Abstract
Amyloid-β protein (Aβ) accumulation is one of the major hallmarks of Alzheimer’s disease (AD) and plays a crucial role in its pathogenesis. Cellular models whereby amyloid precursor protein (APP) is highly expressed are commonly used to test the efficacy of novel neuroprotective compounds. In addition to Aβ, it is known that mutation in the protein presenilin contributes to early onset AD. Recently, a cellular neuroblastoma model where both APP and presenilin are expressed has become available. Since protective effects of nicotine against various neurotoxins have been observed, this study was designed to determine whether nicotine would also protect against cellular damage induced by APP or APP and presenilin. Wild type neuroblastoma (N2a) cell line, and those transfected with amyloid precursor protein (APP), and the combination of APP and presenilin were pretreated with various concentrations of nicotine and the survivability of the cells were determined by MTT assay. Nicotine dose dependently provided protection against cellular loss in all cell lines, with highest protection in the double transfected (44%) followed by single transfected (30%), and wild type (21%). The effects of nicotine in turn were blocked by mecamylamine, a non-selective nicotinic antagonist. These results suggest differential sensitivity of cell lines representing AD pathology to the protective effects of nicotine and provide further support of therapeutic potential of nicotinic agonists in at least a subtype of AD patients.
Keywords: Nicotine, Neuroprotection, Beta Amyloid, APP, Presenilin, N2a Cells, Alzheimer’s Disease
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia, is marked by the occurrence of intracellular neurofibrillary tangles as well as the formation of amyloid plaques [2,4,18]. Although the current treatments of AD, including acetyl cholinesterase inhibitors, have been shown to cause symptomatic relief, they have not been shown to significantly slow the progression of this disease [19,27,32,33]. Consequently, there is a compelling need to find an effective treatment to not only reduce the symptoms associated with AD but also significantly slow its progression.
One of the major limitations with AD research is the availability of effective models whereby major contributory factors are co-expressed. Recently, neuroblastoma cells expressing excessive amyloid precursor protein (APP), as well as APP and presenilin, two important pathological components of AD, have become available. Mutated presenilin protein, by disrupting the processing of amyloid precursor protein (APP), can lead to overproduction of Aβ and is hence implicated in early onset AD [15].
Numerous in-vitro and in-vivo studies have provided evidence for neuroprotective effects of nicotine against various neuronal toxins [5,8,13,23,24,25,31]. Indeed, preclinical studies have suggested that nicotine could have therapeutic benefits in AD by reducing Aβ levels in mouse and rat models [17,29]. It has been suggested that nicotine activates non-amyloidogenic pathway of APP processing that in addition to attenuation of Aβ toxicity results in the release of a large soluble fragment with a range of trophic and protective functions [28, 35].
A major goal of this study was to determine whether nicotine might also protect against cellular toxicity induced by high expression of APP and a combination of APP and presenilin, and whether such effects of nicotine were mediated via nicotinic receptors.
2. Methods
2.1 Drugs
Nicotine bitartrate and Mecamylamine HCl along with analytical reagents were purchased from Sigma Chemical Company (Sigma-Aldrich, St. Louis, MO).
2.2 Cell Culture
To test the possible protective effect of nicotine on cell lines expressing APP and presenilin, the wild type (N2a), single transgenic (N2a-APP695) and the double transgenic (N2a-APPSweΔ9PS1) cell lines were used. Neuroblastoma 2A (N2a) is a mouse neural crest-derived cell line that is widely used for studying neuronal differentiation and axonal growth. The N2a-APP695 is the N2a cell transfected with APP of 695 amino acid residues while the (N2a-APPSweΔ9PS1) is the N2a cells expressing the Swedish mutant gene APP695 and mutant presenilin (PS1 Δ9). The cell lines were generously provided by Dr. Huaxi Xu of Burnham Institute for Medical Research. The cells were cultured in medium containing 57% DMEM containing 15mM HEPES, L-glutamine and sodium bicarbonate with pyridoxine, 47% Opti-MEM, 5% FBS, 1% pen/strep as well as gentamicin. The cells were harvested approximately 4 days later when confluent and plated in a 96 well plate (1.2 × 104/well). Cells were allowed to adhere to plate for 24 hrs.
2.3 Natural Cell Progression
To test the natural progression or survivability of each cell line, MTT assay, described below, was performed to measure cell viability every 24 h for up to 72 h. Initially, approximately 12,000 cells were plated in each well of a 96 well plate and the remaining viable cells at each time point are expressed as percent viability.
2.4 Nicotine Treatment
To test possible protective effects of nicotine in all cell lines, various concentrations of nicotine (freshly prepared) were added to each well. Controls received buffer only. Since cell viability for all cell lines was lowest at 72 h, the effects of nicotine were determined at this time point.
2.5 Pre-Treatment with mecamylamine
To determine possible involvement of nicotinic receptors in action of nicotine, various concentrations of mecamylamine, a non-selective nicotinic receptor antagonist were added 2 h prior to nicotine treatment. Again, cell viability was determined after 72 h.
2.6 MTT Assay
Cell viability was determined by 3, (4,5-diamethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Thus, 30 ul of the MTT solution was added to each well and incubated at 37°C for 3 h. After 3 h, the wells were aspirated and the plates were left to dry overnight. The next day, 50 ul of dimethyl sulfoxide (DMSO) was added to each well to solubilize the formazan crystals. In this assay, the live cells reduce the yellow salt of the MTT solution to an insoluble purple (formazan crystal). The plates were then put on a shaker for 1 h and read spectrophotometrically at 570nm in a plate reader. The data was then analyzed and is represented as percent cell viability.
2.7 Statistical Analysis
Statistical difference between treatment groups was determined by two-way ANOVA followed by post-hoc Tukey comparison test to determine which groups differed. Significant difference was considered a priori at p < 0.05. Data was analyzed using Graphpad Prism 3 (San Diego, CA), and is expressed as mean ± SEM.
3. Results
3.1. Cellular Viability
Figure 1 depicts time-dependent cellular viability after 24, 48 and 72 h for N2a-APP695, N2a-APPSweΔ9PS1 and wild type N2a cell. All cell lines showed a time-dependent reduction in viability F(3,6) = 19.03, p<0.001. However, the N2a wild type cells had the least cell loss compared to singly transfected cells (N2a-APP695) and doubly transfected cells (N2a-APPSweΔ9PS1). Thus, N2a cells had 11% cell loss after 24 h and 29% after 72 h, whereas N2a-APP695 cells had 13% cell loss after 24 h and 36% after 72 h and the N2a-APPSweΔ9PS1 cells had 16% cell loss after 24 h and 45% after 72 h. Based on these results, we chose the 72 h period to test protective potentials of nicotine.
Figure 1.
Natural cell progression of N2a, N2a-APP695 and N2a-APPSweΔ9PS1 cells after 24, 48 and 72 hours. Results represent mean ± SEM of three independent experiments.. * P<0.05 **P<0.01, ***P<0.001 compared to control
3.2. Nicotine Effect
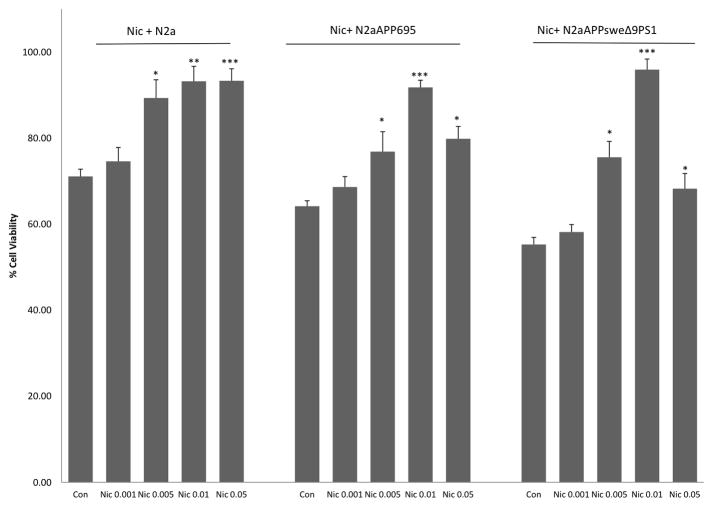
Figure 2 depicts the effects of various concentrations of nicotine on cellular viability in all cell lines. Nicotine provided a dose-dependent protection in all cell lines F(4,8) = 15.90, p<0.01. However, the effects of nicotine were more pronounced in the N2a-APPSweΔ9PS1cells compared to N2a-APP695 cells, and N2a cells. Thus, 0.01 uM nicotine resulted in approx 44% protection in N2a-APPSweΔ9PS1cells compared to approx 30% in N2a-APP695 cells, and approx 21% in N2a cells. This differential effects of nicotine in various cell lines is most likely due to differences in cellular viability between the various cell lines. Moreover, a likely ceiling effect of nicotine is also a contributing factor as the higher nicotine concentration of 0.05 uM was less effective that that of 0.01 uM in at least the single and the double transfected cell lines. Since 0.01 uM nicotine resulted in maximal protection in all cell lines, this concentration was used to determine the effect of mecamylamine pretreatment.
Figure 2.
Effect of nicotine (uM) in N2a, N2a-APP695 and N2a-APPSweΔ9PS1cells. MTT Assay was performed 72h following nicotine pre-treatment. Values are mean ± SEM, n=5. *P<0.05 ** P<0.01 compared to control
3.3. Mecamylamine Effect
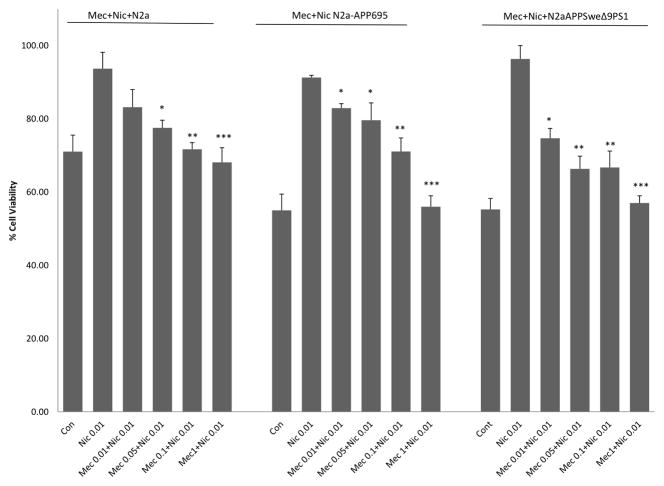
Figure 3 depicts the effects of various concentrations of mecamylamine applied prior to nicotine (0.01uM) in all cell lines. Mecamylamine pre-treatment dose-dependently blocked the effect of nicotine in all cell lines. However, higher concentration of mecamylamine (1.0 uM) was necessary to block the effects of nicotine in N2a-APP695 or N2a-APPSweΔ9PS1, compared to 0.1 uM mecamylamine necessary to block nicotine’s effect in N2a cells. None of the concentrations of mecamylamine alone had any effect in any cell line (data not shown).
Figure 3.
Percent cell viability of mecamylamine + nicotine pre-treatment in N2a, N2a-APP695 and N2a-APPSweΔ9PS1 cells. Mecamylamine (uM) was added 2 hrs before nicotine (0.01 uM) treatment. MTT Assay was performed 72h following nicotine pre-treatment. Values are mean ± SEM, n=5. * P<0.05 **P<0.01, ***P<0.001 compared to nicotine 0.01uM.
4. Discussion
The results of the current study indicate that cells transfected with APP or a combination of APP and presinilin show significant mortality in 72 h period compared to the wild type N2a cells. The doubly transfected cells had the highest mortality suggesting that APP and presenilin together can exacerabte cellular damage or death. Moreover, pretreatment of all cells including the wild type with nicotine, resulted in a dose-dependent protection. Interestingly, doubly transfected cells showed the highest protection by nicotine. Since presenilin has been implicated in early onset AD [15], it may be suggested that nicotine could be of particular benefit in such cases.
The results also strongly implicate involvement of nicotinic receptors in protective effects of nicotine as the effects of nicotine were blocked by the non-selective nicotinic receptor antagonist, mecamylamine. Thus, beyond nicotine, nicotinic agonists could also be of therapeutic potential. Although our results do not allow for specific nicotinic receptor subtype involvement, previous studies utilizing various cell lines including primary and transfected cells have implicated both alpha4-beta2 and homomeric alpha7 nicotinic receptor subtypes in protective effects of nicotine against Aβ [12,13,16,20,34]. The effects of nicotine appear to be at least partially mediated through inhibition of both caspase-dependent and independent apoptosis [34]. An inhibition of nicotine on nitric oxide production has also been reported [30].
A major target of ACh, a primary neurotranmsitter affected in AD, is the nicotinic receptors whose role in cognitive functions is well established [3,11,12,21,26]. Indeed, AD is associated with a marked reduction of nicotinic acetylcholine receptors in key brain regions such as the cerebral cortex and hippocampus [10,14,21]. This loss compounded by the loss of cholinergic cells, is believed to contribute significantly to the cognitive dysfunction in AD [33]. Current therapeutic interventions involving acetyl cholinesterase inhibitors (AChEI) are intended to boost the synaptic availability of ACh in the brain [3,4]. However, as mentioned earlier this strategy has only had a modest effect [19,27,32,33]. Interestingly, it has been demonstrated that ACh or cholinergic neurotransmission are a major target for Aβ-induced damage [3]. Moreover, direct interactions between Aβ and nicotinic receptors have also been noted [10]. Thus, a strategy, whereby the damaging effects of Aβ can be prevented or limited, while simultaneously enhancing nicotinic receptor mediated neurotransmission, may offer a significantly more advantageous approach in AD treatment.
It should be noted that in addition to the established role of APP and the specific involvement of Aβ in formation of plaques, AD pathology also involves phosphorylation of tau protein and the formation of neuronal tangles [21]. Preliminary studies looking at the interaction of nicotine with tau protein and tangle formation have yielded conflicting results [1,6,9,21]. Although some studies have shown that chronic nicotine administration in a mouse model of AD exacerbated tau pathology [6,21], a recent study utilizing selective alpha7 agonist has shown the opposite effect [1]. Thus, it will be of significant interest to further investigate the effects of nicotine or nicotinic agonist in a condition where both Aβ and tau protein can be expressed.
5. Conclusions
In summary, our results indicate a protective effect of nicotine in a cellular model where APP or APP and presenilin are co-expressed. Furthermore, a nicotinic antagonist could block the effects of nicotine. The findings suggest a therapeutic potential for nicotine or nicotinic agonists in AD pathology involving APP or APP and presenilin.
Highlights.
Cells expressing amyloid precursor protein (APP) or a combination of APP and presenilin show significant mortality.
Nicotine dose dependently attenuated cell loss with highest sensitivity in doubly transfected cells.
Nicotine’s effect was blocked by mecamylamine with differential sensitivity in different cell lines.
The results support therapeutic potential for nicotinic agonists in at least a subtype of AD.
Acknowledgments
NIH/NIGMS(2 SO6 GM08016-39) and GU Pilot grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bitner RS, Bunnelle WH, Decker MW, Drescher KU, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther. 2010;334:875–86. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2006;9:35–44. doi: 10.3233/jad-2006-9s305. [DOI] [PubMed] [Google Scholar]
- 3.Buckingham S, Jones A, Brown L, Sattelle D. Nicotinic acetylcholine receptor signaling: Roles in Alzheimer’s Disease and Amyloid Neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings J. Alzheimer’s Disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 5.Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Shen C, Wang YJ, Zhang M, Li J, et al. Nicotine exacerbates tau phosphorylation and cognitive impairment induced by amyloid-beta 25–35 in rats. Eur J Pharmacol. 2010;637:83–8. doi: 10.1016/j.ejphar.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Guan ZZ, Yu WF, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 8.Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S. Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Mol Cell Neurosci. 2003;24:779–786. doi: 10.1016/s1044-7431(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 9.Hellström-Lindahl E, Moore H, Nordberg A. Increased levels of tau protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J Neurochem. 2000;74:777–84. doi: 10.1046/j.1471-4159.2000.740777.x. [DOI] [PubMed] [Google Scholar]
- 10.Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci. 2004;29:427–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama, et al. Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann Neurol. 1997;42:159–63. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- 12.Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Res. 1998;792:331–334. doi: 10.1016/s0006-8993(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 13.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–6. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 14.Kihara T, Shimohama S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol Exp. 2004;64:99–105. doi: 10.55782/ane-2004-1495. [DOI] [PubMed] [Google Scholar]
- 15.Levesque G, George-Hyslop P. The presenilins and Alzheimer Disease. MJM. 1997;3:142–148. [Google Scholar]
- 16.Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. Br J Pharmacol. 2004;141:746–754. doi: 10.1038/sj.bjp.0705653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Zhang J, Zhu H, Qin C, Chen Q. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FASEB J. 2007;21:61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- 18.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGleenon BM, Dynan KB, Passmore AP. Acetylcholinesterase inhibitors in Alzheimer’s disease. J Clin Pharmacol. 1999;48:471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie H, Li Z, Lukas RJ, Shen Y, Song L, Wang X, Yin M. Construction of SH-EP1-alpha4beta2-hAPP695 cell line and effects of nicotinic agonist on beta-amyloid in the cells. Cell Mol Neurobiol. 2008;28:103–12. doi: 10.1007/s10571-007-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, et al. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102:3046–51. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddo S, LaFerla FM. The role of nicotinic acetycholine receptors in Alzheimer’s disease. J Physiol Paris. 2006;99:172–9. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 23.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 24.Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezvani A, Levin E. Cognitive effects of Nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 27.Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 28.Seo J, Kim S, Kim H, Park C, et al. Effects of nicotine on APP secretion and Abeta- or CT (105)-induced toxicity. Biol Psychiatry. 2001;49:240–7. doi: 10.1016/s0006-3223(00)01124-0. [DOI] [PubMed] [Google Scholar]
- 29.Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA. Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer’s disease. Neurobiol Aging. 2011;32:834–44. doi: 10.1016/j.neurobiolaging.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Shimohama S, Akaike A, Kimura J. Nicotine-induced protection against glutamate cytotoxicity, Nicotinic cholinergic receptor-mediated inhibition of nitric oxide formation. Ann N Y Acad Sci. 1996;777:356–61. doi: 10.1111/j.1749-6632.1996.tb34445.x. [DOI] [PubMed] [Google Scholar]
- 31.Stevens TR, Krueger SR, Fitzsimonds RM, Picciotto MR. Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J Neurosci. 2003;23:10093–10099. doi: 10.1523/JNEUROSCI.23-31-10093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada Y, Yonezawa A, Toshiaki K, Katsuki H, Kaneko S, Sugimoto H, et al. Nicotinic Acetylcholine Receptor-Mediated Neuroprotection by Donepezil Against Glutamate Neurotoxicity in Rat Cortical Neurons. J Pharmacol Exp Ther. 2003;306:772–777. doi: 10.1124/jpet.103.050104. [DOI] [PubMed] [Google Scholar]
- 33.Terry AV, Buccafusco JJ. The cholinergic hypothesis of Age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–7. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Mechawar N, Krantic S, Quirion R. α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem. 2011;119:848–858. doi: 10.1111/j.1471-4159.2011.07466.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Liu Q, Chen Q, et al. Nicotine attenuates beta-amyloid-induced neurotoxicity by regulating metal homeostasis. FASEB J. 2006;20:1212–4. doi: 10.1096/fj.05-5214fje. [DOI] [PubMed] [Google Scholar]