al., 1988; Heierhorst et al., 1989; Figueroa et al., 1989). These studies revealed important aspects of the evolutionary history of the peptides.
5. Looking to the future with an eye on the past
5.1. Clues from the actions of UI previously described in vivo and in vitro
Like Janus, it will be fruitful for new work related to the UI/CRF/Urocortin family to look both forwards and backwards at the same time. We suggest that a re-examination of the pharmacological actions of UI and its related peptides, documented some time ago in both mammalian and non-mammalian systems, will provide clues for novel discoveries in the future. For instance, it is now realized that members of the CRF/Urocortin family can serve as autocrine/paracrine regulators via their synthesis and action at distinct anatomical sites (e.g. in the cardiovascular or gastrointestinal system) and that the anatomically restricted actions of the peptide family in mammals (e.g. selectively affecting the mesenteric circulation but not other vasculature) may be due to the site-selective expression of one or both of the CRF1/CRF2 receptors. A similar situation may occur in teleosts or invertebrates, like Aplysia, to confer distinct actions of the peptides in different locations. Thus, springing from older observations in the literature, many of which came from the Lederis laboratory, quite a number of new hypotheses can be put forward to rationalize at the molecular level, the observed physiological–pharmacological data obtained in vivo or in vitro.
5.2. Testing new hypotheses and looking to the future
The new hypotheses relevant to the unique physiology of the UI/CRF/Urocortin family can now be tested with new tools that were not available at the time UI and CRF were discovered. The approaches now available can include: (1) selective targeting of the deletion or over-expression of the peptides or their receptors in a zebrafish system (e.g. selective UI/UII downregulation with specific morpholinos) to test issues relevant to teleost development and physiology, (2) the use of a germ line or tissue-selective gene knockout or an si/shRNA message deletion approaches in mice or other mammalian systems to down-regulate one or other CRF receptor or activating peptide and (3) the use of cell and targeted in vivo receptor expression systems (see below). With current methods, the molecular targets can be restricted either to the peripheral or central nervous systems; or alternatively to selected organs like the gastrointestinal tract. In addition to the ‘molecular’ approaches, much also remains to be done to assess the critical pharmacophores in the different UI/CRF/Urocortin family members (Table 3) responsible for triggering the two CRF receptors via one or more of their G-protein signalling partners. For instance, cell expression systems will enable an understanding of the CRF1/CRF2 receptor dynamics (clustering, homo-heterodimerization/interalization/desensitization) and an analysis of the molecular pharmacology of their distinct signalling properties, furthering the work of the Grammatopoulos group (Markovic et al., 2008; Punn et al., 2006). As alluded to above, an understanding of the receptor- interacting side-chain pharmacophores may pave the way for developing signal-biased agonists or antagonists that can regulate the CRF receptors in a unique way. Such reagents, synthesised as peptide or non-peptide mimetic agonists/antagonists, may solve some of the riddles of the actions of a given UI/CRF/Urotensin peptide in a selected target tissue and may prove to be of future therapeutic utility.
5.3. Lessons re-learned: back to the future
The story of the isolation of the Urotensins amply reinforces the value of comparative endocrinology/pharmacology as an engine of discovery. By evaluating the pharmacological actions of the urophyseal extracts in a variety of species, including eels, frogs, fish and mammals, it became possible not only to devise a tractable bioassay leading to the isolation and sequencing of UI and UII but also to gain insight into aspects of receptor pharmacology that could not have been predicted by working with one species in isolation. The work also emphasizes the essential interaction for discovery between (1) an evaluation of the pharmacological properties of agents in tissues in vivo or in vitro and (2) the results of molecular studies done with expression systems and cloning strategies. Thus, it will be of considerable value to use current molecular techniques to ‘reinvent’ the traditional bioassay and developmental studies that have been so fruitful in furthering the discipline of molecular endocrinology to date. By going back to a more ‘traditional’ physiological–pharmacological approach to assess systemic aspects of the UI/CRF/Urocortin system, but with the use of recently accessible tools, one can predict a future of exciting and unexpected discoveries, heralded by the outcome of studying the actions of extracts of the teleost urophysis in multiple species.
Acknowledgments
Work in the authors’ laboratories, which is relevant to the actions of the UII–UI/CRF/Urocortin peptides, is supported in large part by operating grants from the Canadian Institutes of Health Research (formerly, the Medical Research Council (MRC) of Canada). The MRC of Canada also supported Karl Lederis as an MRC scientist throughout his career in Canada and provided the majority of funds used by his laboratory to identify, sequence and characterize the actions and anatomic distribution of Urotensin I in fish and mammals.
Footnotes
Abbreviations: Amino acids are designated by their one-letter codes (A alanine, R = arginine etc.); CRF, corticotropin-releasing factor (preferred nomenclature suggested by the International Union of Pharmacology (IUPHAR), also termed, corticotropin-releasing hormone or CRH; CRF1/CRF2, IUPHAR nomenclature for CRF receptors-1 and -2; CRH, corticotropin-releasing hormone; GPCR, G-protein-coupled receptor; UI, urotensin I; UII, urotensin II.
References
- Acher R, Light A, Du Vigneaud V. Purification of oxytocin and vasopressin by way of a protein complex. J Biol Chem. 1958;233:116–120. [PubMed] [Google Scholar]
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. (Erratum in: Nature 1999;402:898) [DOI] [PubMed] [Google Scholar]
- Barer R, Heller H, Lederis K. The isolation, identification and properties of the hormonal granules of the neurohypophysis. Proc R Soc Lond B Biol Sci. 1963;158:388–416. doi: 10.1098/rspb.1963.0054. [DOI] [PubMed] [Google Scholar]
- Basu J, Nandi J, Bern HA. The homolog of the pituitary–adrenocortical axis in the teleost fish Tilapia mossambica. J Exp Zool. 1965;159:347–355. doi: 10.1002/jez.1401590307. [DOI] [PubMed] [Google Scholar]
- Bern HA. From fish tail to human brain. Peptides. 2008;29:649–650. doi: 10.1016/j.peptides.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Bern HA, Lederis K. A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts. J Endocrinol. 1969;45(Suppl):xi–xii. [PubMed] [Google Scholar]
- Bern HA, Takasugi N. The caudal neurosecretory system of fishes. Gen Comp Endocrinol. 1962;2:96–110. doi: 10.1016/0016-6480(62)90032-1. [DOI] [PubMed] [Google Scholar]
- Bern HA, Nishioka RS, Chester Joines I, Chan DKO, Rankin JC, Ponniah S. J Endocrinol. 1967;37:xl–xli. [Google Scholar]
- Bern HA, Pearson D, Larson BA, Nishioka RS. Neurohormones from fish tails: the caudal neurosecretory system. I “Urophysiology” and the caudal neurosecretory system of fishes. Recent Prog Horm Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Chan DKO, Chester Jones I, Ponniah S. J Endocrinol. 1969;45:151–159. doi: 10.1677/joe.0.0450151. [DOI] [PubMed] [Google Scholar]
- Conlon JM. Liberation of urotensin II from the teleost urophysis: an historical overview. Peptides. 2008;29 (5):651–657. doi: 10.1016/j.peptides.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Darlison MG, Greten FR, Harvey RJ, Kreienkamp HJ, Stühmer T, Zwiers H, Lederis K, Richter D. Opioid receptors from a lower vertebrate (Catostomus commersoni): sequence, pharmacology, coupling to a G-protein-gated inward-rectifying potassium channel (GIRK1), and evolution. Proc Natl Acad Sci USA. 1997;94 (15):8214–8219. doi: 10.1073/pnas.94.15.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Rybka AE, Townsend PA. The powerful cardioprotective effects of urocortin and the corticotropin releasing hormone (CRH) family. Biochem Pharmacol. 2009;77:141–150. doi: 10.1016/j.bcp.2008.08.033. [DOI] [PubMed] [Google Scholar]
- do Rego JC, Leprince J, Scalbert E, Vaudry H, Costentin J. Behavioral actions of urotensin-II. Peptides. 2008;29:838–844. doi: 10.1016/j.peptides.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Du Vigneaud V, Ressler C, Swan CJM, Roberts CW, Katsoyannis PG. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J Am Chem Soc. 1953;75:4879–4880. [Google Scholar]
- Du Vigneaud V, Gish DT, Katsoyannis PG. A synthetic preparation possessing biological properties associated with argininevasopressin. J Am Chem Soc. 1954;76:4751–4752. [Google Scholar]
- Figueroa J, Morley SD, Heierhorst J, Krentler C, Lederis K, Richter D. Two isotocin genes are present in the white sucker Catostomus commersoni both lacking introns in their protein coding regions. EMBO J. 1989;8:2873–2877. doi: 10.1002/j.1460-2075.1989.tb08435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer J, Lederis K, Rivier J. Urotensin I, a CRF-like neuropeptide, stimulates acth release from the teleost pituitary. Endocrinology. 1983;113:2308–2310. doi: 10.1210/endo-113-6-2308. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bonin H, Ogawa K, Galés C, Bouvier M. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the beta1-adrenergic receptor. Mol Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Lederis K. Effects of urotensin I on the isolated rat tail artery. Pharmacology. 1979a;18:1872–1879. doi: 10.1159/000137233. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Lederis K. Urotensin I effects on intracellular content of cyclic AMP in the rat tail artery. Eur J Pharmacol. 1979b;60:211–220. doi: 10.1016/0014-2999(79)90220-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez GC, Lederis K. Sauvagine-like and corticotropin-releasing factor-like immunoreactivity in the brain of the bullfrog (Rana catesbeiana) Cell Tissue Res. 1988;253:29–37. doi: 10.1007/BF00221736. [DOI] [PubMed] [Google Scholar]
- Gonzalez GC, Martinez-Padron M, Ko D, Lukowiak K, Lederis K. Urotensin I-like immunoreactivity in the central nervous system of Aplysia californica. Peptides. 1991;12:787–793. doi: 10.1016/0196-9781(91)90134-b. [DOI] [PubMed] [Google Scholar]
- Harder S, Dammann O, Buck F, Zwiers H, Lederis K, Richter D, Bruhn TO. Cloning of two thyrotropin-releasing hormone receptor subtypes from a lower vertebrate (Catostomus commersoni): functional expression, gene structure, and evolution. Gen Comp Endocrinol. 2001;124:236–245. doi: 10.1006/gcen.2001.7709. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;2055:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Hausmann H, Meyerhof W, Zwiers H, Lederis K, Richter D. Teleost isotocin receptor: structure, functional expression, mRNA distribution and phylogeny. FEBS Lett. 1995;370 (3):227–230. doi: 10.1016/0014-5793(95)00832-t. [DOI] [PubMed] [Google Scholar]
- Heierhorst J, Morley SD, Figueroa J, Krentler C, Lederis K, Richter D. Vasotocin and isotocin precursors from the white sucker, Catostomus commersoni: cloning and sequence analysis of the cDNAs. Proc Natl Acad Sci USA. 1989;86:5242–5246. doi: 10.1073/pnas.86.14.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H, Lederis K. Paper chromatography of small amounts of vasopressins and oxytocins. Nature. 1958;182:1231–1232. doi: 10.1038/1821231a0. [DOI] [PubMed] [Google Scholar]
- Heller H, Lederis K. Maturation of the hypothalamoneurohypophysial system. J Physiol. 1959;147:299–314. doi: 10.1113/jphysiol.1959.sp006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Muramatsu I, Itoh H, Patel P, Yang SG, Lederis K. Contractile actions of epidermal growth factor-urogastrone in isolated smooth muscle preparations from guinea pig stomach: structure–activity relationships and comparison with the effects of human transforming growth factor-alpha. J Pharmacol Exp Ther. 1989;248:384–390. [PubMed] [Google Scholar]
- Ichikawa T, McMaster D, Lederis K, Kobayashi H. Isolation and amino acid sequence of urotensin I, a vasoactive and ACTH-releasing neuropeptide, from the carp (Cyprinus carpio) urophysis. Peptides. 1982;3:859–867. doi: 10.1016/0196-9781(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Masui T, Hirano T, Iwate T, Ishii S. Vasodepressor substance in the fish urophysis. Annot Zool Jpn. 1968;41:154–158. [Google Scholar]
- Kobayashi Y, Lederis K, Rivier J, Ko D, McMaster D, Poulin P. Radioimmunoassays for fish tail neuropeptides: II. Development of a specific and sensitive assay for and the occurrence of immunoreactive urotensin II in the central nervous system and blood of Catostomus commersoni. J Pharmacol Methods. 1986;15:321–333. doi: 10.1016/0160-5402(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Lederis K. A preliminary report on the ultrastructure of the human neurohypophysis. J Endocrinol. 1963;27:133–135. doi: 10.1677/joe.0.0270133. [DOI] [PubMed] [Google Scholar]
- Lederis K. Chemical and pharmacological properties of urotensin I, a long acting mammalian hypotensive peptide. Aca phyiol Latinoam. 1973;24:481–483. [PubMed] [Google Scholar]
- Lederis K, Bern HA, Medaković M, Chan DKO, Nishioka RS, Letter A, Swanson R, Gunther M, Tesanovic MM, Horne B. Recent functional studies on the caudal neurosecretory system of teleost fishes. Neurosecretion—The Final Neuroendocrine Pathway, VI International Symposium on Neurosecretion; London. 1973; Berlin, Heidelberg, New York: Springer-Verlag; 1974. [Google Scholar]
- Lederis K, Medaković M. Effects and assay of urotensin I on the perfused hind limb of the rat. Gen Comp Endocrinol. 1974a;24:10–16. doi: 10.1016/0016-6480(74)90136-1. [DOI] [PubMed] [Google Scholar]
- Lederis K, Medaković M. Pharmacological observations on the hypotensive action of extracts of teleost fish Urophyses (urotensin I) in the rat. Br J Pharmacol. 1974b;51:315–324. doi: 10.1111/j.1476-5381.1974.tb10665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederis K, Letter A, McMaster D, Moore G, Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982;218:162–165. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- Lederis K, Letter A, McMaster D, Ichikawa T, MacCannell KL, Kobayashi Y, Rivier J, Rivier C, Vale W, Fryer J. Isolation, analysis of structure, synthesis, and biological actions of urotensin I neuropeptides. Can J Biochem Cell Biol. 1983;61:602–614. doi: 10.1139/o83-076. [DOI] [PubMed] [Google Scholar]
- Lederis K, Fryer J, Rivier J, MacCannell KL, Kobayashi Y, Woo N, Wong KL. Neurohormones from fish tails. II: actions of urotensin I in mammals and fishes. Recent Prog Horm Res. 1985;41:553–576. [PubMed] [Google Scholar]
- LeMével JC, Lancien F, Mimassi N, Conlon JM. Central hyperventilatory action of the stress-related neurohormonal peptides, corticotropin-releasing factor and urotensin-I in the trout Oncorhynchus mykiss. Gen Comp Endocrinol. 2009;164:47–51. doi: 10.1016/j.ygcen.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Le Mével JC, Lancien F, Mimassi N, Leprince J, Conlon JM, Vaudry H. Central and peripheral cardiovascular, ventilatory, and motor effects of trout urotensin-II in the trout. Peptides. 2008;29:830–837. doi: 10.1016/j.peptides.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Abdel-Razik AE, Ashton N, Balment RJ. Urotensin II: lessons from comparative studies for general endocrinology. Gen Comp Endocrinol. 2008;157:14–20. doi: 10.1016/j.ygcen.2008.03.010. [DOI] [PubMed] [Google Scholar]
- MacCannell K, Lederis K. Dilatation of the mesenteric vascular bed of the dog produced by a peptide, urotensin I. J Pharmacol Exp Ther. 1977;203:38–46. [PubMed] [Google Scholar]
- MacCannell K, Giraud G, Lederis K, Groves G. Use of a specific mesenteric vasodilator peptide, urotensin I, to reduce afterload in the dog. Can J Physiol Pharmacol. 1980;58:1412–1417. doi: 10.1139/y80-214. [DOI] [PubMed] [Google Scholar]
- MacCannell KL, Lederis K, Hamilton PL, Rivier J. Amunine (ovine CRF), urotensin I and sauvagine, three structurally-related peptides, produce selective dilation of the mesenteric circulation. Pharmacology. 1982;25:116–120. doi: 10.1159/000137732. [DOI] [PubMed] [Google Scholar]
- MacCannell KL, Hamilton PL, Lederis K, Newton CA, Rivier J. Corticotropin releasing factor-like peptides produce selective dilatation of the dog mesenteric circulation. Gastroenterology. 1984;87:94–102. [PubMed] [Google Scholar]
- Maetz J, Bourguet J, Lahlouh B. Urophysis and osmoregulation in the carassius auratus. Gen Comp Endocrinol. 1964;17:401–414. doi: 10.1016/0016-6480(64)90007-3. [DOI] [PubMed] [Google Scholar]
- Mahlmann S, Meyerhof W, Hausmann H, Heierhorst J, Schönrock C, Zwiers H, Lederis K, Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc Natl Acad Sci USA. 1994;91:1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2beta endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol. 2008;22:689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrohan CR, Lu W, Brierley MJ, Dow L, Balment RJ. Fish caudal neurosecretory system: a model for the study of neuroendocrine secretion. Gen Comp Endocrinol. 2007;153:243–250. doi: 10.1016/j.ygcen.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Medaković M, Lederis K. Pharmacological effects of urotensins. IV Blood pressure-lowering effects of urotensin I in spontaneously hypertensive rats. Pharmacology. 1975;13:433–440. doi: 10.1159/000136935. [DOI] [PubMed] [Google Scholar]
- Medaković M, Chan DK, Lederis K. Pharmacological effects of urotensins. I Regional vascular effects of urotensins I and II in the rat. Pharmacology. 1975a;13:409–418. doi: 10.1159/000136932. [DOI] [PubMed] [Google Scholar]
- Medaković M, Chan DK, Lederis K. Pharmacological effects of urotensins. II. Renal effects of urotensin I in the rat. Pharmacology. 1975b;13:419–426. doi: 10.1159/000136933. [DOI] [PubMed] [Google Scholar]
- Montecucchi PC, Henschen A, Erspamer V. Structure of sauvagine, a vasoactive peptide from the skin of a frog. Hoppe-Seyler’s Z Physiol Chem. 1979;360:1178. [Google Scholar]
- Muramatsu I, Miura A, Fujiwara M, Lederis K. Rat isolated mesenteric artery: a sensitive preparation for the bioassay of urotensin I. Gen Comp Endocrinol. 1981;45:446–452. doi: 10.1016/0016-6480(81)90047-2. [DOI] [PubMed] [Google Scholar]
- Muramatsu I, Itoh H, Lederis K, Hollenberg MD. Distinctive actions of epidermal growth factor-urogastrone in isolated smooth muscle preparations from guinea pig stomach: differential inhibition by indomethacin. J Pharmacol Exp Ther. 1988;245:625–631. [PubMed] [Google Scholar]
- Okawara Y, Morley SD, Burzio LO, Zwiers H, Lederis K, Richter D. Cloning and sequence analysis of cDNA for corticotropin-releasing factor precursor from the teleost fish Catostomus commersoni. Proc Natl Acad Sci USA. 1988;85:8439–8443. doi: 10.1073/pnas.85.22.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25 (10):1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Oliver G, Schäfer EA. On the physiological action of extracts of pituitary body and certain other glandular organs: preliminary communication. J Physiol. 1895;18:277–279. doi: 10.1113/jphysiol.1895.sp000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman QJ, Veale WL, Lederis K. Central neurohypophyseal peptide pathways: interactions with endocrine and other autonomic functions. Peptides. 1982;3:515–520. doi: 10.1016/0196-9781(82)90118-8. [DOI] [PubMed] [Google Scholar]
- Pohl S, Darlison MG, Clarke WC, Lederis K, Richter D. Cloning and functional pharmacology of two corticotropin-releasing factor receptors from a teleost fish. Eur J Pharmacol. 2001;430:193–202. doi: 10.1016/s0014-2999(01)01391-7. [DOI] [PubMed] [Google Scholar]
- Poulin P, Lederis K, Pittman QJ. Subcellular localization and characterization of vasopressin binding sites in the ventral septal area, lateral septum, and hippocampus of the rat brain. J Neurochem. 1988;50:889–898. doi: 10.1111/j.1471-4159.1988.tb02996.x. [DOI] [PubMed] [Google Scholar]
- Punn A, Levine MA, Grammatopoulos DK. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol. 2006;20:3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Lederis K, Vale W. In vitro and in vivo ACTH-releasing activity of ovine CRF, sauvagine and urotensin I. Regul Pept. 1983;5:139–143. doi: 10.1016/0167-0115(83)90121-0. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, McMaster D, Chohan K, Shibasaki T, Stell WK, Lederis K. Urotensin I-like immunoreactivity in amacrine cells of the goldfish retina. Neurosci Lett. 1987a;76:96–100. doi: 10.1016/0304-3940(87)90199-6. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt–glucose oxidase–diaminobenzidine method. J Comp Neurol. 1987b;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Suess U, Lawrence J, Ko D, Lederis K. Radioimmunoassays for fish tail neuropeptides: I. Development of assay and measurement of immunoreactive urotensin I in Catostomus commersoni brain, pituitary, and plasma. J Pharmacol Methods. 1986;15:335–346. doi: 10.1016/0160-5402(86)90012-4. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterba G, Juppa H, Schuhmacher U. Untersuchungen zur Funktion des caudalen neurosekretorischen Systems beim Karpfen. Endokrinologie. 1965;48:25–39. (Referenced in Chan et al., 1969) [PubMed] [Google Scholar]
- Tostivint H, Joly L, Lihrmann I, Parmentier C, Lebon A, Morisson M, Calas A, Ekker M, Vaudry H. Comparative genomics provides evidence for close evolutionary relationships between the urotensin II and somatostatin gene families. Proc Natl Acad Sci USA. 2006;103:2237–2242. doi: 10.1073/pnas.0510700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Zelnik PR, Lederis K. Chromatographic separation of urotensins. Gen Comp Endocrinol. 1973;20:392–400. doi: 10.1016/0016-6480(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]


