Abstract
In magnocellular neurones of the supraoptic nucleus (SON), the neuropeptides vasopressin and oxytocin are synthesised and packaged into large dense-cored vesicles (LDCVs). These vesicles undergo regulated exocytosis from nerve terminals in the posterior pituitary gland and from somata/dendrites in the SON. Regulated exocytosis of LDCVs is considered to involve the soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor (SNARE) complex [comprising vesicle associated membrane protein 2 (VAMP-2), syntaxin-1 and soluble N-ethylmaleimide attachment protein-25 (SNAP-25)] and regulatory proteins [such as synaptotagmin-1, munc-18 and Ca2+-dependent activator protein for secretion (CAPS-1)]. Using fluorescent immunocytochemistry and confocal microscopy, in both oxytocin and vasopressin neurones, we observed VAMP-2, SNAP-25 and syntaxin-1-immunoreactivity in axon terminals. The somata and dendrites contained syntaxin-1 and other regulatory exocytosis proteins, including munc-18 and CAPS-1. However, the distribution of VAMP-2 and synaptotagmin-1 in the SON was limited to putative pre-synaptic contacts because they co-localised with synaptophysin (synaptic vesicle marker) and had no co-localisation with either oxytocin or vasopressin. SNAP-25 immunoreactivity in the SON was limited to glial cell processes and was not detected in oxytocin or vasopressin somata/dendrites. The present results indicate differences in the expression and localisation of exocytosis proteins between the axon terminals and somata/dendritic compartment. The absence of VAMP-2 and SNAP-25 immunoreactivity from the somata/dendrites suggests that there might be different SNARE protein isoforms expressed in these compartments. Alternatively, exocytosis of LDCVs from somata/dendrites may use a different mechanism from that described by the SNARE complex theory.
Keywords: hypothalamus, dendritic release, neuropeptides, SNARE
Magnocellular oxytocin and vasopressin neurones project axons to the posterior pituitary gland from which the peptides are released into the circulation, although they also release large amounts of the peptides from the somata and dendrites in the supraoptic (SON) and paraventricular nucleus (1). Dendritic peptide release is induced by a number of physiological stimuli (e.g. reproduction, osmotic challenges and stress) and, depending on the stimulus, electrical activity in the cell bodies can release peptides from nerve terminals in the posterior pituitary with little or no release from the dendrites. Furthermore, some stimuli induce peptide release from dendrites without increasing the electrical activity of the cell body, and without inducing secretion from nerve terminals (2, 3). The mechanisms of dendritic secretion are not well understood, although they could be attributed to different sets of exocytosis proteins to assure the specificity of vesicle fusion.
Electron microscopy revealed large dense-cored vesicles (LDCVs) in the somata and dendrites of magnocellular neurones, with LDCV-filled dilatations in the distal parts of the dendrites. The dendrites are a site of whole vesicle exocytosis, as first shown when tannic acid fixation of SON neurones revealed ‘omega’ fusion profiles at the plasma membrane (4). Conventional neurotransmitter release from axon terminals occurs at sites defined by active zones containing calcium channels (5), exocytosis proteins (6) and proteins such as bassoon and piccolo, which are part of the cytoskeletal matrix that is assembled at the pre-synapse (7, 8). However, there is no functional or morphological evidence for active zones in the somata, dendrites and axon terminals of vasopressin and oxytocin neurones (4, 9).
Regulated exocytosis of vesicles occurs through the interaction of a trio of essential proteins: vesicle associated membrane protein 2 (VAMP-2), syntaxin-1 and soluble N-ethylmaleimide attachment protein-25 (SNAP-25), which form a core complex known as the soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor (SNARE) complex. The SNARE complex interacts with a number of regulatory SNARE proteins such as synaptotagmin-1 and -7 and munc-13 and -18 and the Ca2+-dependent activator protein for secretion (CAPS-1) (10–15). α-SNAP mRNA was identified in dendrites from hypothalamic neurones (16), although the involvement of various core-complex and regulatory exocytosis proteins in the regulation of somato/dendritic peptide release is unknown. Sensitivity of somato/dendritic release to the neurotoxin tetanus toxin (which cleaves VAMP-2) was described in isolated magnocellular neurones (17), suggesting that VAMP-2 proteins similar to those operating in synapses may regulate dendritic exocytosis of oxytocin and vasopressin. Many SNARE proteins have been identified in the terminals of the posterior pituitary (9, 18–23). In the present study, we asked whether the patterns of immunoreactivity for SNARE and associated proteins in the SON considered to be required for exocytosis are different to those found in the posterior pituitary.
Materials and methods
All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated.
Animals
All procedures were approved by the UK Home Office under the Animals (Scientific Procedures) Act 1986, under a project license approved by the local Ethics Committee. Experiments conducted in Canada were performed according to protocols approved by the University of Calgary Animal Care and Use Committee in accordance with guidelines established by the Canadian Council on Animal Care. Only the minimum number of rats necessary to produce reliable scientific data was used. All Sprague–Dawley rats were housed under controlled conditions (12 : 12 h light/dark cycle at 21 °C) with access to food and water.
Slice preparation
The experiments were conducted on random cycling adult female rats (250– 300 g), lactating (days 8–11 after parturition) and post-natal day 8 rats. It is known that somato/dendritic peptide release is enhanced at the two latter states (24, 25). Rats were deeply anaesthetised (pentobartital, 120mg/100g BW by i.p. injection) then perfused through the ascending aorta first with heparin (5000 U/ml; 300 ml) in 0.9% saline solution followed by 300 ml of a 4% paraformaldehyde in 0.1M phosphate buffer (PB) (pH 7.4) solution. The brains were carefully removed and immersed overnight in a solution of 0.2% paraformaldehyde and 15% sucrose in 0.1M PB at 4 °C. The tissue was then placed in a solution of 30% sucrose in 0.1M PB and left until the tissue had sunk (usually 48 h). The tissue was then cut with a freezing microtome (40μm) and rinsed in 0.1M PB at −20 °C.
Single cell preparations
After decapitation of 1–2-month-old rats, each rat brain was rapidly removed and the SON dissected out and transferred to oxygenated Locke solution. Enzymatic dissociation was carried out by incubating the tissue pieces in oxygenated Locke buffer supplemented with 0.5 mg/ml of deoxyribonuclease I and 1 mg/ml protease × for 25 min at room temperature. This was followed by a further incubation in 0.5 mg/ml of deoxyribonuclease I and 1 mg/ml protease XIV for 25 min at room temperature. The tissue pieces were then rinsed twice with normal Locke solution and then maintained in oxygenated normal Locke solution at room temperature for at least 1 h until dissociated mechanically by gentle trituration. The resulting cell suspension was gently plated onto poly-L-lysine treated coverslips (13 mm diameter; Merck, Darmstadt, Germany), placed in multi-well plates. After allowing cells time to settle and attach, the cells were fixed for 30 min at room temperature in 4% paraformaldehyde in 0.1M PB. Cells were then rinsed and stored in 0.1M PB at 4 °C until used for immunocytochemical studies.
Immunocytochemistry
Sections or single cell preparations were first incubated for 30 min at room temperature in a blocking buffer consisting of 1% bovine serum albumin + Triton X-100 (0.1% for single cells and 0.2% for sections) in 0.1M PB with the exception of SNAP25 antibodies, which were processed in 0.01% Triton X-100. Sections or cells were then incubated for 60 min at room temperature then 48 h at 4°C with primary antibodies. After rinsing in 0.1M PB, sections or cells were incubated for 60 min at room temperature with biotinylated-anti-mouse or rabbit IgG (for SNARE or SNARE-associated proteins, dilution 1 : 500; Vector Laboratories, Inc., Burlingame, CA, USA), followed by 60 min at room temperature with Alexa 568-streptavidin conjugate (dilution 1 : 1000; Molecular Probes, Eugene, OR, USA) and anti-rabbit or mouse IgG Alexa 488 [for oxytocin, vasopressin, chromogranin A (CGA), luteinising hormone (LH) or glial fibrillary acidic protein (GFAP), dilution 1 : 500; Molecular Probes].
Both primary and secondary antibodies were diluted in blocking buffer. After further washing, the sections or cells were mounted in a Mowiol (Calbiochem, San Diego, CA, USA) mounting medium, supplemented with 2.5% 1,4-diazabicyclo[2.2.2]octane and fluorescence signals were observed with a Zeiss LSM510 Axiovert confocal laser scanning microscope equipped with argon/krypton lasers (Carl Zeiss, Oberkochen, Germany). Signals were acquired at 1024 × 1024 pixels, using a Zeiss Plan NeoFLUAR 1.4 NA × 63 oil-immersion objective. Scans were made at Nyquist sampling rates to allow deconvolution of images using Huygens software (Scientific Volume Imaging, Hilversum, The Netherlands) and the images were examined using IMAGEJ software (NIH, Bethesda, MD, USA). Emissions from each fluorophore were acquired consecutively to ensure no bleed through of one channel to another. No fluorescent labelling was detected when primary antibodies were omitted.
Table 1 shows a list of the antibodies used, including their sources and effective dilution. Omission of the primary antibodies resulted in no staining. Antibodies were chosen based on published studies and/or manufacturer’s description of specificity (based on abolition of immunostaining following immunogen pre-absorption or western blots). Where possible, we also performed our own control experiments to check primary antibody specificity by pre-incubating the primary antibodies in a five-fold (by concentration) excess of immunogen for 2 h at room temperature and then using this preadsorbed antibody solution as normal. In all cases, this pre-adsorption resulted in no positive immunohistochemical signal. Where the control immunogen was unavailable, we have relied on the suppliers’ descriptions of specificity. For positive controls, anterior pituitary sections were used. The anterior pituitaries were prepared and processed in the same way as the brain section immunohistochemistry, as described above, and were obtained from the same rats used to provide brain sections.
Table 1.
Details of Antibodies.
| Antigen | Dilution | Code, distributor | Host |
|---|---|---|---|
| VAMP-1 | 1 : 50 | 104 001, Synaptic Systems | Mouse |
| VAMP-2 | 1 : 2000 | VAS-SV006, Stressgen | Rabbit |
| VAMP-2 | 1 : 1000 | 104 211, Synaptic Systems | Mouse |
| VAMP-2 | 1 : 500 | V1389, Sigma-Aldrich | Rabbit |
| VAMP-3 | 1 : 100 | 104 102, Synaptic Systems | Rabbit |
| VAMP-4 | 1 : 100 | Ab3348, Abcam | Rabbit |
| SNAP-25 | 1 : 1000 | 567 343, Calbiochem | Rabbit |
| SNAP-25 | 1 : 2000 | VAM-SV012, Stressgen | Mouse |
| SNAP-25 | 1 : 2000 | S9684, Sigma-Aldrich | Rabbit |
| Syntaxin-1 | 1 : 200 | 110 302, Synaptic Systems | Rabbit |
| Munc-18 | 1 : 500 | 116 002, Synaptic systems | Rabbit |
| Munc-13 | 1 : 100 | 126 111, Synaptic Systems | Mouse |
| Synaptotagmin-1 | 1 : 1000 | AB5110, Millipore | Rabbit |
| Synaptotagmin-1 | 1 : 100 | 105 221, Synaptic Systems | Mouse |
| Synaptotagmin-7 | 1 : 200 | 105 172, Synaptic Systems | Rabbit |
| CAPS-1 | 1 : 200 | Kind gift of Professor Tom Martin (65) | Rabbit |
| Oxytocin | 1 : 500 | PS38, Kind gift of Professor Hal Gainer (66) | Mouse |
| Oxytocin | 1 : 200 | PC226L, Calbiochem | Rabbit |
| Vasopressin | 1 : 5000 | PS41, Kind gift of Professor Hal Gainer (66) | Mouse |
| Vasopressin | 1 : 400 | PC234L, Calbiochem | Rabbit |
| Chromogranin A+B | 1 : 100 | Ab8505, Abcam | Rabbit |
| LH-β | 1 : 200 | R23A, Kind gift of Professor Alan McNeilly (67) | Rabbit |
| Bassoon | 1 : 200 | 141 002, Synaptic systems | Rabbit |
| Synaptophysin | 1 : 1000 | 611 880, BD Biosciences | Mouse |
| GFAP | 1 : 1000 | IMG-5083A-1, IMGENEX | Rabbit |
| GFAP | 1 : 100 | IMG-5084A-1, IMGENEX | Mouse |
Manufacturers: Synaptic Systems: Goettingen, Germany; Stressgen: Enzo Life Sciences, Exeter, UK; Sigma-Aldrich: Dorset, UK; Abcam: Cambridge, UK; Calbiochem: Feltham, UK; Millipore: Watford, UK; BD Biosciences: Oxford, UK; IMGENEX: Upper Heyford, UK. Donors: Tom Martin: University of Wisconsin- Madison, Madison, WI, USA; Hal Gainer: NINDS Bethesda, MD, USA; Alan McNeilly: University of Edinburgh, Edinburgh, UK.
After titrating the antibody concentration and determining the conditions necessary for each antibody (e.g. concentration of Triton-X100 or need for visualisation with biotinylated secondary and streptavidin-fluorophore as opposed to secondary-fluorophore conjugate), each antibody was tested in sections from at least five rats, and three different single cell preparations.
Results
Immunohistochemistry for SNAREs and associated proteins in SON somata-dendrites and axon terminals of the posterior pituitary gland
VAMP-2
All the immunohistochemistry against the SNARE and regulatory proteins was conducted on labelled vasopressin and oxytocin cells, although examples are shown for one cell type only. Previous studies have demonstrated vasopressin and oxytocin are packaged in LDCVs (26) and immunoreactive signals are abundant in somata and dendrites of both isolated magnocellular neurones and in sections containing the SON and posterior pituitary. Because VAMP-2 is located in the secretory vesicle coat, we expected VAMP-2 to be located with peptide-containing LDCVs and therefore to observe co-localisation of each peptide and VAMP-2 signals. Unexpectedly, we did not detect VAMP-2 immunoreactivity within the somata or dendrites of oxytocin and vasopressin SON neurones in either sections or single cell preparations (Fig. 1). In the hypothalamic sections, VAMP-2 immunoreactivity was sufficiently heavy to outline the cells (Fig. 1A–F). In both hypothalamic sections (Fig. 1A–F) and acutely isolated magnocellular neurones (Fig. 1G–I), there was punctuate VAMP-2 immunoreactivity but only on the extracellular side of the plasma membrane of the oxytocin or vasopressin somata and dendrites. This extracellular VAMP-2 immunoreactivity was found to be co-localised with the synaptic vesicle marker synaptophysin (Fig. 1J–M), suggesting that these sites are probably pre-synaptic terminals. VAMP-2 immunoreactivity was present in the axon terminals of magnocellular neurons in the posterior pituitary (Fig. 1N–P).
Fig. 1.
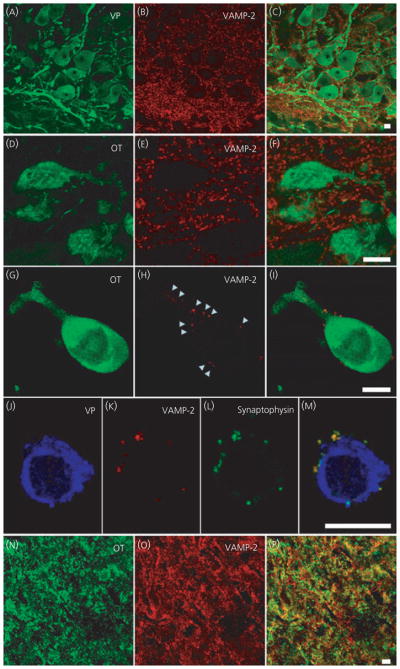
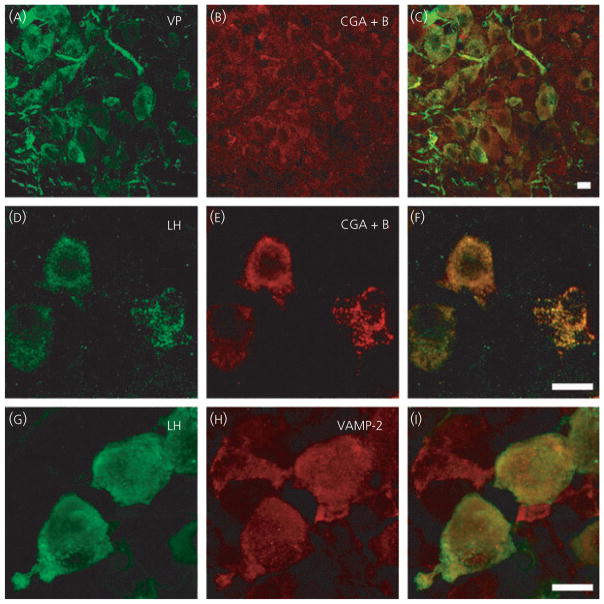
Vesicle associated membrane protein 2 (VAMP-2) immunoreactivity in supraoptic nucleus (SON) oxytocin (OT) and vasopressin (VP) neurones. Hypothalamic SON sections (A–F) and isolated neurones (G–M) show strong punctuate staining of VAMP-2 around VP and OT somata and dendrites; however, the overlays did not show co-localisation with the peptide. Labelling of VAMP-2 was seen on the outside of the plasma membrane of the neurones and co-localisation of VAMP-2 with synaptophysin (J–M) suggests labelling of pre-synaptic terminals. In the axon terminals in posterior pituitary (N–P), VAMP-2 is co-localised with the peptide, as shown by the yellow in the overlay (P). Arrowheads highlight examples of punctuate labeling around the outside an isolated cell. Scale bars = 10 μm.
To determine whether the lack of intracellular VAMP-2 immunoreactivity in magnocellular neurone somata and dendrites was the result of insufficient sensitivity of the immunohistochemistry, we examined sections from lactating and post-natal day 8 rats. These two physiological states have been demonstrated to be models of high somato/dendritic secretion (24, 25) and we anticipated a greater expression of proteins involved in release. However, we still did not observe intracellular VAMP-2 immunoreactivity in either oxytocin or vasopressin neurones (data not shown). We also confirmed that the technique, antibody and tissues used in the present study were capable of demonstrating intracellular VAMP-2 immunoreactivity co-incident with a peptide or LDCV marker. Using hypothalamic sections from the same rats, we found both peptides to be co-localised with the dense-cored vesicle marker chromogranin A+B (Fig. 2A–C). Moreover, in anterior pituitary sections from these rats, immunoreactivity for another peptide hormone, LH, showed co-localisation with both chromogranin A+B (Fig. 2D–F) and VAMP-2 immunoreactivity (Fig. 2G–I).
Fig. 2.
Positive controls for vesicle associated membrane protein 2 (VAMP-2) antibody and technique. Hypothalamic sections (A–C) show vasopressin (VP) and the large dense-cored vesicle protein chromogranin A (CGA)+B co-localised (C). In anterior pituitary sections (D–I), luteinising hormone (LH) is co-localised with both CGA+B and VAMP-2, as shown in the overlay images (F, I), demonstrating that the tissue and technique is not responsible for the absence of oxytocin and vasopressin co-localisation with VAMP-2 in cell somata and dendrites shown in previous figure. Scale bars = 10 μm.
Sections containing the SON and isolated SON neurones were probed with one of three different VAMP-2 antibodies and each of these was repeated at least three times (Table 1); all of which showed a consistent lack of intracellular immunoreactivity in somata and dendrites but a strong signal in putative pre-synaptic terminals on these cells.
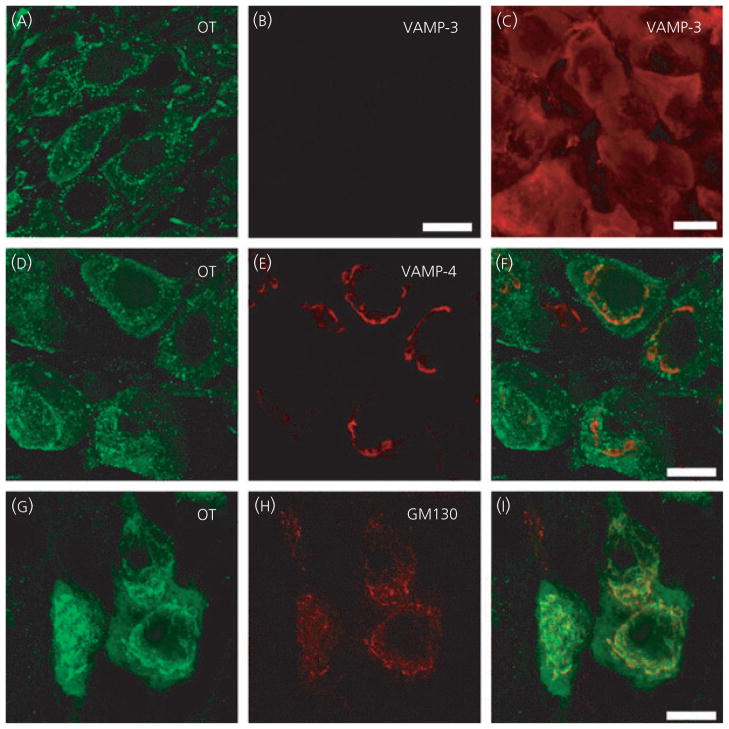
Because we showed that peptide release from SON was sensitive to tetanus toxin, which suggests a role of a toxin-sensitive member of the VAMP family in somato/dendritic release, we also examined antibodies against VAMP-1 and VAMP-3. There was no immunoreactivity for either VAMP-1 or VAMP-3 in the SON somata/dendrites (Fig. 3A–B; VAMP-1 not shown) or axon terminals in the posterior pituitary (data not shown). However, there was abundant VAMP-3 immunoreactivity in anterior pituitary sections from the same rat (Fig. 3C). Although both oxytocin and vasopressin somata were immunoreactive for VAMP-4 (shown with oxytocin immunostaining only; Fig. 3D–F), the VAMP-4 signal was exclusively localised to the perinuclear area and showed only partial co-localisation with the peptides in this area. If VAMP-4 was involved in formation of the SNARE complex and be involved in exocytosis, we would expect VAMP-4 to be co-localised with peptide signal throughout the somata and dendrites and to be located at the plasma membrane. VAMP-4 is considered to play a role in chaperoning and shuttling proteins around the Golgi apparatus and maturation of secretory vesicles (27), and the cell regions showing VAMP-4 immunoreactivity were similar to the regions immunoreactive for the Golgi marker GM130 (Fig. 3G–I).
Fig. 3.
Other members of the vesicle associated membrane protein (VAMP) family in supraoptic nucleus neurones. In hypothalamic sections (A–I), oxytocin neurones (OT) show no VAMP-3 immunoreactivity but there is abundant signal in anterior pituitary section cells (C). OT neurones show VAMP-4 immunoreactivity in the perinuclear region, although this is not co-localised with the oxytocin signal throughout the cytoplasm (D–F). The VAMP-4 immunoreactivity appears to be located in the same region labeled by the Golgi marker GM130 (G–I). Scale bars = 10 μm.
SNAP-25
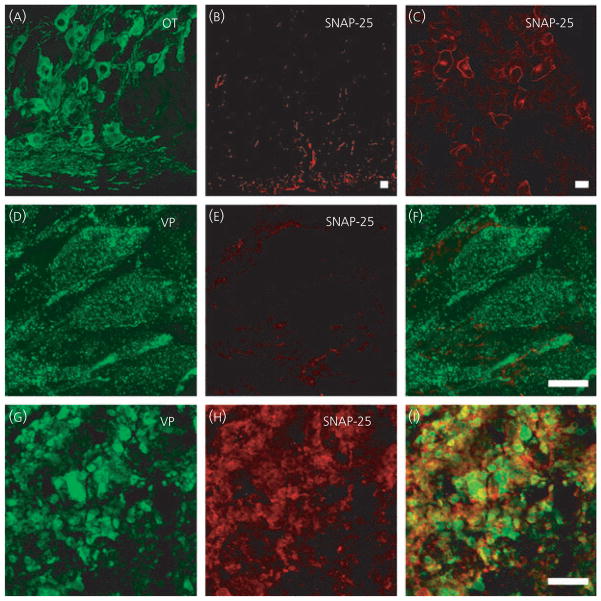
Previous studies have shown SNAP-25 to be localised to the cytosolic face of those regions of plasma membrane that are sites of secretion, such as the active zone of the synapse (28, 29). However, the release of oxytocin and vasopressin from somata and dendrites is not located to specific areas of the cell membrane or associated with anatomical structures such as active zones (4, 9). Consistent with this, we did not find immunoreactivity for the active zone marker Bassoon (data not shown). We found SNAP-25 immunoreactivity in the hypothalamic sections, particularly in the ventral part of the sections (Fig. 4B) in all three physiological states tested, although examination at high magnification showed no intracellular signal in either the oxytocin or vasopressin somata and dendrites (Fig. 4D–F). Instead, the SNAP-25 signal was found to be co-localised with the glial cell marker GFAP (data not shown). Again, this result was found in at least three trials of each of three different SNAP-25 antibodies (Table 1), in both hypothalamic and pituitary sections and fixed, isolated SON neurones. There was SNAP-25 immunoreactivity in the axon terminals of both oxytocin and vasopressin (Fig. 4G–I) neurones and strong immunoreactivity in the anterior pituitary (Fig. 4C).
Fig. 4.
Soluble N-ethylmaleimide attachment protein-25 (SNAP-25) in supraoptic nucleus neurones. SNAP-25 immunoreactivity is present in hypothalamic sections (A,B, D–F), but not in oxytocin (OT) or vasopressin (VP) neurones. In posterior pituitary sections (G–I), SNAP-25 is co-localised with the peptide and SNAP-25 immunoreactivity is shown in cells of the anterior pituitary (C). Scale bars = 10 μm.
Syntaxin-1 and munc18
Syntaxins are the third family of SNARE proteins considered to be essential for exocytosis in addition to VAMP-2 and SNAP-25 (29). Syntaxin-1 is the isoform predominantly associated with exocytosis and is primarily concentrated at sites of neurotransmitter release in neurones. Syntaxin-1 has also been found in the endoplasmic reticulum and on recycling organelles, including synaptic vesicles, and is considered to be involved in vesicle trafficking (30, 31). In the present study, syntaxin-1 immunoreactivity was found throughout the cytoplasm of somata and dendrites (Fig. 5A–C, D–F) and axon terminals of both oxytocin and vasopressin cells (Fig. 5G, I).
Fig. 5.
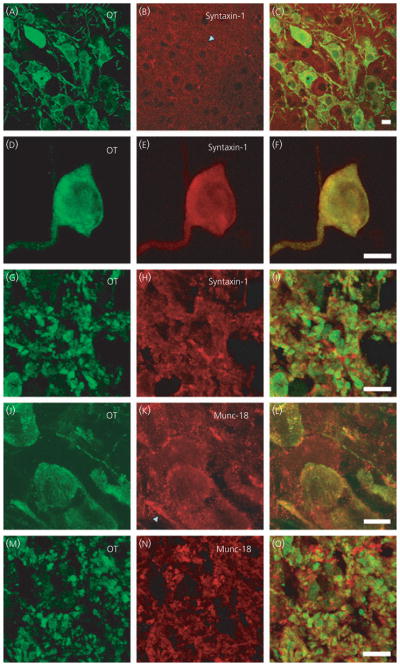
Syntaxin-1 and munc-18 in supraoptic nucleus (SON) neurones. Syntaxin-1 immunoreactivity is present in hypothalamic sections (A–C), isolated SON neurones (D–F) and sections of the posterior pituitary (G–I) and partially co-localised with the peptide in all compartments. In hypothalamic (J–L) and posterior pituitary sections (M–O), munc-18 is also present in somata, dendrites and axon terminals of the magnocellular neurones. Scale bars = 10 μm. OT, oxytocin.
Munc-18 is associated with the SNARE complex, in particular with syntaxin-1 (32, 33). By binding to syntaxin-1, munc-18 influences the stability of syntaxin-1 and conformation of the SNARE complex to influence if exocytosis will take place. We observed a pattern of expression of munc-18 similar to that for syntaxin-1 (Fig. 5J–O); both are distributed throughout the cytoplasm of oxytocin and vasopressin neurones somata and dendrites and axon terminals. We also used an antibody specific for munc-13, another regulator of syntaxin-1 shown to be involved in secretion of other peptides such as insulin (34, 35), but found no immunoreactivity in the hypothalamic or pituitary sections (data not shown).
Synaptotagmin-1 and CAPS-1
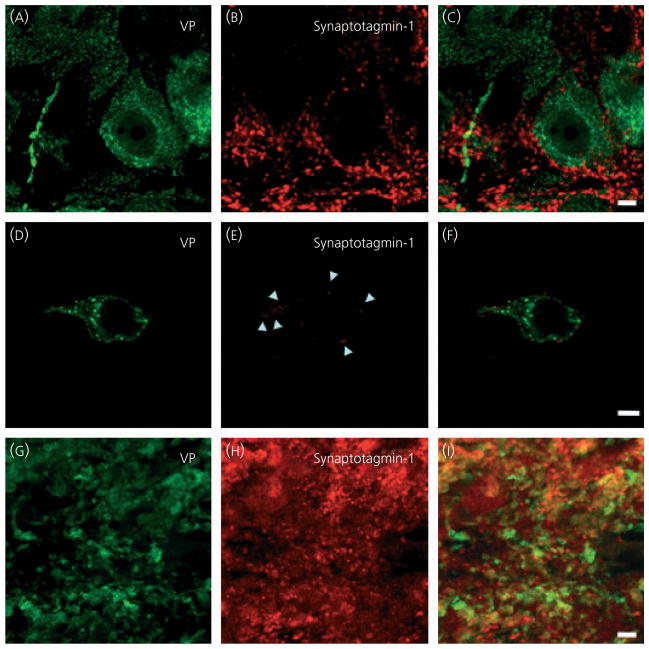
Synaptotagmin-1 is a member of the synaptotagmin family of which there are six members involved in protein trafficking. Synaptotagmin-1 is an integral membrane glycoprotein expressed in the coat of both synaptic vesicles and dense-cored vesicles (36). It has a cytoplasmic tail with two calcium-binding sites, and the calcium-dependence of exocytosis occurs in part via calcium binding to synaptotagmin- 1; thus, synaptotagmin-1 is proposed to be the calcium sensor for exocytosis (28, 37). Because somato/dendritic secretion of oxytocin and vasopressin is calcium-dependent (38, 39), we expected to observe immunoreactivity and co-localisation with the peptide in the somata and dendrites. Although the hypothalamic sections showed synaptotagmin-1 immunoreactivity, this was found only outside the plasma membrane of the neurones in a punctate pattern like VAMP-2 (Fig. 6). As with VAMP-2, we also saw co-localisation of synatotagmin-1 with synaptophysin (data not shown), suggesting the synaptagmin-1 signal was confined to pre-synapses on the plasma membrane of oxytocin and vasopressin somata and dendrites. We also used an antibody specific for synaptotagmin-7, another Ca2+-dependent regulator of exocytosis (40), but found no immunoreactivity in hypothalamic or pituitary sections (data not shown).
Fig. 6.
Synaptotagmin-1 in supraoptic nucleus (SON) neurones. In hypothalamic sections (A–C) and isolated SON neurones (D–F), synaptotagmin-1 immunoreactivity is present around, but not in magnocellular neurones. In posterior pituitary sections (G–I), synaptotagmin-1 is present in peptide immunoreactive axon terminals. Scale bars = 10 μm. VP, vasopressin.
Another protein proposed as a calcium sensor for vesicle (especially LDCV) exocytosis is CAPS (15, 41). Mammalian CAPS-1 has been described in the cytosol and plasma membrane as well as located on LDCVs but not synaptic vesicles (42, 43). We found abundant immunoreactivity for CAPS-1 in the cytoplasm of somata and dendrites in both oxytocin and vasopressin neurones, as well as the axon terminals in the posterior pituitary (Fig. 7). The patterns of immunoreactivity described above for syntaxin-1, munc-18, synaptotagmin- 1 and CAPS-1 was also found in lactating and PDN8 rats (data not shown).
Fig. 7.
Ca2+-dependent activator protein for secretion (CAPS-1) in supraoptic nucleus (SON) neurones. In hypothalamic sections (A–C) and isolated SON neurones (D–F), CAPS-1 immunoreactivity is present in magnocellular neurones (A, D). In posterior pituitary sections (G–I), CAPS-1 is also present in axon terminals. Scale bars = 10 μm. OT, oxytocin.
Discussion
Fusion of peptide-containing vesicles with the plasma membrane is promoted by the formation of a complex of vesicle and plasma membrane SNARE proteins, which is regulated by Ca2+ sensors. At neural synapses, the vesicle bound protein VAMP-2 forms a complex with the plasma membrane proteins SNAP-25 and syntaxin. However, although immunocytochemistry on SON and pituitary sections showed the presence of VAMP-2, SNAP-25 and syntaxin-1 in axon terminals, we found no immunoreactivity for VAMP-2 or SNAP-25 within the somata and dendrites of SON neurones. Our data suggest that different isoforms of these proteins may be utilised by somata/dendrites in the SON compared to the axon terminals in the posterior pituitary.
The detection of VAMP-2 in LDCV fractions of posterior pituitary homogenates (18, 22), the sensitivity of axon terminal exocytosis to botulinum toxin B (18) and tetanus toxin (44) strongly suggest the involvement of VAMP-2 in oxytocin and vasopressin release from this compartment. However, a previous study using electron microscopy demonstrated VAMP-2 immunoreactivity without significant association with LDCVs (9) in the axon terminals. In the present study, peptide immunoreactivity was co-localised with VAMP-2 immunoreactivity, although the abundance of peptides in the terminals meant we could not determine whether they were expressed in the same population of vesicles or in separate vesicles. Magnocellular neurones can sort and regulate transport of LDCVs to different compartments of the cell based on the vesicle contents (45), so it is possible that LDCVs expressing VAMP-2 are preferentially shuttled to the axon terminals.
The most unexpected findings of the present study were the absence of detectable VAMP-2 and SNAP-25 from the somato/dendritic compartment of magnocellular neurones. Being located in the LDCV membrane, we expected VAMP-2 to be co-localised with both oxytocin and vasopressin, as we found in the terminals of in the posterior pituitary. In the SON, the labelling pattern of VAMP-2 surrounded the dendrites and somata and resembles that of afferent pre-synaptic contacts. Similar patterns have previously been observed by others using VAMP-2 antibodies (46). Because VAMP proteins are considered to be essential components of the exocytosis fusion apparatus and somato/dendritic oxytocin release has been shown to be tetanus toxin sensitive (17), the lack of VAMP-2 (or VAMP-1 and VAMP-3) immunoreactivity suggests that magnocellular neurones use a different VAMP isoform for the release of LDCVs. VAMP-2 is the predominant VAMP isoform involved in exocytosis in the mammalian brain. The three VAMP-2 antibodies used in the present study were raised using synthetic peptides corresponding to residues 2–18, residues 2–27 (which are in the N-terminal located in the vesicle coat but exposed to the cytoplasm), and residues 36–56. Tetanus toxin requires residues 40–87 to be present to cleave VAMP-2 (47), such that any VAMP isoform sensitive to tetanus toxin would be expected to be recognised by the third VAMP-2 antibody.
Although, in the present study, neither VAMP-1, nor VAMP-3 were detected in magnocellular neurones, a previous study found both in the terminal homogenate but not in a LDCV fraction (18). VAMP-4 was not found in oxytocin and vasopressin neurones beyond the perinuclear space and this distribution is not compatible with a protein expressed in vesicle coats and involved in exocytosis at the plasma membrane. Instead, VAMP-4 is considered to play a role in trans-Golgi network to endosome transport and has been shown to form SNARE complexes with syntaxin-6 and -13 in early endosomes (48). Consistent with this, previous studies have found no evidence of VAMP-4 in the posterior pituitary (20, 49). Other candidates, such as VAMP-7 and VAMP-8, are considered to be tetanus toxin-insensitive (50, 51). Thus, VAMP-1, -3, -4, -7 and -8 are not viable substitutes for VAMP-2. To our knowledge, there are currently no other tetanus toxin-sensitive members of the VAMP family described in the literature.
The membrane-associated protein SNAP-25 was also absent in the dendrites and somata of SON neurones, indicating that a different isoform of SNAP-25 may be used in these neurones, although this has to be determined in more detail. SNAP-25 has been identified in dendrites from neonatal hypothalamic neurones (52) and dendrites of dopamine neurones of the substantia nigra (53). Although formerly thought to be restricted to exocytosis in non-neuronal cells, SNAP-23 is expressed in the brain (54) and a recent study has shown SNAP-23 in somata/dendrites of hippocampal neurones (55). In these neurones, SNAP-23 is involved in trafficking post-synaptic glutamate receptors. In pancreatic acinar cells, SNAP-23 can functionally substitute for SNAP-25 in the regulation of insulin release (56). Whether SNAP-23 (or different isoform of SNAP-25) is involved in somato/dendritic vasopressin and oxytocin release remains to be shown.
Syntaxin-1 labelling was found within dendrites and somata of SON neurones. Syntaxin-1 has been hypothesised to be the most important membrane SNARE (57), with a clearly defined location on the plasma membrane (58), and inhibition of its function causes a complete block of exocytosis, rather than just an impairment as seen with inhibition of SNAP-25 (59). Munc-18, known to be closely associated with syntaxin-1, was also detected in the somata and dendrites of SON neurones. In chromaffin cells, a lack of munc-18 blocks docking of LDCVs (60), whereas the over-expression of munc-18 in Drosophila inhibits exocytosis in a syntaxin dependent manner (61).
Synaptotagmin-1 immunoreactivity was not detected in dendrites or cell bodies of magnocellular neurones, although it is abundantly expressed in LDCVs of endocrine cells, such as PC12 cells (62), and has also been detected in dendrites of unidentified hypothalamic cells (52). Interestingly, a recent study of the expression of SNARE proteins in dendrites of dopamine neurones in the substantia nigra found no immunohistochemical signal for synaptotagmin-1 and synaptotagmin-2 (53), although there are reports of the involvement of synaptotagmin-4 and synaptotagmin-7 in somato/dendritic dopamine release (63). We found no immunoreactive signal for synaptotagmin-7 but did not test for synaptotagmin-4.
Regarding immunoreactivity for another calcium-sensing protein involved with exocytosis, CAPS-1 was present within and throughout the all compartments of SON neurones, consistent with reports in other endocrine and neuroendocrine cell types. In mammals, CAPS-1 is exclusively located on LDCVs in neural, neuroendocrine and endocrine tissues; however, it appears to be exclusively located on LDCVs. CAPS can bind to all three core SNARE proteins (64) and is considered to prime vesicle exocytosis (65).
Taken together, the data obtained in the present study suggest that the molecular machinery available for vasopressin and oxytocin release from the somata and dendrites of magnocellular SON neurones (Fig. 8B) differs substantially from the SNARE proteins used for the release of classic neurotransmitters at their synaptic terminals (Fig. 8A). It is tempting to speculate that magnocellular SON neurones produce multiple types of LDCV populations routed to different compartments of the cell. The absence of VAMP-2 and SNAP-25 immunoreactivity suggests that different SNARE protein isoforms might be expressed on LDCVs directed to the somata and dendrites. On the other hand, there may be only one type of LDCVs in all compartments that lacks VAMP-2 immunoreactivity and the VAMP-2 immunoreactivity is associated with other vesicle types, such as small electron-lucent vesicles (9). Alternatively, dendritic exocytosis may use a different mechanism from that described by the SNARE complex theory. However, this remains to be determined in more detail.
Fig. 8.
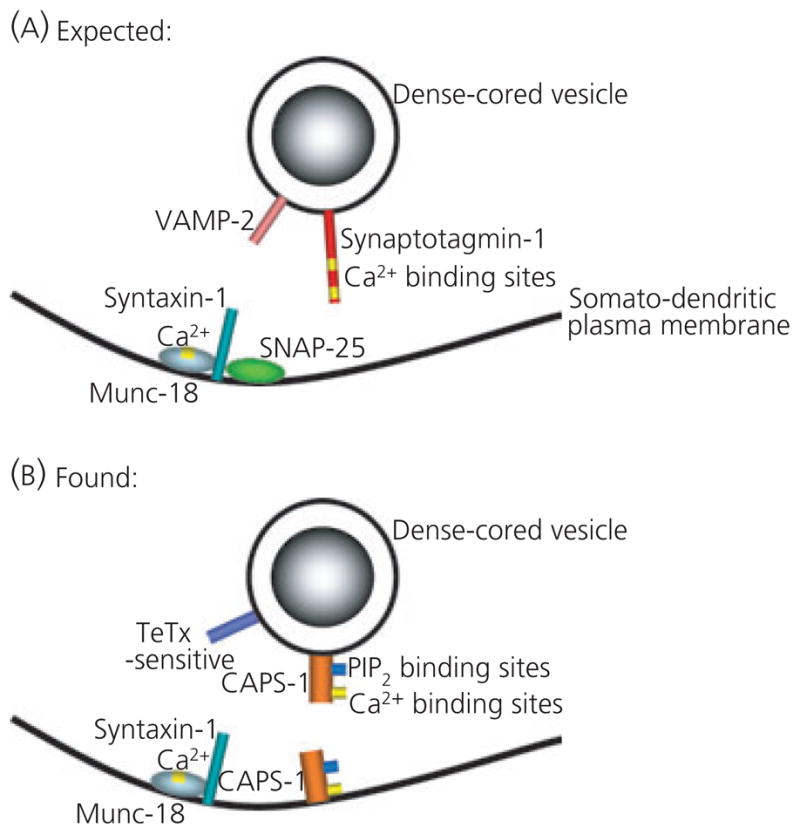
(A) Proposed soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor (SNARE) and SNARE-associated regulatory proteins involved in exocytosis in many cells types, including exocytosis from synapses and large dense-cored vesicle release from neuroendocrine cells (adapted from 10). (B) Composition of SNARE and SNARE-associated regulatory proteins that may be involved in somato/dendritic release of oxytocin and vasopressin in the supraoptic nucleus. CAPS-1, Ca2+-dependent activator protein for secretion; SNAP-25, soluble N-ethylmaleimide attachment protein-25; VAMP-2, vesicle associated membrane protein 2.
Acknowledgments
We thank Professor Gareth Leng for critically reading the manuscript and Ms Trudi Gillespie (IMPACT, University of Edinburgh) for technical assistance with the confocal microscopy. This study was supported by grants from the Biotechnology and Biological Sciences Research Council (M.L.), Canadian Institutes for Health Research (Q.J.P.) and MEXT Japan (T.O.). Q.J.P. is an Alberta Heritage Foundation for Medical Research Scientist.
References
- 1.Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 4.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 5.Llinás R, Sugimori M, Hillman DE, Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 6.Sudhof TC. The synaptic vesicle cycle. Ann Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 7.tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25:203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Volknandt W, Gundelfinger E, Zimmermann H. A comparison of synaptic protein localization in hippocampal mossy fiber terminals and neurosecretory endings of the neurohypophysis using the cryo-immunogold technique. J Neurocytol. 2000;29:19–30. doi: 10.1023/a:1007108012667. [DOI] [PubMed] [Google Scholar]
- 10.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 11.Montecucco C, Schiavo G, Pantano S. SNARE complexes and neuroexocytosis: how many, how close? Trends Biochem Sci. 2005;30:367–372. doi: 10.1016/j.tibs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen JB. SNARE complexes prepare for membrane fusion. Trends Neurosci. 2005;28:453–455. doi: 10.1016/j.tins.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Stojilkovic SS. Ca2+-regulated exocytosis and SNARE function. Trends Endocrinol Metab. 2005;16:81–83. doi: 10.1016/j.tem.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 15.Speidel D, Varoqueaux F, Enk C, Nojiri M, Grishanin RN, Martin TF, Hofmann K, Brose N, Reim K. A family of Ca2+-dependent activator proteins for secretion: comparative analysis of structure, expression, localization, and function. J Biol Chem. 2003;278:52802–52809. doi: 10.1074/jbc.M304727200. [DOI] [PubMed] [Google Scholar]
- 16.Morris JF, Christian H, Ma D, Wang H. Dendritic secretion of peptides from hypothalamic magnocellular neurosecretory neurones: a local dynamic control system and its functions. Exp Physiol. 2000;85:131S–138S. doi: 10.1111/j.1469-445x.2000.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 17.De Kock CPJ, Wierda KDB, Bosman LWJ, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurgutis P, Shuang RQ, Fletcher A, Stuenkel EL. Characterization and distribution of SNARE proteins at neuroendocrine nerve endings. Neuroendocrinology. 1996;64:379–392. doi: 10.1159/000127141. [DOI] [PubMed] [Google Scholar]
- 19.Walch-Solimena C, Takei K, Marek KL, Midyett K, Sudhof TC, De Camilli P, Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993;13:3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsson G, Meister B. Molecular components of the exocytotic machinery in the rat pituitary gland. Endocrinology. 1996;137:5344–5356. doi: 10.1210/endo.137.12.8940356. [DOI] [PubMed] [Google Scholar]
- 21.Navone F, Di Gioia G, Jahn R, Browning M, Greengard P, De Camilli P. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol. 1989;109:3425–3433. doi: 10.1083/jcb.109.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pupier S, Leveque C, Marqueze B, Kataoka M, Takahashi M, Seagar MJ. Cysteine string proteins associated with secretory granules of the rat neurohypophysis. J Neurosci. 1997;17:2722–2727. doi: 10.1523/JNEUROSCI.17-08-02722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Bhalla A, Dean C, Chapman ER, Jackson MB. Synaptotagmin IV: a multifunctional regulator of peptidergic nerve terminals. Nat Neurosci. 2009;12:163–171. doi: 10.1038/nn.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 25.Chevaleyre V, Dayanithi G, Moos FC, Desarmenien MG. Developmental regulation of a local positive autocontrol of supraoptic neurons. J Neurosci. 2000;20:5813–5819. doi: 10.1523/JNEUROSCI.20-15-05813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JF. Distribution of neurosecretory granules among the anatomical compartments of the neurosecretory processes of the pituitary gland: a quantitative ultrastructural approach to hormone storage in the neural lobe. J Endocrinol. 1976;68:225–234. doi: 10.1677/joe.0.0680225. [DOI] [PubMed] [Google Scholar]
- 27.Steegmaier M, Klumperman J, Foletti DL, Yoo JS, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1999;10:1957–1972. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahn R, Sudhof TC. Synaptic vesicles and exocytosis. Annu Rev Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- 29.Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- 30.von KK, Schmitz F. The expression pattern and assembly profile of synaptic membrane proteins in ribbon synapses of the developing mouse retina. Cell Tissue Res. 2003;311:159–173. doi: 10.1007/s00441-002-0674-0. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Arca S, Proux-Gillardeaux V, Alberts P, Louvard D, Galli T. Ectopic expression of syntaxin 1 in the ER redirects TI-VAMP- and cellubrevin- containing vesicles. J Cell Sci. 2003;116:2805–2816. doi: 10.1242/jcs.00467. [DOI] [PubMed] [Google Scholar]
- 32.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta-cell. J Biol Chem. 2000;275:41521–41527. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
- 34.Sheu L, Pasyk EA, Ji J, Huang X, Gao X, Varoqueaux F, Brose N, Gaisano HY. Regulation of insulin exocytosis by Munc13-1. J Biol Chem. 2003;278:27556–27563. doi: 10.1074/jbc.M303203200. [DOI] [PubMed] [Google Scholar]
- 35.Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large densecore vesicles in bovine chromaffin cells. EMBO J. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 37.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 38.Di Scala-Guenot D, Strosser MT, Richard P. Electrical stimulations of perifused magnocellular nuclei in vitro elicit Ca2+-dependent, tetrodotoxin- insensitive release of oxytocin and vasopressin. Neurosci Lett. 1987;76:209–214. doi: 10.1016/0304-3940(87)90717-8. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig M, Landgraf R. Does the release of vasopressin within the supraoptic nucleus of the rat brain depend upon changes in osmolality and Ca2+/K+? Brain Res. 1992;576:231–234. doi: 10.1016/0006-8993(92)90685-3. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda M, Ogata Y, Saegusa C, Kanno E, Mikoshiba K. Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem J. 2002;365:173–180. doi: 10.1042/BJ20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 42.Berwin B, Floor E, Martin TF. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:137–145. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 43.Grishanin RN, Klenchin VA, Loyet KM, Kowalchyk JA, Ann K, Martin TF. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J Biol Chem. 2002;277:22025–22034. doi: 10.1074/jbc.M201614200. [DOI] [PubMed] [Google Scholar]
- 44.Dayanithi G, Weller U, Ahnert-Hilger G, Link H, Nordmann JJ, Gratzl M. The light chain of tetanus toxin inhibits calcium-dependent vasopressin release from permeabilized nerve endings. Neuroscience. 1992;46:489–493. doi: 10.1016/0306-4522(92)90068-d. [DOI] [PubMed] [Google Scholar]
- 45.Landry M, Vila-Porcile E, Hoekfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–589. [PubMed] [Google Scholar]
- 46.Deleuze C, Alonso G, Lefevre IA, Duvoid-Guillou A, Hussy N. Extrasynaptic localization of glycine receptors in the rat supraoptic nucleus: further evidence for their involvement in glia-to-neuron communication. Neuroscience. 2005;133:175–183. doi: 10.1016/j.neuroscience.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Hall C, Barbieri JT. Substrate recognition of VAMP-2 by botulinum neurotoxin B and tetanus neurotoxin. J Biol Chem. 2008;283:21153–21159. doi: 10.1074/jbc.M800611200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc Natl Acad Sci USA. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsson G, Bean AJ, Meister B. Isoform-specific exocytotic protein mRNA expression in hypothalamic magnocellular neurons: regulation after osmotic challenge. Neuroendocrinology. 1999;70:392–401. doi: 10.1159/000054501. [DOI] [PubMed] [Google Scholar]
- 50.Coco S, Raposo G, Martinez S, Fontaine JJ, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T. Subcellular localization of tetanus neurotoxin- insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J Neurosci. 1999;19:9803–9812. doi: 10.1523/JNEUROSCI.19-22-09803.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- 52.Schwab Y, Mouton J, Chasserot-Golaz S, Marty I, Maulet Y, Jover E. Calcium- dependent translocation of synaptotagmin to the plasma membrane in the dendrites of developing neurones. Brain Res Mol Brain Res. 2001;96:1–13. doi: 10.1016/s0169-328x(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 53.Witkovsky P, Patel JC, Lee CR, Rice ME. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience. 2009;164:488–496. doi: 10.1016/j.neuroscience.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen D, Minger SL, Honer WG, Whiteheart SW. Organization of the secretory machinery in the rodent brain: distribution of the t-SNAREs, SNAP-25 and SNAP-23. Brain Res. 1999;831:11–24. doi: 10.1016/s0006-8993(99)01371-2. [DOI] [PubMed] [Google Scholar]
- 55.Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadoul K, Berger A, Niemann H, Weller U, Roche PA, Klip A, Trimble WS, Regazzi R, Catsicas S, Halban PA. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J Biol Chem. 1997;272:33023–33027. doi: 10.1074/jbc.272.52.33023. [DOI] [PubMed] [Google Scholar]
- 57.McMahon HT, Suedhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell SJ, Ryan TA. Munc18-dependent regulation of synaptic vesicle exocytosis by syntaxin-1A in hippocampal neurons. Neuropharmacology. 2005;48:372–380. doi: 10.1016/j.neuropharm.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 59.Graham ME, Barclay JW, Burgoyne RD. Syntaxin/Munc18 interactions in the late events during vesicle fusion and release in exocytosis. J Biol Chem. 2004;279:32751–32760. doi: 10.1074/jbc.M400827200. [DOI] [PubMed] [Google Scholar]
- 60.Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–592. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 61.Wu MN, Schulze KL, Lloyd TE, Bellen HJ. The ROP-syntaxin interaction inhibits neurotransmitter release. Eur J Cell Biol. 2001;80:196–199. doi: 10.1078/0171-9335-00143. [DOI] [PubMed] [Google Scholar]
- 62.Shin OH, Rizo J, Sudhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- 63.Mendez JA, Bourque MJ, Fasano C, Kortleven C, Trudeau LE. Somatodendritic dopamine release requires synaptotagmin 4 and 7 and the participation of voltage-gated calcium channels. J Biol Chem. 2011;286:23928–23937. doi: 10.1074/jbc.M111.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daily NJ, Boswell KL, James DJ, Martin TF. Novel interactions of CAPS (Ca2+-dependent activator protein for secretion) with the three neuronal SNARE proteins required for vesicle fusion. J Biol Chem. 2010;285:35320–35329. doi: 10.1074/jbc.M110.145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wassenberg JJ, Martin TF. Role of CAPS in dense-core vesicle exocytosis. Ann NY Acad Sci. 2002;971:201–209. doi: 10.1111/j.1749-6632.2002.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNeilly AS, Jonassen JA, Fraser HM. Suppression of follicular development after chronic LHRH immunoneutralization in the ewe. J Reprod Fertil. 1986;76:481–490. doi: 10.1530/jrf.0.0760481. [DOI] [PubMed] [Google Scholar]