Summary
A new paradigm has emerged relating the pathogenesis of rheumatoid arthritis (RA), focused on the balance between T helper type 17 cells and regulatory T cells (Tregs). In humans, both subpopulations depend on transforming growth factor (TGF)-β for their induction, but in the presence of inflammatory cytokines, such as interleukin (IL)-6, the generation of Th17 is favoured. Tocilizumab is a therapeutic antibody targeting the IL-6 receptor (IL-6R), which has demonstrated encouraging results in RA. The aim of this study was to evaluate the effect of tocilizumab on Th1 cells, Th17 cells, IL-17 and interferon (IFN)-γ double secretors Th17/Th1 cells, and Tregs in RA patients. Eight RA patients received tocilizumab monthly for 24 weeks and blood samples were obtained every 8 weeks to study T cell populations by flow cytometry. The frequency of Th17 cells, Th1 cells and Th17/Th1 cells was evaluated in peripheral blood mononuclear cells (PBMCs) activated in vitro with a polyclonal stimulus. Tregs were identified by their expression of forkhead box protein 3 (FoxP3) and CD25 by direct staining of PBMCs. Although no changes were detected in the frequency of Th1 or Th17 cells, the percentages of peripheral Tregs increased after therapy. In addition, the infrequent Th17/Th1 subpopulation showed a significant increment in tocilizumab-treated patients. In conclusion, tocilizumab was able to skew the balance between Th17 cells and Tregs towards a more protective status, which may contribute to the clinical improvement observed in RA patients.
Keywords: interleukin-6, regulatory T cells, rheumatoid arthritis, Th17 cells, tocilizumab
Introduction
Interleukin (IL)-6 is a pleiotropic cytokine produced by several cell types, including T and B lymphocytes, and synovial fibroblasts. This cytokine binds to membrane-anchored or soluble IL-6 receptor (IL-6R), which associates and delivers signals through gp130, a widely expressed membrane protein, allowing IL-6 to act on many tissues [1]. IL-6 has been associated with rheumatoid arthritis (RA) pathogenesis, as elevated levels of IL-6 on RA patients’ sera and synovial fluid correlate with high disease activity [2]. IL-6 contributes to the inflammatory and degenerative process in RA through multiple mechanisms. For instance, it promotes an increase in acute phase proteins, osteoclastogenesis and bone resorption, plasmatic cell differentiation, and T cell proliferation [3]. Tocilizumab is a humanized anti-IL-6R antibody that has demonstrated effectiveness in reducing disease activity and delaying joint destruction in RA patients [4].
Recent evidence suggests that keeping an adequate balance between pathogenic T helper type 17 (Th17) cells and protective regulatory T cells (Tregs) is critical for preventing the development of RA and other inflammatory diseases [5]. Pioneer experiments performed with murine naive T cells suggested that Th17 and Tregs have a common but mutually exclusive developmental pathway. It was demonstrated that both lineages depend on transforming growth factor (TGF)-β for their development; however, when IL-6 was added to the culture medium Treg differentiation was repressed, while Th17 differentiation was completed [6]. There is no consensus regarding the cytokines required for Th17 and Treg differentiation in humans. Based on in-vitro studies, some authors have proposed that, as in the mouse, IL-6 is a suppressor of Treg induction, while it potentiates Th17 development together with TGF-β, IL-1β, IL-23 and IL-21 [7,8]. In order to explore the effects of IL-6 on human Tregs, Th17 and Th1 cells in vivo, we analysed the frequency of these cells in the peripheral blood of active RA patients treated for 6 months with tocilizumab.
Materials and methods
Patients
We recruited eight female patients meeting the American College of Rheumatology (ACR) criteria for RA, who exhibited an active disease as defined by a Disease Activity Score for 28 joints (DAS28) > 5·1, regardless of receiving treatment with disease-modifying anti-rheumatic drugs (Table 1). At study entry, patients presented a mean ± standard deviation (s.d.) age of 48 ± 9 years and disease duration of 3·5 ± 2·6 years. Patients received tocilizumab intravenously (8 mg/kg of body weight), monthly, for 24 weeks. The ACR20, ACR50 [9] and European League Against Rheumatism (EULAR) criteria [10] were used to define response to treatment. Blood samples were drawn at study entry and every 8 weeks. Seven female healthy individuals were recruited as controls (mean ± s.d., age 44·1 ± 8·3 years). The study was approved by the Ethical Committee of the Hospital Clínico, Universidad de Chile, and patients and donors gave their written consent.
Table 1.
Clinical characteristics of rheumatoid arthritis patients at baseline and after 6 months of therapy with tocilizumab.
| Baseline (mean ± s.d.) | 6 months (mean ± s.d.) | P | |
|---|---|---|---|
| ESR (mm/h) | 34·13 ± 21·91 | 8·5 ± 17·58 | 0·0078 |
| CRP (mg/l) | 31·98 ± 32·07 | 3,79 ± 7·53 | 0·0234 |
| HAQ | 2·14 ± 0·59 | 0·91 ± 0·91 | 0·0206 |
| DAS28 | 6·43 ± 0·83 | 2·43 ± 1·61 | 0·0078 |
ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; HAQ: Health Assessment Questionnaire; DAS28: Disease Activity Score for 28 joints; s.d.: standard deviation.
Flow cytometry
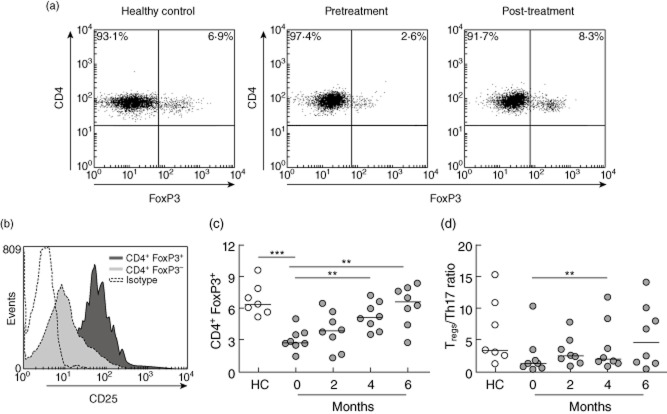
Peripheral blood mononuclear cells (PBMCs) were obtained from blood samples by density gradient with Ficoll-Paque (GE Healthcare, Uppsala, Sweden) and cryopreserved in liquid nitrogen until use. For IFN-γ and IL-17-producing CD4+ T cell evaluation, cells were stimulated with 15 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin (Sigma-Aldrich, St Louis, MO, USA) for 5 h at 37°C and 5% CO2, in the presence of 3 μg/ml brefeldin A (eBioscience, San Diego, CA, USA). Cells were stained with anti-CD3-fluorescein isothiocyanate (FITC) and anti-CD8-phycoerythrin-cyanin 5 (PE-Cy5) antibodies (BD Biosciences, San Jose, CA, USA). Subsequently, cells were permeabilized with a fixation/permeabilization kit (eBioscience), incubated with anti-IFN-γ-PE (BD Biosciences) and IL-17-allophycocyanin (APC) (eBioscience) antibodies and fixed for flow cytometry. For Treg determination, PBMCs were incubated with anti-CD4-FITC and anti-CD25-PE-Cy7 antibodies (eBioscience), permeabilized with a forkhead box protein 3 (FoxP3) fixation/permeabilization buffer set (eBioscience) and incubated with anti-FoxP3-PE antibody or rat immunoglobulin (Ig)G2a-PE antibody (eBioscience) as an isotype control. Data were acquired in a FACSCalibur flow cytometer (BD Biosciences) and analysed using WinMDI version 2·9 software. A region was set in CD3+CD8– cells to define the CD4+ T cell population, and Tregs were defined as CD4+ FoxP3+ cells, which contained cells expressing high levels of CD25 (Fig. 2a,b).
Figure 2.
Regulatory T cells (Tregs) in rheumatoid arthritis (RA) patients treated with tocilizumab. (a) Representative dot-plots of CD4+ forkhead box protein 3 (FoxP3)+ Tregs from a healthy control and an RA patient before and after 6 months of therapy. (b) Representative histogram showing that the CD4+ FoxP3+ population expresses higher levels of CD25 than the CD4+ FoxP3– population. (c) Percentages of Tregs in the blood of healthy controls (HC) and RA patients at baseline and after 6 months of therapy with tocilizumab. (d) Treg/Th17 ratios on HC and on RA patients at baseline and after 6 months of therapy. Horizontal lines represent median values. **P < 0·01; ***P < 0·001.
Statistical analyses
To compare cell populations at baseline with those obtained after therapy, the two-tailed Wilcoxon signed-rank test was used. Differences between RA patients and healthy controls were analysed using the two-tailed Mann–Whitney U-test. The Spearman test was applied for correlations between clinical and immunological variables. P < 0·05 was considered significant. For statistical analyses and graphics, Prism version 5 software (GraphPad, San Diego, USA, USA) was used.
Results
A significant decrease in clinical parameters of disease activity and severity [erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), DAS28 and Health Assessment Questionnaire (HAQ) scores] was observed in this group of RA patients after 6 months of tocilizumab therapy (Table 1). In agreement with these results, seven of eight and five of eight patients achieved ACR20 and ACR50 response criteria, respectively. According to the EULAR criteria, seven of eight patients showed a good response, while one patient exhibited a moderate response.
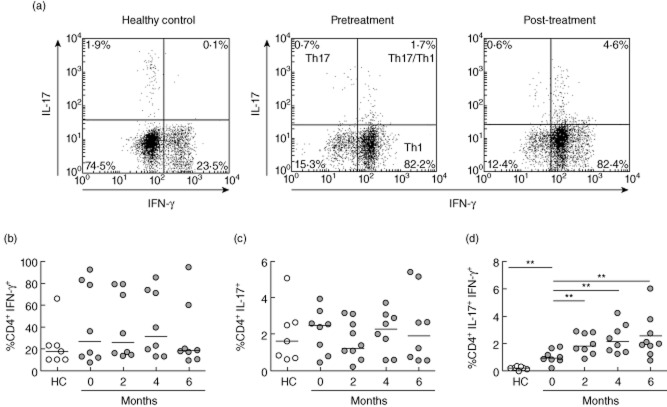
We assessed the frequency of the main CD4+ T cell effector subpopulations involved in RA pathogenesis, Th1 and Th17 cells as determined by the production of IFN-γ and IL-17, respectively, after a polyclonal stimulus of PBMCs obtained from blood of RA patients receiving tocilizumab therapy, and compared them to healthy controls (Fig. 1a). As described previously [11], no significant differences in the percentages of Th1 and Th17 cells were observed between RA patients at baseline and healthy controls (Fig. 1b,c). Unexpectedly, no decrease was detected in the frequency of these cell subpopulations after 6 months of IL-6R blockade (Fig. 1b,c). As anti-IL-6R therapy did not affect the number of total CD4+ T cells per ml of blood (data not shown), we concluded that changes in percentages of different populations represent changes in their absolute frequencies.
Figure 1.
T helper type 1 (Th1), Th17 and Th17/Th1 populations in rheumatoid arthritis (RA) patients treated with tocilizumab. (a) Representative dot-plots of CD4+ T cells expressing interferon (IFN)-γ (Th1), IL-17 (Th17) and both cytokines simultaneously (Th17/Th1) after a polyclonal stimulus, in peripheral blood mononuclear cells (PBMCs) from a healthy control and an RA patient before and after 6 months of therapy. (b–d) Percentages of Th1 cells (b), Th17 cells (c) and Th17/Th1 cells (d) in PBMCs of RA patients at baseline and after 6 months of therapy. These populations were also determined in healthy controls (HC). Horizontal lines represent median values. **P < 0·01.
Interestingly, a subpopulation of CD4+ T cells was identified that simultaneously secrete IFN-γ and IL-17 when PBMCs of RA patients were stimulated in vitro with PMA and ionomycin (Fig. 1a). This subpopulation has been described in inflamed tissues and designated ‘Th17/Th1 cells’ [12]. Of note, Th17/Th1 cells were present in significantly higher frequencies in RA patients than in healthy controls, where they were almost undetectable (P = 0·0022) (Fig. 1d). Surprisingly, Th17/Th1 cells showed a significant increase as early as 2 months after therapy was initiated, and remained elevated until the end of the protocol (P = 0·0078 for 2, 4 and 6 months of tocilizumab therapy) (Fig. 1d).
Conversely, the proportion of Tregs was reduced in PBMCs from RA patients at baseline in relation to healthy controls (P = 0·0003) (Fig. 2c). Remarkably, treatment with tocilizumab induced a significant and sustained increase in the Treg subpopulation after 4 and 6 months of therapy (P = 0·0078 for both comparisons) (Fig. 2c). Of note, no significant associations between changes in clinical parameters and changes in T cell populations along time were detected (data not shown).
Finally, the ratio between Tregs and Th17 cell frequencies in PBMCs from RA patients and healthy controls was analysed. A lower Treg/Th17 ratio was observed in RA patients in comparison to healthy controls, although this difference was not statistically significant (P = 0·0721). In RA patients, this ratio showed a trend to increase throughout the tocilizumab therapy, approaching the mean ratio exhibited by healthy controls; however, only the difference between baseline and 4 months of therapy was statistically significant (P = 0·1953, P = 0·0078 and P = 0·0547, for baseline versus 2, 4 and 6 months, respectively) (Fig. 2d).
Discussion
In recent years, IL-6 has been considered a key cytokine for tailoring the fate of naive T cells toward the proinflammatory Th17 phenotype, to the detriment of Treg peripheral induction [6]. The aim of this study was to assess the effect of an IL-6 antagonist on the balance between Th17 and Treg subpopulations in RA patients. Our data demonstrate that 6 months of therapy with tocilizumab induced an increase in the frequency of Tregs in the blood of RA patients, while no significant variations were detected in the percentages of Th17 cells.
Our results are in line with studies demonstrating an inhibitory effect of IL-6 over the Treg population [8], as we observed that inhibiting IL-6 function led to an increase in peripheral Tregs, which are diminished in the blood of RA patients in comparison to healthy controls, as described previously [13]. This increase in Tregs may be important for the positive clinical response to tocilizumab observed in most patients.
In parallel, we did not observe significant changes on the frequency of Th17 cells following tocilizumab therapy. These results are in agreement with those obtained in murine models of arthritis, in which anti-IL-6R antibodies administered at disease onset prevented arthritis induction and Th17 differentiation, whereas the same treatment applied at later stages was not able to reproduce these effects [14,15]. Those results, as well as our data, suggest that IL-6, although necessary for Th17 induction, is dispensable to sustain the Th17 response, a role that is probably assumed by IL-7 in mouse and in humans [16]. Conversely, a recent report described a drop in Th17 frequencies, together with an increase in Tregs, in RA patients after three doses of tocilizumab [17]. Noticeably, the data presented herein show a tendency for a decrease in Th17 cells after 2 months of treatment, although the frequencies of this population were recovered at later time-points.
Another interesting finding of our study is the presence of an infrequent population of CD4+ T cells that simultaneously produce IFN-γ and IL-17 (Th17/Th1) in in-vitro-stimulated PBMCs of RA patients, which is almost absent in healthy controls. These cells have been found in joints of RA and juvenile idiopathic arthritis (JIA) patients and also, at low frequencies, in the blood of healthy or diseased individuals [11,12,18,19]. Our results confirm previous data showing elevated Th17/Th1 cell frequencies in the blood of RA patients compared to healthy individuals [18]. It has been suggested that Th17/Th1 cells are derived from Th17 cells exposed to a proinflammatory milieu [12,19]. Although there is much controversy regarding Th17/Th1 cells functions in health and disease, studies in mice have suggested that IFN-γ expression in Th17 cells is necessary for autoimmune damage [20], and Th17/Th1 cell frequencies in joints have been associated positively with inflammatory parameters in JIA [21]. Interestingly, in this study we found that IL-6 inhibition provokes a sharp increase in the percentage of Th17/Th1 cells in almost all patients. This phenomenon can be caused by an increase in the rate of conversion from Th17 to Th17/Th1 cells, as it has been described that IL-6 is able to stabilize the phenotype of differentiated Th17 cells [22]. Alternatively, IL-6 blockade may impair the recruitment of Th17 and Th17/Th1 cells to inflamed joints via the chemokine receptor CCR6, as tocilizumab decreases serum levels of its ligand, CCL20 [23], thus leading to accumulation of these cells in the blood. A definitive conclusion regarding this issue, and the explanation of why Th17 cells did not follow the pattern of Th17/Th1 cells, could be drawn after evaluating these subpopulations in synovial fluid of tocilizumab-treated patients.
Overall, our results indicate that blocking IL-6 in vivo is sufficient to skew the balance between Th17 and Tregs in peripheral blood, favouring a more protective response in the context of an inflammatory disease such as RA. Although the scope of our work is limited, given the restricted number of patients, we believe that this study constitutes an important step to understanding the role of IL-6 in RA pathogenesis. It is likely that a more extensive use of IL-6 antagonists in RA, together with forthcoming in-vitro studies, will extend the reach of the findings presented herein.
Acknowledgments
We are very grateful to Nancy Fabres, Juana Orellana, Camila de Gatica, Francisca Escudero and Javiera Jury for their excellent technical assistance. We also thank Dr Carolina Ribeiro for English writing assistance. We would like to thank Roche Laboratories for providing tocilizumab doses. This work was supported by Oficina de Apoyo a la Investigación Clínica, Hospital Clínico, Universidad de Chile (grant 450/11), Fondecyt-Chile (grant 1100102) and Fundación Ciencia Translacional-Chile.
Disclosure
Dr L. Soto has received consultant fees from Roche Laboratories.
References
- 1.Kishimoto T. Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 2.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi T, Tanaka Y, Amano K. Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients – REACTION 52-week study. Rheumatology (Oxf) 2011;50:1908–1915. doi: 10.1093/rheumatology/ker221. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxf) 2009;48:602–606. doi: 10.1093/rheumatology/kep028. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. et al. [DOI] [PubMed] [Google Scholar]
- 7.Volpe E, Servant N, Zollinger R. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. et al. [DOI] [PubMed] [Google Scholar]
- 8.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felson DT, Furst DE, Boers M. Rationale and strategies for reevaluating the ACR20. J Rheumatol. 2007;34:1184–1187. [PubMed] [Google Scholar]
- 10.van Gestel AM, van Prevoo ML, van ‘t Hof MA, van de Rijswijk MH, van Putte LB, Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- 11.Yamada H, Nakashima Y, Okazaki K. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. et al. [DOI] [PubMed] [Google Scholar]
- 12.Annunziato F, Cosmi L, Santarlasci V. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson CA, Brown AK, Bejarano V. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxf) 2006;45:1210–1217. doi: 10.1093/rheumatology/kel089. et al. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto M, Serada S, Mihara M. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. et al. [DOI] [PubMed] [Google Scholar]
- 15.Iwanami K, Matsumoto I, Tanaka-Watanabe Y. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. et al. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Leung S, Wang C. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. et al. [DOI] [PubMed] [Google Scholar]
- 17.Samson M, Audia S, Janikashvili N. Inhibition of IL-6 function corrects Th17/Treg imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. et al. [DOI] [PubMed] [Google Scholar]
- 18.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 19.Nistala K, Adams S, Cambrook H. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosmi L, Cimaz R, Maggi L. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:2504–2515. doi: 10.1002/art.30332. et al. [DOI] [PubMed] [Google Scholar]
- 22.Jones GW, McLoughlin RM, Hammond VJ. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. et al. [DOI] [PubMed] [Google Scholar]
- 23.Kawashiri SY, Kawakami A, Iwamoto N. Proinflammatory cytokines synergistically enhance the production of chemokine ligand 20 (CCL20) from rheumatoid fibroblast-like synovial cells in vitro and serum CCL20 is reduced in vivo by biologic disease-modifying antirheumatic drugs. J Rheumatol. 2009;36:2397–2402. doi: 10.3899/jrheum.090132. et al. [DOI] [PubMed] [Google Scholar]