Summary
B-1 cells are innate-like lymphocytes characterized by spontaneous production of ‘natural’ polyspecific antibodies, often of self-specificity, and thought to be responsible for tissue homeostasis, mucosal protection, maintaining resting serum immunoglobulin (Ig)M levels and for early immunoglobulin production following infection. Although defined most clearly in mice, a human B-1 cell counterpart, defined by the phenotype CD19 or 20+CD27+CD43+CD69 or 70–, has been proposed recently, facilitating a study of their role in human humoral immunodeficiencies, such as common variable immunodeficiency (CVID). This study examined circulating B-1 cells in 27 CVID patients in comparison to age-matched controls (n = 28). Phenotypic putative B-1 cell proportions varied widely, but there was an overall 60–70% decrease in CVID (0·039 ± 0·033% of lymphocytes, mean ± standard deviation) compared with controls (0·110 ± 0·159% of lymphocytes, P = 0·0012). This decrease was, however, explained largely by concomitant loss of total CD27+ memory B cells characteristic of CVID, although those with higher memory B cell proportions appeared to show a true decrease. No age-related effects were apparent in B-1 cell proportions. However, among CVID patients, there was a strong positive correlation between the B-1 cell proportion and serum IgM levels, a relationship that was not evident for IgA, nor was there a relationship between memory B cell proportions and serum IgM. Patients with CVID have fewer circulating putative phenotypic B-1 cells, which largely reflected the overall decrease in memory B cells. However, B-1 cell proportions correlated with resting serum IgM levels, suggesting a possible role in IgM deficiency in CVID.
Keywords: antibody deficiency, B cells, B1 cells, B-1 cells, common variable immunodeficiency
Introduction
Conventional B cells (B-2 cells) are produced from bone marrow precursors to migrate to the peripheral lymphoid tissue where they are able to bind foreign antigen and, after receiving T cell-derived co-stimulatory signals and cytokines, differentiate into memory B cells and antibody-forming cells, the latter comprising mainly bone marrow-resident plasma cells. However, in mice, a primordial type of innate-like B cell termed the ‘B-1 cell’, which arises early in embryonic development, has been defined, differing from conventional B cells in a number of key ways: (i) a preferential serosal localization; (ii) expression of a restricted variable region surface immunoglobulin repertoire; (iii) self-renewal capacity; (iv) spontaneous production of immunoglobulin (Ig)M and generation of resting serum IgM concentrations; and (v) production of low-affinity polyreactive antibodies with autoantigen specificity, which cross-react with microbial antigens [1]. The majority also express the T cell marker CD5 (B-1a cells), although some do not (B-1b cells). Functionally, they appear to be important for tissue homeostasis, where the self-specificity of secreted IgM assists in clearance of apoptotic cell debris, but they also respond to pathogens following stimulation of innate pattern-recognition receptors to produce polyspecific IgM and IgA (at mucosal surfaces), which could play a role in immune defence [1].
The existence of this population in humans has been debated, but recently B lineage cells from cord and peripheral blood expressing the phenotype CD27+CD43+CD70– had many characteristic features of B-1 cells, including spontaneous IgM secretion, efficient T cell stimulation and tonic intracellular signalling [2], potentially enabling, for the first time, assessment of this cellular subset in patients with immunodeficiency. A number of human immunodeficiency conditions are characterized by B cell abnormalities, from complete absence in conditions such as in X-linked agammaglobulinaemia to more subtle perturbations in B or T cell biology which affect the B cell's ultimate ability to differentiate appropriately into antibody-forming cells. The most common such condition to require treatment with replacement immunoglobulin is common variable immunodeficiency (CVID), a heterogeneous group of conditions whose pathogenesis is poorly understood but which have the unifying feature of failure of total immunoglobulin production, resulting in recurrent sinopulmonary and, to a lesser extent, gastrointestinal infections, often accompanied by inflammatory and autoimmune complications [3,4]. Genetic explanations are present in a small minority of patients, while in most the pathogenesis is unknown. Given the possible role of B-1 cells in host defence and the facility to detect them phenotypically, we examined this cellular subset in patients with CVID.
Methods
Twenty-seven adult CVID patients (16 female, 11 male; aged 27–74 years, median 53) were enrolled from immunology out-patient clinics of Westmead Hospital, Sydney, a major teaching hospital of the University of Sydney. The diagnosis of CVID was based on a history of respiratory, gastrointestinal or skin infections, a serum IgG below normal range (< 7 g/l) and the exclusion of secondary causes of hypogammaglobulinaemia. Pneumococcal vaccination responses were measured in very few of our patients, and could not be used either as a diagnostic criterion or in analysis. All patients were receiving regular intravenous immunoglobulin (IVIG) infusions. Only one (female) patient had a peripheral B cell proportion of less than 1% (0·74%). Twenty-eight (12 female, 16 male) age-matched (30–62 years, median 52) Sydney residents were included as controls. All patients and controls gave informed consent and the study was approved by the Ethics Committee, Western Sydney Local Health District.
Peripheral blood was collected in lithium heparin, in the case of CVID patients timed to immediately precede the IVIG infusion. Whole blood was lysed at room temperature using ammonium chloride lysing solution (Kinetik, Queensland, Australia) and stained for 10 min with the following antibodies: CD27-phycoerythrin (PE) (clone 1A4CD27; Immunotech, Marseille, France), CD19-peridinin chlorophyll-cyanin 5·5 (PerCP-Cy5·5) (clone SJ25C1; BD Bioscience, CA, USA), CD43-allophycocyanin (APC) (clone 1G10; BD Pharmingen, CA, USA) and CD69-fluorescein isothiocyanate (FITC) (clone L78; BD Bioscience), the latter chosen over CD70 due to ready supply, and its equivalence as an activation marker [2]. CD19 was used to mark B cells, given its equivalence to CD20 in marking peripheral blood B cells, and given the supplementary data presented in Griffen et al. demonstrating universal expression by putative B-1 cells [2]. Samples were acquired on a fluorescence activated cell sorter (FACS) Calibur flow cytometer (BD Biosciences Pharmingen) within 24 h of processing, and listmode data analysed using Cellquest software (BD Biosciences Pharmingen). Typically, between 5000 and 10 000 (median 8400) B cell events were collected; three patients had fewer events due to low B cell proportions. Lymphocyte counts were obtained via the ADVIA120 haematology system (Siemens, NY, USA).
Statistics were performed using GraphPad Prism for Macintosh (GraphPad Software, La Jolla, CA, USA). The Mann–Whitney U-test analysed the mean difference between continuous variables, with P-values below 0·05 considered significant. Linear regression analysis was used to examine the relationship between B-1 cells and IgM.
Results
Human phenotypic B-1 cells are reduced in CVID
Blood cells were stained for CD19, CD27, CD43 and CD69 and analysed as illustrated in Fig. 1. Cells expressing the B-1 phenotype (CD19+CD27+CD43+CD69–) constituted a small but distinct population, which could be readily quantified. These cells were relatively rare in the peripheral blood of controls, comprising 0·11 ± 0·16% [mean ± standard deviation (s.d.)] of lymphocytes (1·2 ± 1·9% of B cells), and varied widely between individuals (Fig. 2a). Phenotypic B-1 cell proportions in CVID patients also varied widely, but there was an approximate two-thirds reduction in frequency, with B-1 cells constituting on average 0·039 ± 0·033% of lymphocytes (0·66% of B cells) (P = 0·0012 for proportion of lymphocytes, P = 0·018 for B cells) (Fig. 2a). This difference was noted throughout the adult age range (Fig. 2b) without the age-related changes in B-1 cell proportions reported previously [2], either when the B-1 cell population was expressed as a percentage of lymphocytes (Fig. 2b) or of memory B cells (data not shown).
Figure 1.
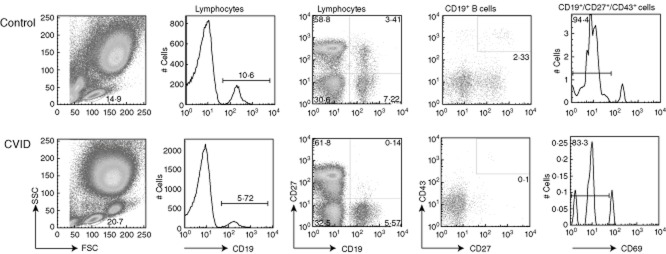
Gating strategy for detection of putative B-1 cells in a representative control (upper plots) and a common variable immunodeficiency (CVID) patient (lower plots). The gate for each column is shown. Initial gating was on lymphocytes by light scatter characteristics. CD27+CD43+ plots were gated on CD19+ (second column) lymphocyte events, and cellular proportion determined. Finally, CD19+CD27+CD43+ cells staining positive for CD69 (very few events) were excluded. The CD27+ memory B cell compartment was calculated by gating on lymphocytes, as shown (far right).
Figure 2.
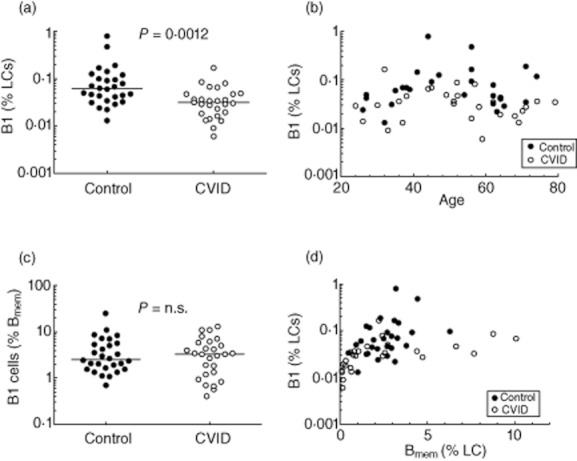
Putative phenotypic B-1 cells in common variable immunodeficiency (CVID) patients in comparison to controls. (a) A significant reduction in B-1 cells, as a proportion of lymphocytes, was noted in CVID patients (note logarithmic scale). (b) The decrease in B-1 cells appears to be present at all ages, and no clear trend for B-1 cells with respect to age was noted in either group. (c) When B-1 cells were expressed as a proportion of CD27+ memory B cells, no differences were noted between CVID patients and controls, suggesting that the decrease in B-1 cells related largely to a decrease in total CD27+ memory B cells. (d) B-1 cell proportions plotted against CD27+ memory B cell proportions (as a percentage of lymphocytes) show minimal differences between CVID patients and controls, with the possible exception of a few CVID patients with normal to high proportions of memory B cells (e.g. > 4% of lymphocytes) who may have reduced proportions.
The reduction in phenotypic B-1 cell proportions in CVID patients was related largely to loss of total memory B cells
Because memory B cells are often defined by expression of CD27 [5], B-1 cells as defined by Griffin et al. [2] could be seen simply as a subset of memory B cells, known to be reduced in most CVID patients. We therefore sought to determine whether the reduction in B-1 cells simply represented a reduction in total CD27+ B cells or were regulated independently. In support of the former hypothesis we found that B-1 cells, when expressed as a proportion of memory B cells, did not differ between CVID patients and controls (Fig. 2c). Further, B-1 cell proportions also seemed similar to controls when expressed as a function of total memory B cell proportions (Fig. 2d). Conversely, those with normal or higher memory B cell proportions did seem to show a true decrease in B-1 cell proportions, although the numbers were relatively small (Fig. 2d).
B-1 cell proportions show a strong correlation with IgM levels
Given the role of B-1 cells in ‘natural’ IgM production [6], we next examined whether there was any relationship between circulating B-1 cell proportions and serum IgM levels, taken within the preceding 3 months. We found a strong positive correlation (Fig. 3a) (P < 0·0001), a relationship that was not apparent with IgA (Fig. 3b) or between circulating total memory B cell proportions and IgM (data not shown).
Figure 3.
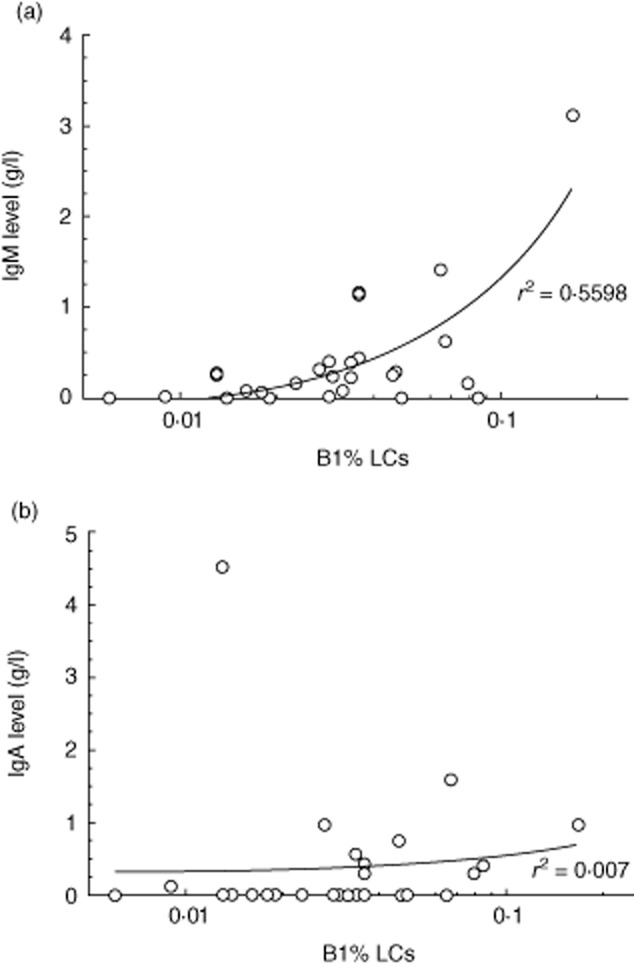
Relationship to most recent serum immunoglobulin (Ig)M level (a) and IgA (b) against the proportion of putative B-1 cells, with linear regression line as shown (note logarithmic scale).
Discussion
A number of cellular perturbations have been described which might contribute to pathogenesis of CVID, or its complications [7]. The most frequent is the finding of reduction in isotype-switched and total memory B cell subsets in the majority of patients [8–10], which can be predictive of autoimmune and inflammatory complications, particularly in patients who demonstrate accumulation of CD21–CD38– B cells of an ‘anergic’ or ‘exhausted’ phenotype [9,11]. Other cellular abnormalities include loss of plasmablasts [10], decreased naive T cells [10,12], regulatory T cells [13], invariant natural killer (iNK) T cells [14] and Toll-like receptor (TLR) signalling defects [15,16]. The plethora of cellular abnormalities, however, has failed to provide a unifying hypothesis for the final common pathway, namely loss of B cell responses and resting serum immunoglobulin concentrations, perhaps reflecting the heterogeneity of the condition.
In this study we describe another lymphocyte abnormality associated with CVID, namely paucity of putative phenotypic B-1 cells (Fig. 1). Although we found a highly significant reduction, firm conclusions must be tempered by the phenotypic overlap with conventional (B-2) B cells bearing a memory phenotype (CD27 expression), which are known to be depleted in many patients with CVID. Therefore, when expressed in relationship to total memory B cells, there was no evident decrease (Fig. 2c), with the possible exception of CVID patients with preserved or high memory B cell proportions (Fig. 2d). In terms of possible disease pathogenesis, these observations would suggest either that the same (largely unknown) processes which result in the lack of CD27+ B-2 cells in CVID are also affecting B-1 cells or, alternatively, that CD27+CD43+CD69– B cells are simply a subset of conventional memory B-2 cells rather than a separate lineage, and are affected by a singular pathogenesis in CVID. The latter view must be considered, given the reservations by some of the veracity of the original phenotypic description [17,18]. These papers have questioned the possibility that CD3+ T cells might contaminate the analysis gate and that the B-1 phenotype might represent doublets. We subsequently tested a subset of our cohort (five controls, four CVID patients) for CD3 expression, and found that only about 10% of B-1 cells co-expressed CD3. Thus, although some T cells might be present in this gate, the clear majority of cells are true singlet putative B-1 cells. Interestingly, the T cell proportion appeared higher in those CVID patients showing the greatest depletion of memory B cells (and thus the fewest CD27+ B cell events), suggesting that those few contaminating T cells remained, while the true phenotypic B-1 cells were lost. As this phenomenon would act only to reduce differences between CVID and controls, and as we found that the differences were nevertheless statistically significant, this noteworthy phenomenon should not affect the conclusions of this study.
Conversely, we made the novel observation of a strong relationship between putative B-1 cells and serum IgM levels (Fig. 3); potentially a highly relevant observation, as in chimaeric mice 80% of the resting IgM was found to be the product of B-1 cells [6]. In our study, correlation seemed unique to B-1 cells and serum IgM levels, and was not found with total memory B cells, nor did it apply to resting serum IgA levels. To be relevant pathogenically it implies some steady state relationship between circulating B-1 cells, their tissue localization and function, and these variables remain largely unexplored. However, the relationship between B-1 cells and serum IgM is enticing, and while needing confirmation in larger cohorts may give some pathogenic clues for at least one hitherto unexplained feature of the CVID phenotype, namely loss of IgM production. If supported, such findings could direct future research endeavours towards understanding developmental pathways of B-1 cells in human immunobiology and immunodeficiency.
Acknowledgments
We would like to acknowledge the invaluable assistance of Marjorie Bennetts for the collection of samples, and to the patients and controls for their willingness to co-operate with this study. We would also like to thank Stuart Tangye for his critical reading of the manuscript.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 2.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70– [Erratum appears in J Exp Med 2011 January 17;208(1):67] J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical Immunology. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 4.Hammarstrom L, Vorechovsky I, Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120:225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangye SG, Liu YJ, Aversa G, de Phillips JH, Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouillot G, Carmagnat M, Gerard L. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30:746–755. doi: 10.1007/s10875-010-9424-3. et al. [DOI] [PubMed] [Google Scholar]
- 8.Piqueras B, Lavenu-Bombled C, Galicier L. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. et al. [DOI] [PubMed] [Google Scholar]
- 9.Warnatz K, Denz A, Drager R. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. et al. [DOI] [PubMed] [Google Scholar]
- 10.Wehr C, Kivioja T, Schmitt C. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. et al. [DOI] [PubMed] [Google Scholar]
- 11.Rakhmanov M, Gutenberger S, Keller B, Schlesier M, Peter H-H, Warnatz K. CD21low B cells in common variable immunodeficiency do not show defects in receptor editing, but resemble tissue-like memory B cells. Blood. 2010;116:3682–3683. doi: 10.1182/blood-2010-05-285585. [DOI] [PubMed] [Google Scholar]
- 12.Livaditi O, Giamarellos-Bourboulis EJ, Kakkas I. Grouping of patients with common variable immunodeficiency based on immunoglobulin biosynthesis: comparison with a classification system on CD4-naive cells [Erratum appears in Immunol Lett 2008 April 15;117(1):121] Immunol Lett. 2007;114:103–109. doi: 10.1016/j.imlet.2007.09.006. et al. [DOI] [PubMed] [Google Scholar]
- 13.Horn J, Manguiat A, Berglund LJ. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156:446–454. doi: 10.1111/j.1365-2249.2009.03913.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulcher DA, Avery DT, Fewings NL. Invariant natural killer (iNK) T cell deficiency in patients with common variable immunodeficiency. Clin Exp Immunol. 2009;157:365–369. doi: 10.1111/j.1365-2249.2009.03973.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marron TU, Yu JE, Cunningham-Rundles C. Toll-like receptor function in primary B cell defects. Front Biosci (Elite edn) 2012;4:1853–1863. doi: 10.2741/507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu JE, Knight AK, Radigan L. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. J Allergy Clin Immunol. 2009;124:349–356. doi: 10.1016/j.jaci.2009.05.019. et al, 56.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Descatoire M, Weill J-C, Reynaud C-A, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Andres M, Grosserichter-Wagener C, van Teodosio C, Dongen JJM, van Orfao A, Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]


