Abstract
Purpose
The prognosis for children with IPAH unresponsive to therapy is poor. We investigated the plasma proteome for a molecular basis of good versus poor outcome to long-term vasodilator therapy.
Experimental design
Plasma was collected at baseline or shortly after therapy initiation and following chronic vasodilator therapy, then divided into those with good outcome (n = 8), and those with a poor outcome (n = 7). To identify proteins unique to either outcome, we used differential gel electrophoresis and mass spectrometry. Results were confirmed by commercial enzyme-linked immunosorbent assay.
Results
Before and after therapy, SAA-4 was 4-fold lower in those with good outcome compared to those with poor outcome, while serum paraoxonase/arylesterase-1 was increased 2-fold in those with good outcome versus poor outcome. After therapy, haptoglobin and hemopexin were 1.45- and 1.8-fold lower, respectively, in those with a good versus poor outcome. Among those with a good outcome, SAP was 1.3-fold lower prior to therapy.
Conclusions and clinical relevance
SAP and SAA-4 regulate circulating mononuclear phagocytes. As such, they may contribute to the differential response to chronic vasodilator therapy in the context of inflammation in IPAH.
Keywords: Inflammation, Pulmonary hypertension, Vasodilators
1 Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a rare, life-threatening disorder characterized clinically by sustained elevations of pulmonary artery pressure (PAP) [1]. The histopathological hallmark of IPAH is remodeling of the pulmonary arterial vasculature with medial and adventitial thickening of the arteries and arterioles [2]. This is frequently observed in tandem with intimal proliferation, in situ thrombi, deposition of extracellular matrix, and inflammation [3, 4]. The structural and hemodynamic changes within the pulmonary arterial circulation lead to increased pulmonary vascular resistance, right heart failure and ultimately, death [1]. Despite significant progress in our comprehension of the pathophysiology of IPAH, the molecular mechanisms underlying IPAH and the optimal treatment strategies that might attenuate or even reverse this vasculopathy remain unclear. Recently, proteomic approaches have been utilized to study pulmonary arterial hypertension (PAH) [5–17]. Investigation of the lung tissue, myocardial tissue, cell, plasma, and serum proteomes in these studies has produced a large panel of putative biomarkers primarily focused on the pathogenesis of PAH. In this study, we endeavored to define plasma proteomic differences in the context of a significant clinical problem in PAH, the response or lack of response to chronic therapy leading to a good or poor long-term outcome.
Historically, vascular remodeling associated with IPAH has been ascribed to endothelial cell damage, loss of vasodilator function, and concomitant vasoconstriction of the pulmonary arterial bed [3]. Although vasodilator therapy is given to these patients, current targeted therapies may also reverse remodeling in select cases [18]. By mechanisms that are unclear, children have a better outcome to vasodilator therapy than adults [19]. However, a subgroup of pediatric IPAH patients is unresponsive to therapy, which is associated with a poor prognosis [20]. In one study of children with IPAH, the 5-year survival rate for responders was 88% compared with 33% in nonresponders [21]. Age also appears to be an important factor within the pediatric IPAH population with the 5-year survival rate reported to be 88% in children less than 6 years of age versus 25% in children more than 6 of age [21], although a recent study found no correlation of outcome with age [22]. Older age at diagnosis is a predictor of worse outcome [23] and younger children treated aggressively with intravenous therapy are more likely to be able to be weaned from intravenous therapy [24]. Uncovering the molecular basis for durable response to vasodilator therapy is therefore of critical importance.
Although they offer clinical benefits, vasodilators are not curative. Their mechanisms of action are poorly understood, and currently there is no algorithm that reliably predicts a priori response to long-term vasodilator therapy in these patients. We used gel-based proteomics to investigate whether plasma from children with IPAH (responders and nonresponders to long-term therapy) contained proteins that may help to explain the molecular basis for responsiveness to chronic vasodilator therapy. We report that the plasma of patients with IPAH who are responsive to vasodilator therapy with a good clinical outcome have a distinct proteomic profile and have differential levels of at least two proteins that are known to bind and regulate subsets of peripheral blood mononuclear cells.
2 Materials and methods
2.1 Study subjects
All subjects were studied by informed consent under protocols approved by the Institutional Review Board at the University of Colorado Health Sciences Center, Denver, Colorado, in accordance with guidelines recommended by the National Institutes of Health. All patients were diagnosed with IPAH between 1995 and 2005 using standard definitions and were treated using a similar therapeutic strategy [25]. For this study, all children presented with severe disease with evidence of right heart failure, and were started on intravenous prostacyclin. All children were treated with combination therapy including the addition of an endothelin receptor antagonist or type-5 phosphodi-esterase inhibitor as these therapies became available. All patients were nonreactive to initial acute vasodilator testing with inhaled nitric oxide and oxygen at the time of initial cardiac catheterization using the pediatric definition [23, 26]. Samples were retrospectively chosen from a bank of plasma, and were chosen in two groups of seven to eight patients each, with those with a good outcome (able to wean from intravenous prostanoids or near normalization of PAP and pulmonary vascular resistance index (PVRI) and no death; n = 8) or very poorly to therapy (death or no improvement in hemodynamics on intravenous therapy; n = 7; Table 1).
Table 1.
Patient demographics and clinical measurements
| Poor outcome nonresponders | Good outcome responders | |
|---|---|---|
| Total number of patients | 7 | 8 |
| Age (year old): median (range) | 14 (11–17) | 5 (1–15) |
| Gender (male/female) | 3/4 | 3/5 |
| Race (number) | Caucasian (4) | Caucasian (7) |
| Hispanic (2) | Hispanic (0) | |
| Asian (0) | Asian (1) | |
| Black (1) | Black (0) | |
| Etiology (number) | Idiopathic PAH (7) | Idiopathic PAH (8) |
| Concomitant therapy (number) | IV prostacyclin (7) | IV prostacyclin (8) |
| ERA (5) | ERA (6) | |
| PDE-5i (4) | PDE-5i (3) | |
| Right heart catheterization at initial blood sample | (mean, SD) | (mean, SD) |
| Mean right atrial pressure (mmHg) | 7 ± 3 | 6 ± 2 |
| Mean pulmonary arterial pressure (mmHg) | 83 ± 18 | 63 ± 14a) |
| Pulmonary vascular resistance index (unitxm2) | 29 ± 22 | 17 ± 6 |
| Right heart catheterization at follow-up sample | ||
| Mean right atrial pressure (mmHg) | 6 ± 4 | 4 ± 2 |
| Mean pulmonary arterial pressure (mmHg) | 60 ± 10 | 26 ± 10a),b) |
| Pulmonary vascular resistance index (unitxm2) | 17 ± 4 | 5 ± 3a),b) |
| Death | 4/7 | 0/8 |
| Discontinuation of intravenous prostanoids. | 1/7 | 8/8 |
mmHg, millimeters mercury; SD, standard deviation; IV, intravenous; ERA, endothelin receptor antagonist; PDE, phosphodi-esterase inhibitor.
p <0.05 good response versus poor response.
p <0.05 initial versus follow-up catheterization.
2.2 Plasma collection and processing
Blood samples for this study were obtained using an IRB-approved, either at baseline (prior to therapeutic intervention or within several months), or following chronic (>6 months) vasodilator therapy. Between 5 and 10 mL blood volumes were collected in heparinized tubes, placed on ice and centrifuged at 1000 × g for 10 min within 30 min of collection. Plasmas were delipidated and depleted of albumin and IgG as previously described [27]. Proteins were precipitated from plasma by a methanol/chloroform extraction protocol [28]. Dried protein pellets were allowed to dissolve overnight in rehydration buffer (7 M urea, 2 M thiourea, 4% w/v CHAPS) [29] and each was diluted to the same protein concentration after protein assay. Protein concentrations were determined by the method of Bradford [30]. Plasma supernatants were aliquoted into fresh Eppendorf tubes, snap frozen in liquid nitrogen, and stored at −80°C until analyzed.
2.3 Cy dye labeling and 2D electrophoresis
The overall procedure was performed as described previously [31–33]. Fifty micrograms μg of total protein extract from responders or nonresponders were labeled with either Cy3 or Cy5. Sample labeling utilized dye swapping on consecutive runs to minimize dye bias within the two groups. Labeled samples of responders and nonresponders were combined with 50 μg of Cy2-labeled internal standard (a pool consisting of equal amounts of all samples in the study) and run on a single 2D gel. Preparative gels were run using a mixture of 50 μg of Cy2-labeled pooled internal standard and 950 μg of unlabeled pooled internal standard. The preparative gel was also poststained with SYPRO Ruby (Molecular Probes, Eugene, OR, USA) in order to allow visualization of the unlabeled protein spots and ensures map matching. All Cy labeling was done according to the manufacturer’s protocol, where 200 pmol of dye was used to label 50 μg of protein (Cy dyes DIGE fluors, GE Healthcare, Piscataway, NJ, USA), under standard minimal dye labeling conditions [31–33].
Each set (seven sets total) of analytical samples were passively rehydrated into Immobiline DryStrips 24 cm pH 3–10 (GE Healthcare) overnight or for at least 18 h, followed by iso-electric focusing using an IPGphor IEF unit (Amersham Biosciences/GE Healthcare). Focusing was conducted at 20°C, with 50 μA per strip, as follows: (i) 500 V for 500 Vh, (ii) 1000 V for 1000 Vh, (iii) 8000 V for 24 000 Vh, (iv) 8000 V for 64 000 Vh, and (v) 8000 V for 64 000 Vh [33].
For the preparative gel, the focusing parameters were the same, but utilized the following step and hold voltages: (i) 250 V for 1000 Vh, (ii) 500 V for 1000 Vh, (iii) 1000 V for 1000 Vh, (iv) 8000 V for 66 000 Vh, (v) 8000 V for 66 000 Vh, and (vi) 8000 V for 66 000 Vh [33].
After focusing, strips were incubated at room temperature for 15 h in reducing and alkylating solutions as previously described [34]. Strips were then loaded onto second dimension 8–16% tris-glycine gels (Jules Gels, Milford, CT, USA), sealed with agarose (SDS equilibrium buffer, 0.5% w/v agarose, and 0.25% v/v of saturated aqueous bromophenol blue) and run at 20 W per gel on the Ettan Dalt System (Amersham/GE Healthcare) for approximately 4–6 h [33].
2.4 Gel imaging
Imaging was done on a Typhoon 9400 Variable Mode Laser Imager (Amersham/GE Healthcare) [33]. The gels that were used for protein identification were then fixed for 1 h in 7.5% acetic acid/10% methanol, and stained overnight with Sypro Ruby protein gel stain (Invitrogen/Molecular Probes). Following destaining (7.5% acetic acid/30% methanol), gels were reimaged at 100-μm resolution (laser excitation 532 nm, emission 560 nm, LP Gen. Purple).
2.5 Gel image analysis
Imaging of gels and subsequent analysis was performed essentially as described previously [35]. Fluorescent images of the gels were analyzed by decyder software (v. 5.0; Amersham/GE Healthcare) for spot detection and relative quantification of proteins. Spot detection was performed in the differential in-gel analysis module with the number of spots set at 2000. Spot boundaries and volumes were detected automatically and normalized volume ratios were calculated for each protein spot. Gel matching utilized the biological variation analysis (BVA) module for assessment of protein abundance fold changes and statistical calculations. Preparative gel images were also matched to analytical images in BVA. One Cy2 image (with the highest spot count) was selected as the reference image, and all gels were mapped to this reference image. Matching was further improved by land marking. Statistical analysis was performed using student t-test for every matched spot set, comparing the average and standard deviation of protein abundance for spots of interest. A p value of <0.05 was considered significant. The corresponding spots were then matched to a Sypro stained image of the preparative gel, which was first mapped to the reference image [33]. Spots chosen for picking were selected that met the criteria of ≥1.2-fold change with p <0.05 in all 7/7 analytical gels.
2.6 Spot picking and tryptic digestion
Proteins of interest were excised from preparative gels using a robotic spot picker (Ettan SpotPicker software v 1.10, GE Healthcare/Amersham Bioscience) fitted with a 1.0-mm deep, 1.4 mm in diameter picker head, and placed in 96-well plates, then transferred to an Ettan Digester (software v 1.10, GE Healthcare/Amersham Bioscience). Excised plugs were sequentially washed with 100 μL of 50 mM ammonium bicarbonate, 100 μL of 75% ACN, 100 μL of 100% ACN, and dried at room temperature. Sequencing grade modified trypsin in 25 mM ammonium bicarbonate (1:4 v/v; Promega, Madison, WI, USA) was added to each gel plug, plates were sealed with film and after a 30-min incubation at 4°C were left at room temperature overnight for digestion [33]. Following digestion, digested peptides were extracted with 1.0% formic acid solution and then again with 50% ACN and 1.0% formic acid.
2.7 Mass spectrometry
All peptide digests were analyzed by MALDI-TOF-MS exactly as described previously [35]. A 0.5 μL of each sample was pipeted onto a 100-well MALDI target (Applied Biosystems, Foster City, CA, USA) and combined with matrix solution (CHCA, 0.5 μL, 5 mg/mL in 80:20:0.1 water/ACN/TFA v/v/v). Mass spectra were collected on a Voyager DE-STR mass spectrometer (Applied Biosystems) operated in the positive ion, reflectron mode. Two hundred and ten laser shots meeting predetermined criteria were averaged to yield each peptide mass fingerprint (i.e. 35 laser shots at six different positions within a spot). Spectra were internally calibrated based on the monoisotopic masses of several trypsin autolysis peptides (i.e. m/z 515.3306, 842.5100, 1045.5642, 2211.1046). Raw data were converted to ASCII files and each spectrum was processed in ProTS Data (v 1.1.1.0, Biodesix Inc., Steamboat Springs, CO, USA). Software parameters were as follows: background (1000 m/z, 200 points), noise (1000 m/z, 500 points), and peak detection (S/N ≥ 4, 1000 m/z, six-point peak width with reduced sensitivity at low m/z). Monoisotopic masses were added to a peak list and submitted to the Mascot Daemon (v 1.8.0, Matrix Science, London, UK) and searched against both the NCBI nonredundant (2 712 766 sequences, released on August 15, 2005) and SwissProt databases (204 086 sequences, released on January 10, 2006). Search parameters were as follows: mammalia taxonomy; fixed modification of carbamidomethylation on cysteines; trypsin as the enzyme allowing with one missed cleavage; a minimum of two peptides required for identification; and a peptide mass tolerance of ±50 ppm. Proteins assignment required that (i) the probability-based molecular weight search score was significant (p <0.05) and (ii) the isoelectric point (pI) and molecular mass of the proposed identification matched the observed protein migration on the gel within 20%. The percentage of sequence coverage for mass fingerprinting is presented in Tables 2 and 3.
Table 2.
Proteins differential between children with good and poor outcome
| Protein name | Fold differencea) | Spot no. | Mascot score | pI | MW (kDa) | Percentage coverage |
|---|---|---|---|---|---|---|
| Prior to long-term treatment | ||||||
| Alpha-2-macroglobulin | 2.34 | 432 | 280 | 5.6 | 160 | 41 |
| Complement factor B | −1.88 | 1039 | 192 | 6.7 | 73 | 36 |
| Serotransferrin | −1.61 | 1107 | 200 | 38 | ||
| Fibrinogen beta chain | 1.34 | 1138 | 253 | 7.9 | 65 | 65 |
| Complement factor I | 1.48 | 1172 | 116 | 7.4 | 64 | 36 |
| Serum paraoxonase/arylesterase1 (PON-1) | 2.12 | 1265 | 162 | 5 | 50 | 71 |
| Apolipoprotein A-IV | 2.83 | 1289 | 352 | 5.3 | 42 | 72 |
| Apolipoprotein-L1/A-IV | 1.63 | 1292 | 55 | 5.7 | 44 | 22 |
| Alpha-1-microglobulin/Inter-alpha-trypsin inhibitor (together) | 1.25 | 1352 | 142 | 5.9 | 39 | 37 |
| Complement C4 alpha chain complement C4-A beta chain | 1.51 | 1356 | 113 | 5.4 | 33 | 22 |
| Hemoglobin subunit beta | −4.25 | 1518 | 189 | 6.8 | 16 | 85 |
| Serum amyloid A-4 protein | −4.04 | 1530 | 95 | 7.0 | 15 | 49 |
| Vitronectin | 2.31 | 1551 | 54 | 5.4 | 13.5 | 11 |
| Clusterin | 1.8 | 1632 | 208 | 6 | 40 | 47 |
| Apolipoprotein A-I | 2.03 | 1633 | 374 | 5.2 | 28.1 | 80 |
| After long-term treatment | ||||||
| Alpha-2-macroglobulin | 1.93 | 281 | 366 | 5.6 | 160 | 58 |
| Hemopexin | −1.84 | 926 | 240 | 6.4 | 49 | 74 |
| Vitamin D binding protein | −2.16 | 1148 | 380 | 5.2 | 51 | 70 |
| Haptoglobin | −1.45 | 1315 | 90 | 6.1 | 41 | 36 |
Fold difference of responders from nonresponders. For example, SAA-4 was 4.04-fold lower in responders compared to nonresponders. Conversely, serum PON-1 was 2.12-fold higher in responders compared to nonresponders.
Table 3.
Proteins differential in children with good outcome before and after therapy
| Protein | Fold differencea) | Spot no. | Mascot score | pI | MW (kDa) | Percentage coverage |
|---|---|---|---|---|---|---|
| Alpha-1-antichymotrypsin | −1.19 | 1031 | 285 | 4.9 | 49 | 52 |
| Serum amyloid P-component | −1.27 | 1421 | 162 | 5.0 | 25 | 37 |
| Apolipoprotein A-I | 2.8 | 1487 | 361 | 5.2 | 28 | 81 |
Fold difference of responders before and after therapy. For example, serum amyloid P was 1.27-fold lower in responders before therapy compared to after therapy.
2.8 ELISAs
The plasma concentration of serum amyloid A (SAA), and serum amyloid P (SAP) in responders and nonresponders was measured using commercially available ELISAs, according to the manufacturers’ instructions (Abnova #KA0518, Hy-Cult Biotech HK331, respectively).
2.9 Statistical analyses
The Student t-test was used for comparisons by ELISAs. A p value of <0.05 was considered significant. Clinical variables were compared using an unpaired t-test. Analysis was performed using Prism 4 for Mac.
3 Results
3.1 Clinical data
The clinical data from the patient cohort are presented in Table 1. Diagnosis of IPAH was made using standard methodology [36]. All patients with IPAH had severe disease and were treated with continuous intravenous prostacyclin, the most aggressive PAH therapy available. In retrospect, patients were categorized as having a good or poor long-term outcome based on change in therapy and death rate. Similar to previous reports, those with a good outcome were younger and were able to be weaned from intravenous therapy while meeting strict criteria [24] (Table 1). Furthermore, hemodynamics in those with a good outcome at the earliest catheterization had lower mean PAP but similar PVRI. All children with a good outcome weaned from intravenous prostacyclin therapy to oral or inhaled PAH therapy whereas four of seven with a poor outcome died on intravenous prostacyclin therapy. The average time between the initial plasma sample and the follow-up sample was 1.8 ± 1.8 years in those with a poor outcome and 1.9 ± 1.8 years in those with a good outcome.
3.2 Plasma protein spot maps and analysis
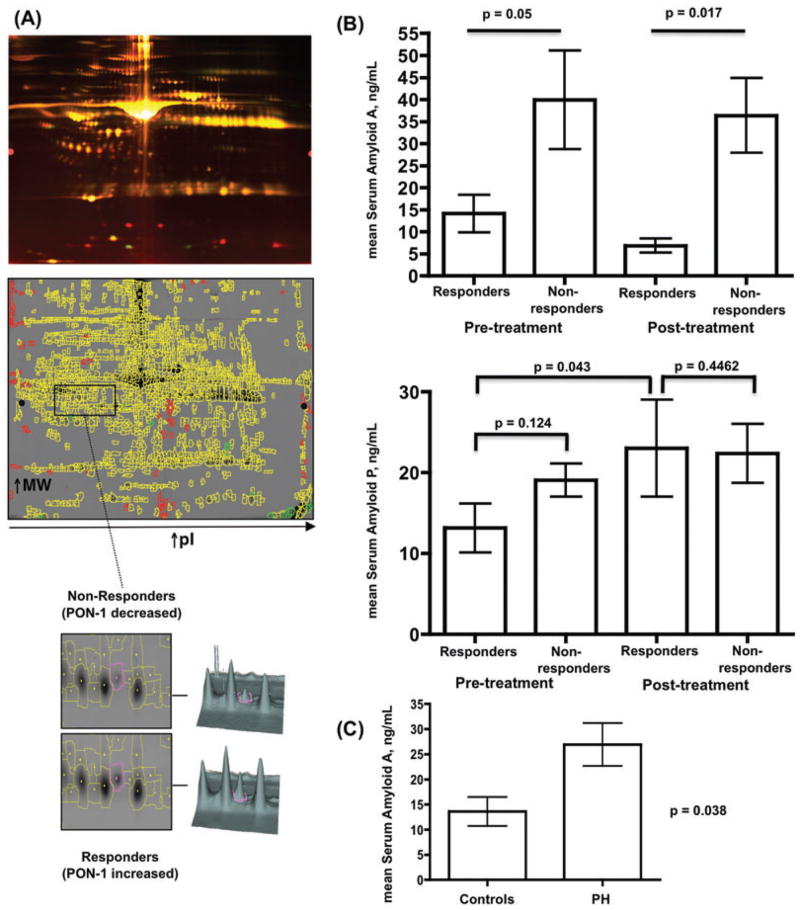
DIGE has been used to examine a variety of proteomes, including IPAH [7, 12]. However, no proteomic studies are published that examined response to long-term therapy in pediatric IPAH. To begin to address this, we examined plasma from 14 children and young adults diagnosed with IPAH before and after chronic vasodilator therapy. Patients were selected on the basis of their response/nonresponse (n = 7 per group) to long-term therapy defined as restoration of mean PAP to near normal levels following >6 months of vasodilator therapy (Table 1). Because of the high abundance of albumin and immunoglobulins, we depleted [27] each plasma sample prior to analysis by two dimensional difference in-gel electrophoresis (DIGE, representative images Fig. 1A) and MALDI-TOF-MS. We first analyzed plasma obtained prior to vasodilator therapy, and for which the samples were grouped into responders/nonresponders. Between 1034 and 1789 spots were codetected in Differential In-Gel Analysis (DIA) workspaces of DeCyder software. From the master gel, a total of 98 spots were differentially expressed at least ±1.2-fold in all seven gels with a p value <0.05. From the preparative gel, a total of 45 spots were identified. From these a total of 15 unique protein identifications were made with high confidence (Table 2). The sequence coverage of the identified proteins ranged from 11% to 85%.
Figure 1.
Proteomic analysis of long-term outcome response to vasodilator therapy. (A, upper image) Representative pseudocolored DIGE image showing separation of plasma proteins from responders and nonresponders. (Middle image) Representative spot map overlying a DIGE gel. Yellow spots indicate proteins common to both responders and nonresponder samples, while green and red spots highlight differential proteins. (Lower image) Representative spot-pick images for PON-1. (B, upper graph) Before and after therapy, serum amyloid A is significantly lower in responders than nonresponders. (Middle graph) There was no significant difference in serum amyloid P between responders and nonresponders, but SAP was significantly increased after therapy in responders (p = 0.043). (C) Plasma serum amyloid A is significantly increased in PAH patients (n = 31) versus controls (n = 11).
Next, we examined plasma after vasodilator therapy had established durable good/poor–response/nonresponse groupings in the patient cohort. Between 906 and 1189 spots were codetected in DIA workspaces of DeCyder software. From the master gel, a total of 68 spots were differentially expressed at least ±1.2-fold in all seven gels with a p value <0.05. From the preparative gel, a total of 18 spots were identified. From these a total of four unique protein identifications were made with high confidence (Table 2). The sequence coverage of the identified proteins ranged from 36% to 74%.
Finally, we performed an examination of plasma in children with a good response before and after vasodilator therapy to identify proteins that might help explain the response. Between 834 and 1089 spots were codetected in DIA workspaces of DeCyder software. From the master gel, a total of 28 spots were differentially expressed at least ±1.2-fold in all seven gels with a p value <0.05. From the preparative gel, a total of three unique protein identifications were made with high confidence (Table 3). The sequence coverage of the identified proteins ranged from 37% to 81%.
3.3 Protein identification in good versus poor outcome patients
Using spot intensity, we identified and quantified proteins differentially expressed between the responder/nonresponder groups. We found that, prior to treatment, the plasma of children with a good response had 4.04-fold less SAA-4, while having 2.12-fold increased serum paraoxonase-1 (PON-1; representative gel, Fig. 1, data Table 2; PON-1 previously identified in PAH serum [14]). Other identified proteins of difference included complement C4 (previously identified in IPAH plasma [17]), apolipoprotein A-I (previously identified in PAH plasma [8]), apolipoprotein A-IV (previously identified in PAH plasma [14]), alpha-2-macroglobulin, clusterin, and vitronectin. We did not identify any posttranslational modifications of these proteins.
Next, we examined plasma collected after vasodilator therapy in good versus poor outcome children (Table 2). We found vitamin D binding protein, haptoglobin, alpha-2-macroglobulin, and hemopexin were differentially expressed and previously identified in PAH plasma/serum [7, 14].
Finally, we undertook a comparison of plasma from good outcome patients before and after onset of vasodilator therapy to identify proteins associated with the response. We found alpha-1-antichymotrypsin (1.2-fold increased after therapy), SAP (1.3-fold increased after therapy), and apolipoprotein-I (2.8-fold decreased after therapy), to be significantly different.
3.4 Validation of plasma SAA and SAP levels
To independently validate our DIGE data, we performed commercial ELISAs on freshly thawed aliquots of the same plasma samples (but not depleted) that were used for DIGE. As shown in Fig. 1, SAA-4 was significantly (p <0.05) higher at baseline and after therapy in the nonresponder group. SAP was lower on average at pretreatment in the responder group versus nonresponders, but was at similar levels to the non-responder group posttreatment (Fig. 1). Among responders, SAP was lower pretreatment. In addition, we verified the correct band sizes for the validated proteins and absence of SAA-4 and SAP degradation products by immunoblot analyses (data not shown). Finally, we found that plasma SAA-4, but not SAP, was significantly increased in children with PAH compared to controls (Fig. 1).
4 Discussion
The results of this study provide potentially important insights into plasma proteome changes accompanying outcome to chronic vasodilator therapy in pediatric IPAH. As there is no cure for IPAH, there is a clear and present need for increased understanding of the mechanisms leading to response to therapy and thus outcome. To that end, we embarked on the first study to directly interrogate differences in plasma proteomes from PAH patients responsive/nonresponsive to long-term treatment. We identified several plasma proteins associated with differential response, some of which have been previously identified as being distinctly expressed in PAH patients versus controls.
SAA is a family of conserved acute phase proteins expressed by macrophages, vascular smooth muscle cells, and endothelial cells in response to stimulation by TNF alpha and IL-6 [37]. SAA-1 and SAA-2 expression can increase 10-to 1000-fold during acute phase periods [38]. SAA-3 is a pseudogene not expressed in humans and SAA-4 is constituitively expressed [39]. In the systemic vasculature, SAA stimulates vascular proteoglycan synthesis in a pro-atherogenic manner [40]. Such a role constitutes an early event in the “response to retention” hypothesis of atherosclerosis development [41]. Higher serum SAA levels decrease the activity of PON-1, an enzyme with well-established potent anti-oxidative and anti-inflammatory properties [42]. Here, we too found that children with a good outcome had lower SAA-4 levels than those with a poor outcome and higher risk of death, both before and after therapy, and that higher SAA-4 in nonresponders with poor outcome was associated with decreased PON-1 (Table 2). Low PON-1 predicts arterial stiffness in renal transplant patients [43]. Intriguingly, vascular stiffening is a major patho-biological component of IPAH [44], and is a better predictor of outcome in pediatric IPAH compared to measurement of pulmonary vascular resistance alone [45]. Although we did not directly test PON-1 activity, levels of PON-1 appear to correlate to its activity [46]. Recently, SAA has been shown to stimulate vascular smooth muscle cell calcium entry and downstream signaling associated with coronary muscle dysfunction under inflammatory conditions such as atherosclerosis [47]. Similarly, we recently reported that plasma endothelin-1 induces pulmonary artery smooth muscle cells to produce hyaluronic acid to which THP-1 monocytes readily adhere [48]. These studies raise the possibility that blood borne factors in PAH patients modulate vascular remodeling in PAH through their effects on both resident vascular cells and peripheral blood mononuclear cells, though local production of SAA at sites of lung inflammation might also be critically important [49].
We also demonstrated a small yet statistically significant increase in SAP in responders with good outcome after treatment with PAH specific therapy. This result (1.27-fold) was verified by ELISA, which showed a 2-fold difference. There was no significant difference in responders versus nonresponders with regard to SAP plasma levels. SAP was first identified as a plasma glycoprotein that is a component of systemic amyloid deposits [50], and has recently been identified at high concentration in arterial atherosclerotic lesions [51]. SAP binds Ca2+-dependent ligands (danger- or damage-associated molecular patterns) at sites of injury such that Fc gamma receptor (FcγR) expressing cells can be activated to phagocytose [52]. In this way, SAP likely facilitates the local restricted innate immune cell activation at sites of tissue damage. Therapeutic administration of SAP has been shown to reduce fibrocyte numbers in bleomycin lung fibrosis [53], and inhibit kidney fibrosis associated with monocyte-macrophage regulatory mechanisms by binding primarily to FcγRIII and FcγRI [54]. Fibrocytes are pro-inflammatory/pro-fibrotic cells that differentiate from peripheral blood mononuclear cells, traffic to pulmonary blood vessels, and participate in the ensuing remodeling [55,56]. We recently found increased numbers of fibrocytes in pediatric IPAH patients compared to controls [57]. Our present study is the first to demonstrate quantitative changes in SAP in IPAH. Although our finding that SAP is increased in responders after therapy may be associative, it is tempting to suggest that increased SAP might limit fibrocyte differentiation, which could, in turn, decrease the extent of inflammation and fibrosis.
The limitations of the study include the relatively small number of samples tested, sample bias, as well as the DIGE method chosen for the proteomic analysis. To the first point, pediatric IPAH is a rare disease, and the number of accessible patients available is limited. We had an obvious difference in mean age between those with a good versus poor outcome, as has been previously reported [23, 24]. Future studies will certainly include larger study samples to expand the list of differentially expressed proteins and to examine differences in age, gender, disease class, and even protein posttranslational modification. Furthermore, there was an inherent selection bias in choosing the samples for the study. All samples were chosen in retrospect based on known outcome. Some of the pretreatment samples were from patients that had recently begun therapy and therefore were not completely treatment naïve, and patients were determined to be responders in a “post hoc” fashion. Thus, our study should be considered as primarily hypothesis generating. In our small study, no differences in age or gender with regard to SAP or SAA-4 could be discerned, and there were no apparent correlations between age and plasma levels of either SAA-4 or SAP. However, since age appears to be a critical factor determining response to long-term therapy in PAH patients, this requires further study. The prospect of serial analysis of plasma protein profile changes over time within individuals is particularly exciting in this context. To the latter point, no single proteomic technique is presently capable of examining the entire plasma proteome, with its ~1012 dynamic range of protein concentration [58]. We attempted to minimize this limitation by depleting samples of albumin and IgG prior to analysis. Since the fold changes we observed by DIGE using depleted samples were nearly identical to those we obtained by ELISA using raw plasma, it is unlikely that the depletion protocol introduced quantitative errors.
The choice of sample size in our study was based on previous investigations into the plasma proteome [5,6,8], as well as other proteomes [7,9,12,13]. The carefully controlled selection of subjects in this study, as well as standardized blood collection, processing and storage protocol, collectively minimized the variation of external factors. Some of the proteins contributing to this concept of a “responder” plasma proteome are known to be involved in inflammation and regulation of monocytes [48, 49], as well as plasma protein transport [59] and even angiogenesis [60]. This implies that the response to vasodilators, with regard to circulating proteins, is almost certainly a multifactorial, complex process that likely involves the contribution of many cell types. Indeed, our finding of reduced haptoglobin and hemopexin in responders versus nonresponders after therapy suggests that within nonresponders an ongoing intravascular hemolysis may be present [61]. Such a phenomenon may or may not impact the biology of the peripheral blood mononuclear cells. Ergo, cautious optimism must be exercised whenever conclusions are made using results from one tissue compartment (blood) and applied to other tissues (lung). Furthermore, the plasma proteome changes we observed in response to therapy may be related to unaccounted-for variability in therapeutic regimen, age, gender, diet, etc.
Despite its limitations, our study suggests that differences in the plasma proteome can potentially differentiate outcome to PAH therapy. The ability to discriminate response to therapy has major implications for our understanding of the disease process and for our ability to durably treat it. In addition, this study provides the basis for identification of more potential biomarkers and therapeutic targets for IPAH from a serially accessible resource. By understanding the differences in the plasma proteome, future investigations can focus on disease related processes in this complex biological fluid.
Clinical Relevance.
Idiopathic pulmonary arterial hypertension (IPAH) is a rare, incurable disorder characterized clinically by sustained elevations of pulmonary artery pressure. The vascular remodeling associated with IPAH is a complex process that has been ascribed to endothelial and smooth muscle cell damage leading to a loss of vasodilator function and proliferation of smooth muscle cells. Although targeted vasodilator therapy is the mainstay of treatment, IPAH currently has no cure. Most pediatric IPAH patients are diagnosed late in their disease and are unresponsive to therapy with a poor prognosis. Uncovering the molecular basis for durable response to therapy is therefore of critical importance. Our findings support the emerging concept that inflammation is a principal component of the pathobiology of pulmonary hypertension. Using nonbiased gel-based proteomic analysis, we identified quantitative differences in plasma proteins known to modulate inflammation between children with a good or poor outcome to chronic therapy, in particular, serum amyloid A-4 (SAA-4) and serum amyloid P (SAP). These proteins could be further studied in animal models to determine their potential causal contributions to the characteristic inflammation and pulmonary vascular remodeling seen in IPAH.
Acknowledgments
This study was funded by National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research (SCCOR) Grant HL-084923-02; NIH Program Project Grant HL-014985-35; the Jayden DeLuca Foundation; M01-RR00069 General Clinical Research Center National Center for Research Resources National Institutes of Health; and the Leah Bult Pulmonary Hypertension Research Fund. We thank Neil Davie, Ph.D. for his initial contributions to this work while in Denver. We acknowledge the assistance of the UC Denver Proteomics Mass Spectrometry Facility.
Abbreviations
- IPAH
idiopathic pulmonary artery hypertension
- PAH
pulmonary artery hypertension
- PAP
pulmonary artery pressur
- PBMC
peripheral blood mononuclear cells
- SAA
serum amyloid A
- SAP
serum amyloid P
Footnotes
The authors have declared no conflict of interest.
References
- 1.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23:298–304. doi: 10.1097/MOP.0b013e3283464a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haworth SG. Idiopathic pulmonary arterial hypertension in childhood. Cardiol Rev. 2010;18:64–66. doi: 10.1097/CRD.0b013e3181ce03df. [DOI] [PubMed] [Google Scholar]
- 3.Tuder RM, Abman SH, Braun T, Capron F, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S3–S9. doi: 10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol. 2007;2:369–399. doi: 10.1146/annurev.pathol.2.010506.092033. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Salam VB, Wharton J, Cupitt J, Berryman M, et al. Proteomic analysis of lung tissues from patients with pulmonary arterial hypertension. Circulation. 2010;12:2058–2067. doi: 10.1161/CIRCULATIONAHA.110.972745. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Zhang Y, Li N, Liu Z, et al. Potential diagnostic biomarkers in serum of idiopathic pulmonary arterial hypertension. Respir Med. 2009;103:1801–1806. doi: 10.1016/j.rmed.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Zhou L, Li Q, Liu J, et al. Up-regulation of two actin-associated proteins prompts pulmonary artery smooth muscle cell migration under hypoxia. Am J Respir Cell Mol Biol. 2009;41:467–475. doi: 10.1165/rcmb.2008-0333OC. [DOI] [PubMed] [Google Scholar]
- 8.Yuditskaya S, Tumblin A, Hoehn GT, Wang G, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113:1122–1128. doi: 10.1182/blood-2008-03-142604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, et al. Fhl-1, a new key protein in pulmonary hypertension. Circulation. 2008;118:1183–1194. doi: 10.1161/CIRCULATIONAHA.107.761916. [DOI] [PubMed] [Google Scholar]
- 10.Terrier B, Tamby MC, Camoin L, Guilpain P, et al. Identification of target antigens of antifibroblast antibodies in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1128–1134. doi: 10.1164/rccm.200707-1015OC. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich S, Taraseviciene-Stewart L, Huber LC, Speich R, et al. Peripheral blood B lymphocytes derived from patients with idiopathic pulmonary arterial hypertension express a different RNA pattern compared with healthy controls: a cross sectional study. Respir Res. 2008;9:20. doi: 10.1186/1465-9921-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyrick BO, Friedman DB, Billheimer DD, Cogan JD, et al. Proteomics of transformed lymphocytes from a family with familial pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:99–107. doi: 10.1164/rccm.200703-499OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laudi S, Steudel W, Jonscher K, Schöning W, et al. Comparison of lung proteome profiles in two rodent models of pulmonary arterial hypertension. Proteomics. 2007;7:2469–2478. doi: 10.1002/pmic.200600848. [DOI] [PubMed] [Google Scholar]
- 14.Yu M, Wang XX, Zhang FR, Shang YP, et al. Proteomic analysis of the serum in patients with idiopathic pulmonary arterial hypertension. J Zhejiang Univ Sci B. 2007;8:221–227. doi: 10.1631/jzus.2007.B0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamé MW, Jones AD, Wilson DW, Segall HJ. Monocrotaline pyrrole targets proteins with and without cysteine residues in the cytosol and membranes of human pulmonary artery endothelial cells. Proteomics. 2005;17:4398–4413. doi: 10.1002/pmic.200402022. [DOI] [PubMed] [Google Scholar]
- 16.Schott P, Singer SS, Kögler H, Neddermeier D, et al. Pressure overload and neurohumoral activation differentially affect the myocardial proteome. Proteomics. 2005;5:1372–1381. doi: 10.1002/pmic.200401005. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Salam VB, Paul GA, Ali JO, Gibbs SR, et al. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics. 2006;6:2286–2294. doi: 10.1002/pmic.200500510. [DOI] [PubMed] [Google Scholar]
- 18.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–1208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 19.Barst RJ, Ertel SI, Beghetti M, Ivy DD. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J. 2011;37:665–677. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, et al. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–665. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 21.Barst RJL, Gersony W. Long-term vasodilator treatment improves survival in children with primary pulmonary hypertension. Cardiol Young. 1993;3:89. [Google Scholar]
- 22.van Loon RL, Roofthooft MT, Delhaas T, van Osch-Gevers M, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol. 2010;106:117–124. doi: 10.1016/j.amjcard.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term PAH disease management. Circulation. 2011;125:113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 24.Melnick L, Barst RJ, Rowan CA, Kerstein D, et al. Effectiveness of transition from intravenous epoprostenol to oral/inhaled targeted pulmonary arterial hypertension therapy in pediatric idiopathic and familial pulmonary arterial hypertension. Am J Cardiol. 2010;105:1485–1489. doi: 10.1016/j.amjcard.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 25.Tissot C, Ivy DD, Beghetti M. Medical therapy for pediatric pulmonary arterial hypertension. J Pediatr. 2010;157:528–532. doi: 10.1016/j.jpeds.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barst RJ. Pharmacologically induced pulmonary vasodilatation in children and young adults with primary pulmonary hypertension. Chest. 1986;89:497–503. doi: 10.1378/chest.89.4.497. [DOI] [PubMed] [Google Scholar]
- 27.Fu Q, Bovenkamp DE, Van Eyk JE. A rapid, economical, and reproducible method for human serum delipidation and albumin and IgG removal for proteomic analysis. Methods Mol Biol. 2007;357:365–371. doi: 10.1385/1-59745-214-9:365. [DOI] [PubMed] [Google Scholar]
- 28.Ho JT, White JF, Grisshammer R, Hess S. Analysis of a G protein-coupled receptor for neurotensin by liquid chromatography-electrospray ionization-mass spectrometry. Anal Biochem. 2008;376:13–24. doi: 10.1016/j.ab.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhingra V, Li Q, Allison AB, Stallknecht DE, et al. Proteomic profiling and neurodegeneration in West-Nile-virus-infected neurons. J Biomed Biotechnol. 2005;2005:271–279. doi: 10.1155/JBB.2005.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Tannu NS, Hemby SE. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat Protoc. 2006;1:1732–1742. doi: 10.1038/nprot.2006.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alban A, David SO, Bjorkesten L, et al. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. doi: 10.1002/pmic.200390006. [DOI] [PubMed] [Google Scholar]
- 33.Zurawel A, Moore EE, Peltz ED, Jordan JR, et al. Proteomic profiling of the mesenteric lymph after hemorrhagic shock: differential gel electrophoresis and mass spectrometry analysis. Clin Proteomics. 2011;8:1–14. doi: 10.1186/1559-0275-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Boylan MT, Evans CA, Whetton AD, et al. Application of two-dimensional difference gel electrophoresis to studying bone marrow macrophages and their in vivo responses to ionizing radiation. J Proteome Res. 2005;4:1371–1380. doi: 10.1021/pr050067r. [DOI] [PubMed] [Google Scholar]
- 35.Hunsucker SW, Solomon B, Gawryluk J, Geiger JD, et al. Assessment of post-mortem-induced changes to the mouse brain proteome. J Neurochem. 2008;105:725–737. doi: 10.1111/j.1471-4159.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenzweig EB, Feinstein JA, Humpl T, Ivy DD. Pulmonary arterial hypertension in children: diagnostic work-up and challenges. Prog Pediatr Cardiol. 2009;27:4–11. doi: 10.1016/j.ppedcard.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King VL, Thompson J, Tannock LR. Serum amyloid A in atherosclerosis. Curr Opin Lipidol. 2011;22:302–307. doi: 10.1097/MOL.0b013e3283488c39. [DOI] [PubMed] [Google Scholar]
- 38.Hua S, Song C, Geczy CL, Freedman SB, et al. A role for acute-phase serum amyloid A and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Rep. 2009;14:187–196. doi: 10.1179/135100009X12525712409490. [DOI] [PubMed] [Google Scholar]
- 39.Obici L, Raimondi S, Lavatelli F, Bellotti V, et al. Susceptibility to AA amyloidosis in rheumatic diseases: a critical overview. Arthritis Rheum. 2009;61:1435–1440. doi: 10.1002/art.24735. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PG, Thompson JC, Webb NR, de Beer FC, et al. Serum amyloid A but not C-reactive protein stimulates vascular proteoglycan synthesis in a pro-atherogenic manner. Am J Pathol. 2008;173:1902–1910. doi: 10.2353/ajpath.2008.080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tannock LR, King VL. Proteoglycan mediated lipoprotein retention: a mechanism of diabetic atherosclerosis. Rev Endocr Metab Disord. 2008;9:289–300. doi: 10.1007/s11154-008-9078-0. [DOI] [PubMed] [Google Scholar]
- 42.Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res. 2011;42:219–225. doi: 10.1016/j.arcmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Gungor O, Kircelli F, Demirci MS, Tuncel P, et al. Serum paraoxonase 1 activity predicts arterial stiffness in renal transplant recipients. J Atheroscler Thromb. 2011;18:901–905. doi: 10.5551/jat.9175. [DOI] [PubMed] [Google Scholar]
- 44.Kang KW, Chang HJ, Kim YJ, Choi BW, et al. Cardiac magnetic resonance imaging-derived pulmonary artery distensibility index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ J. 2011;75:2244–2251. doi: 10.1253/circj.cj-10-1310. [DOI] [PubMed] [Google Scholar]
- 45.Hunter KS, Lee PF, Lanning CJ, Ivy DD, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boesch-Saadatmandi C, Rimbach G, Schrader C, Kofler BM, et al. Determinants of paraoxonase activity in healthy adults. Mol Nutr Food Res. 2010;54:1842–1850. doi: 10.1002/mnfr.201000190. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T, Ikeda K, Yamamoto Y, Iida H, et al. Effects of serum amyloid a and lysophosphatidylcholine on intracellular calcium concentration in human coronary artery smooth muscle cells. Int Heart J. 2011;52:185–193. doi: 10.1536/ihj.52.185. [DOI] [PubMed] [Google Scholar]
- 48.Yeager ME, Belchenko DD, Nguyen CM, Colvin KL, et al. Endothelin-1, the unfolded protein response, and persistent inflammation-role of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2012;46:14–22. doi: 10.1165/rcmb.2010-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connolly M, Marrelli A, Blades M, McCormick J, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2010;184:6427–6437. doi: 10.4049/jimmunol.0902941. [DOI] [PubMed] [Google Scholar]
- 50.Cathcart ES, Wollheim FA, Cohen AS. Plasma protein constituents of amyloid fibrils. J Immunol. 1967;99:376–385. [PubMed] [Google Scholar]
- 51.Stewart CR, Haw A, 3rd, Lopez R, McDonald TO, et al. Serum amyloid P colocalizes with apolipoproteins in human atheroma: functional implications. J Lipid Res. 2007;48:2162–2171. doi: 10.1194/jlr.M700098-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–6741. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 53.Pilling D, Roife D, Wang M, Ronkainen SD, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castaño AP, Lin SL, Surowy T, Nowlin BT, et al. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bucala R, Spiegel LA, Chesney J, Hogan M, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 56.Stenmark KR, Frid MG, Yeager ME. Fibrocytes: potential new therapeutic targets for pulmonary hypertension? Eur Respir J. 2010;36:1232–1235. doi: 10.1183/09031936.00137410. [DOI] [PubMed] [Google Scholar]
- 57.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, et al. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J. 2012;39:104–111. doi: 10.1183/09031936.00072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surinova S, Schiess R, Hüttenhain R, Cerciello F, et al. On the development of plasma protein biomarkers. J Proteome Res. 2011;10:5–16. doi: 10.1021/pr1008515. [DOI] [PubMed] [Google Scholar]
- 59.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. 2008;87:617–629. doi: 10.1016/j.ejcb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Dobryszycka W. Biological functions of haptoglobin—new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997;35:647–654. [PubMed] [Google Scholar]
- 61.Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin Chim Acta. 2001;312:13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]