Abstract
OBJECTIVE
Hyperlipidemia has been associated with erectile dysfunction (ED). We investigated structural changes, including possible smooth muscle hyperplasia, in the penis of a hyperlipidemia-associated ED animal model.
MATERIALS AND METHODS
Hyperlipidemia was induced by high-fat diet. Penile tissues of normal and hyperlipidemic rats were stained with Alexa-488-conjugated phalloidin and/or with antibodies against rat endothelial cell antigen (RECA), neuronal nitric oxide synthase (nNOS), and collagen-IV (Col-IV), followed by image and statistical analyses.
Main Outcome Measures: Corpus cavernosum content of smooth muscle, endothelium, collagen-IV, and nNOS.
RESULTS
Phalloidin intensely stained all smooth muscle in the penis, revealing the circular and longitudinal components of cavernous smooth muscle (CSM). CSM content was significantly higher in hyperlipidemic than in normal rats. Cell numbers in both circular and longitudinal CSM were significantly higher in hyperlipidemic than in normal rats. Cavernous endothelial content was significantly lower in hyperlipidemic than in normal rats. nNOS-positive nerves within dorsal nerves, around dorsal arteries, and in the corpora cavernosa were all significantly lower in hyperlipidemic than in normal rats.
CONCLUSION
Hyperlipidemia is associated with reduced nNOS-positive nerves, reduced endothelium, and increased smooth muscle in the penis. The increased smooth muscle is due to hyperplasia. Together, these structural changes may explain why hyperlipidemic men are more likely to develop ED.
Keywords: hyperlipidemia, phalloidin, cavernous smooth muscle, cavernous endothelium, nNOS-positive nerves, collagen-IV, erectile dysfunction
INTRODUCTION
Nearly 100 million men in the United States have mean lipid values outside the optimal ranges [1]. Hyperlipidemic men are more likely to have erectile dysfunction (ED) than normolipidemic men [2-4]. Current management of hyperlipidemia-associated ED calls for PDE5 inhibitors as the first-line treatment despite the lack of validated evidence demonstrating their efficacy [5]. Even when combined with cholesterol-lowering drug atorvastatin, PDE5 inhibitor sildenafil was unable to fully restore erectile function [6]. Thus, alternative treatment options need be explored and such efforts require a better understanding of the pathological process [5, 6].
In 2003 we published a study in which a hyperlipidemia-ED rat model was established for the experimental treatment with vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) [7]. In the penile corpus cavernosum of these rats we observed a reduced nerve content and fewer endothelial cells - features consistent with decreased erectile function. However, we also observed an increased cavernous smooth muscle (CSM) content, which may indicate a better erectile function. In 2010 we published another study in which the same rat model was used for experimental stem cell therapy [8]. Although this time the rats were induced to develop hyperlipidemia and examined by a different investigator (Huang versus Gholami), the same histological changes as those in the 2003 study were observed. Thus, an increased CSM content appears to be a consistent feature in hyperlipidemic rats and this observation prompted us to further examine whether the CSM increase is due to hyperplasia or hypertrophy.
In a recent study we found that, by using Alexa-488-conjugated phalloidin, which binds specifically to F-actin, individual smooth muscle cells could be clearly visualized (Lin et al., submitted). Furthermore, by combining phalloidin stain with immunofluorescence (IF) stain for neuronal nitric oxide synthase (nNOS), rat endothelial cell antigen (RECA), and type IV collagen (Col-IV), we were able to visualize the microanatomical relationship between smooth muscles and each of these three components. In the present study we demonstrate how this improved penile histology can be used to obtain quantitative data pertaining to ED-associated changes in the cellular and extracellular compartments. Specifically, we report that the increased CSM content in the hyperlipidemia-ED rats is due to smooth muscle hyperplasia.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at our institution. Twenty 3-month-old male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). They were randomized into two groups: The normal diet group received a standard rat chow while the high-fat diet group received a diet consisting of 2% cholesterol and 10% lard (Zeigler Brothers, Gardner, PA). After 6 months, hyperlipidemia and erectile dysfunction developed in the high-fat diet group as reported previously [7, 8]. All animals were sacrificed with bilateral thoracotomy and their penises harvested for histological analysis
Penile samples were fixed in 2% formaldehyde and 0.002% picric acid in 0.1M phosphate buffer for 4 h, followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in Optimum Cutting Temperature Compound (Sakura Finetek, Torrence, CA) and stored at -70 °C until use. Sections were cut at 5 μm, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min. To stain F-actin, tissue sections were incubated with Alexa-488-conjugated phalloidin (1:100 in 1% BSA, Invitrogen, Carlsbad, CA) for 20 min at room temperature, followed by incubation with 4’,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich, St. Louis, MO). For immunofluorescence stain, tissue sections were placed in 4% paraformaldehyde for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated at 4 °C with mouse anti-RECA (ABD SEROTEC, Raleigh, NC), rabbit anti-Collagen IV (Abcam, Cambridge, MA), or rabbit anti-nNOS (Santa Cruz Biotechnology, Santa Cruz, CA). Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the sections were incubated with Alexa-594 conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Invitrogen). After rinses with PBS, the slides were further stained with DAPI. When indicated, the immunostained tissues were also stained with Alexa-488-conjugated phalloidin as described above before the DAPI stain.
The stained tissues were examined with a Nikon Eclipse E600 fluorescence microscope and photographed with a Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments, Melville, NY). Computerized histomorphometric analysis was performed using Image-Plus 5.1 software (Media Cybernetics, Bethesda, MD). To analyze CSM content, the corpus cavernosum was photographed at 20x magnification, and the ratio between the phalloidin-stained area (pixel number of green stain) and the entire corpus cavernosum was calculated. To analyze cell number in the longitudinal CSM and fiber number in the circular CSM, the corpus cavernosum was photographed at 1000x magnification, followed by counting positively stained cells and fibers. To analyze nNOS content, the number of positively stained dots within dorsal nerves, around dorsal arteries and in corpus cavernosum was counted at 200x magnification. To analyze endothelial or Col-IV content, the integrated optical density (IOD) of positively stained area was obtained at 100x magnification.
Data were analyzed with Prism 4 (GraphPad Software, San Diego, CA). Analysis of variance (ANOVA) was used to determine the difference between the means of different treatment groups, followed by paired T test. Difference was considered significant when p<0.05. All data are shown as mean ± standard deviation (SD).
RESULTS
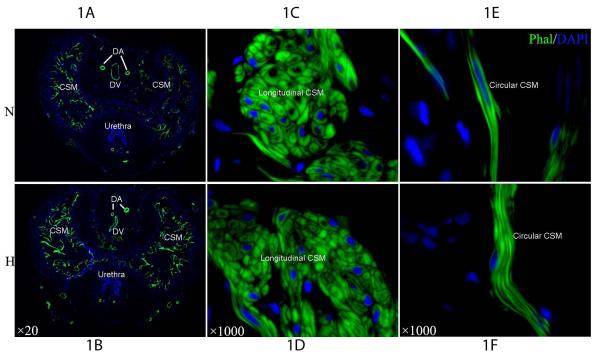
Alexa488-phalloidin intensely stained all smooth muscles in the rat penis, including the two dorsal arteries, the dorsal vein, and the CSM (Fig. 1). The CSM content was significantly higher in hyperlipidemic than in normal rats (Fig. 1A&B; Table 1). At higher magnification it became clear that the CSM was divided into a circular and a longitudinal compartment (Inserts in Fig. 1C-H). The cell number in the longitudinal CSM was significantly higher in hyperlipidemic than in normolipidemic rats (Fig. 1E&F; Table 1). The fiber number in the circular CSM was also significantly higher in hyperlipidemic than in normolipidemic rats (Fig. 1G&H; Table 1).
Figure 1.
IF stain with Alexa488-phalloidin for comparison of cavernous smooth muscle
Table1.
Comparison of cavernous smooth muscle content and cell number
| CSM content (% Total area) |
Longitudinal CSM cells (Number/area) |
Circular CSM fibers (Number/thickness) |
|
|---|---|---|---|
| N | 9.24±0.01 | 214.62±23.16 | 3.40±0.55 |
| H | 12.8±0.01a | 321.22±20.01b | 6.60±0.89b |
CSM: cavernous smooth muscle; N: Normal rats; H: Hyperlipidemic rats
P<0.05 compared with N
P<0.01 compared with N
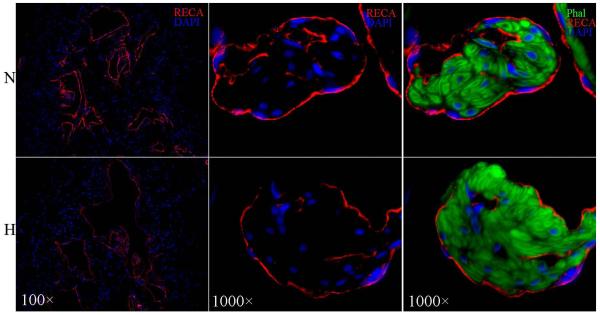
The cavernous endothelium, identified by IF staining with anti-RECA antibody, covers the sinusoidal side of CSM (Fig. 2). Under identical staining and photographic conditions, the endothelium was found thinner in hyperlipidemic than in normolipidemic rats (Fig. 2A&B). Furthermore, while the endothelium in normolipidemic rats was a continuous lining that wraps around CSM bundles, that in hyperlipidemic rats was discontinuous (Fig. 2C&D). Both the thinning and discontinuity of the endothelium in hyperlipidemic rats are reflected in a significantly lower endothelial content (Table 2).
Figure 2.
IF staining with anti-RECA antibody for comparison of cavernous endothelial content
Table 2.
Comparison of endothelium and collagen-IV in corpora cavernosa
IOD: Integrated Optical Density; N: Normal rats; H: Hyperlipidemic rats
P<0.01 compared with N
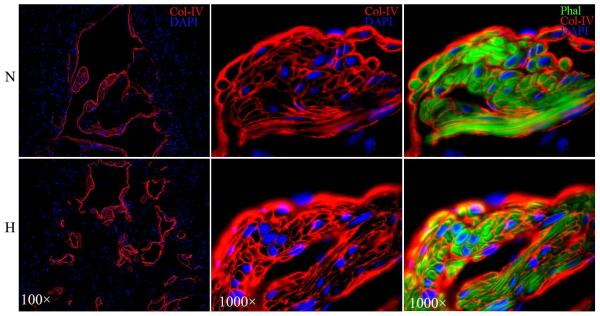
Col-IV, identified by IF staining with anti-Col-IV antibody, was localized between the endothelium and CSM as well as surrounding each CSM cells (Fig. 3). Under identical staining and photographic conditions, Col-IV was found more abundantly expressed in hyperlipidemic than in normolipidemic rats. However, this difference was offset by the difference in CSM content (Tables 1&2).
Figure 3.
IF staining with anti-Col-IV antibody for comparison of Col-IV content
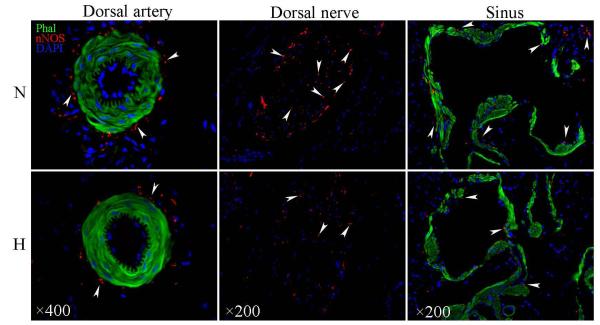
nNOS-positive nerves were identified by IF staining with anti-nNOS antibody. The numbers of these nerves were significantly lower in hyperlipidemic than in normolipidemic rats (Fig. 4; Table 3) as seen within the dorsal nerves (Fig. 4A&B), around dorsal arteries (Fig. 4C&D), and in the cavernous tissue (Fig. 4E&F).
Figure 4.
IF stain with anti-nNOS antibody for Comparison of nNOS-Positive Nerves
Table 3.
Comparison of nNOS content
| Within dorsal nerve (Number of dots) |
Around dorsal artery (Number of dots) |
In corpora cavernosa (Number of dots) |
|
|---|---|---|---|
| N | 578.6±52.6 | 56.9±6.3 | 50.2±5.5 |
| H | 433.4±41.3a | 41.8±5.4a | 32.4±4.9b |
N: Normal rats; H: Hyperlipidemic rats
P<0.05 compared with N
P<0.01 compared with N
DISCUSSION
ED is usually associated with a reduced CSM content, for example, in arteriogenic [9] and neurogenic ED [10, 11] ED rat models. However, in 2003 we reported that rats with hyperlipidemia-associated ED had increased CSM content [7], and this has been independently confirmed in two separate studies by Ryu et al [12] and Ryu et al [13]. Most recently, in an experimental stem cell therapy for hyperlipidemia-associated ED we again observed an increased CSM content in hyperlipidemic rats [8]. Thus, it appears that increased muscularity occurs not only in blood vessels [14] but also in the corpus cavernosum. Since, increased muscularity in blood vessels is known due to hyperplasia, this study was intended to discern whether smooth muscle hyperplasia also occurred in the corpus cavernosum.
In a recent study we demonstrated an improved penile histology by the use of phalloidin stain (Lin et al., submitted). Specifically, we reported the finding that the CSM was comprised of a circular and a longitudinal component, and we also reported the visualization of individual CSM cells. In the present study we took advantage of the phalloidin stain and demonstrated that both the circular and longitudinal CSM compartments were affected by hyperlipidemia, and the resulting increases were due to hyperplasia. Thus, similar to the situation with the vascular smooth muscle (VSM) cells in blood vessels [14], increased CSM cellular proliferation occurred as a response to hyperlipidemia.
It has been shown that hyperlipidemia causes the destruction of the endothelial barrier, thereby exposing the VSM directly to blood circulation [14]. This leads to increased VSM cellular proliferation due to the presence of growth factors in the blood, particularly those derived from the platelets [14]. In our previous studies [7, 8] and present study we also observed a damaged cavernous endothelium as well as a lowered cavernous endothelial content in hyperlipidemic rats. Similar findings have also been reported in hyperlipidemic mice by Ryu et al [15]. Thus, hyperlipidemia-associated CSM hyperplasia was likely due to increased exposure of CSM to growth factors in the blood. Furthermore, similar to the situation with the VSM, hyperplasia in the CSM is probably associated with decreased contractility as the cells transit from a quiescent contractile phenotype to a proliferative synthetic phenotype [14].
The cavernous ECM is mainly composed of collagen types I, III, and IV, with types I and IV being predominant [16]. While Col-I and Col-III are primarily localized to the non-muscle non-endothelial areas of the corpus cavernosum, Col-IV is closely associated with the endothelial basement membrane and the smooth muscle basal lamina (Lin et al, submitted). In blood vessels Col-IV is also closely associated with the endothelium and smooth muscle, and plays important roles in maintaining the contractile phenotype of vascular SMC [17]. In the penile corpus cavernosum, reduced Col-IV level has been associated with psychogenic ED [18]; however, the overall importance of Col-IV in erectile function remains unknown. In the present study we found an increased level of Col-IV in the corpora cavernosa of hyperlipidemic rats. However, relative to the increased number of CSM cells, the Col-IV level was slightly lower in the hyperlipidemic rats.
In our 2003 paper we reported that the penile dorsal nerves of hyperlipidemic rats had a remarkable decrease in the number and size of nonmyelinated axons [7]. In our 2010 paper we reported that the penile dorsal nerves of hyperlipidemic rats had significantly fewer nNOS-positive fibers [8]. In the present study we confirmed the latter study’s finding, and additionally we observed that the reduction of nNOS-positive nerves also occurred around the dorsal artery and in the corpora cavernosa. Thus, hyperlipidemia appears to cause an overall reduction of nNOS-positive nerves in various parts of the penis.
Reduced nNOS-positive nerves and endothelium can both negatively affect erectile function due to the reduced bioavailability of nitric oxide. The diminished endothelium also causes the CSM to lose contractility as the cells become synthetic. Together, these structural changes can possibly explain why hyperlipidemic men are at higher risk of having ED.
ACKNOWLEDGEMENTS
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (DK045370).
REFERENCES
- 1.Cleeman JI. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, Blair SN. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930–7. doi: 10.1093/oxfordjournals.aje.a117181. [DOI] [PubMed] [Google Scholar]
- 3.Roumeguere T, Wespes E, Carpentier Y, Hoffmann P, Schulman CC. Erectile dysfunction is associated with a high prevalence of hyperlipidemia and coronary heart disease risk. Eur Urol. 2003;44:355–9. doi: 10.1016/s0302-2838(03)00306-3. [DOI] [PubMed] [Google Scholar]
- 4.Nikoobakht M, Nasseh H, Pourkasmaee M. The relationship between lipid profile and erectile dysfunction. Int J Impot Res. 2005;17:523–6. doi: 10.1038/sj.ijir.3901350. [DOI] [PubMed] [Google Scholar]
- 5.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–78. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 6.Dadkhah F, Safarinejad MR, Asgari MA, Hosseini SY, Lashay A, Amini E. Atorvastatin improves the response to sildenafil in hypercholesterolemic men with erectile dysfunction not initially responsive to sildenafil. Int J Impot Res. 2010;22:51–60. doi: 10.1038/ijir.2009.48. [DOI] [PubMed] [Google Scholar]
- 7.Gholami SS, Rogers R, Chang J, et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003;169:1577–81. doi: 10.1097/01.ju.0000055120.73261.76. [DOI] [PubMed] [Google Scholar]
- 8.Huang YC, Ning H, Shindel AW, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MC, El-Sakka AI, Graziottin TM, Ho HC, Lin CS, Lue TF. The effect of vascular endothelial growth factor on a rat model of traumatic arteriogenic erectile dysfunction. J Urol. 2002;167:761–7. doi: 10.1016/S0022-5347(01)69141-9. [DOI] [PubMed] [Google Scholar]
- 10.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–9. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu JK, Shin HY, Song SU, et al. Downregulation of angiogenic factors and their downstream target molecules affects the deterioration of erectile function in a rat model of hypercholesterolemia. Urology. 2006;67:1329–34. doi: 10.1016/j.urology.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Ryu JK, Cho CH, Shin HY, et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther. 2006;13:705–15. doi: 10.1016/j.ymthe.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Harker L. Hyperlipidemia and atherosclerosis. Science. 1976;193:1094–100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JK, Zhang LW, Jin HR, et al. Derangements in endothelial cell-to-cell junctions involved in the pathogenesis of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2009;6:1893–907. doi: 10.1111/j.1743-6109.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 16.Luangkhot R, Rutchik S, Agarwal V, Puglia K, Bhargava G, Melman A. Collagen alterations in the corpus cavernosum of men with sexual dysfunction. J Urol. 1992;148:467–71. doi: 10.1016/s0022-5347(17)36630-2. [DOI] [PubMed] [Google Scholar]
- 17.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–25. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 18.Raviv G, Wespes E, Vanegas JP, et al. Difference in glycohistochemical lectin staining of collagen fibers in the corpora cavernosa of normal and impotent men. J Androl. 1996;17:187–93. [PubMed] [Google Scholar]