Summary
Interleukin (IL)-17-mediated immune response has been shown to play a critical role in inflammation-associated disease. However, its role in the pathogenesis of chronic hepatitis B virus (HBV) in paediatric patients remains unknown. We investigated the frequency of T helper type 17 (Th17) cells and evaluated the association between the Th17 and clinical characters in paediatric patients with chronic hepatitis B (CHB). The frequency of Th17 cells was detected by flow cytometry analyses from 65 paediatric patients with CHB and nine healthy controls. The degree of hepatic inflammation was graded using the histological activity index (HAI). Compared with healthy controls, the frequency of Th17 cells in peripheral blood was significantly higher in paediatric patients with CHB. The proportion of Th17 cells was higher in the patients with higher HAI score (G2–G3) compared to those subjects with lower HAI score (G0–G1), but the frequency of Th17 cells had no correlation with serum HBV DNA loads or alanine aminotransferase levels. Compared with the younger age group (age 1–6 years), Th17 cell frequency was higher in the older age group (age 7–18 years). Peripheral Th17 cell frequency is associated closely with inflammation activity of liver tissues in paediatric patients with CHB.
Keywords: hepatitis B virus, interleukin-17, paediatric patients
Introduction
Hepatitis B virus (HBV) infection remains a serious global health problem [1]. There are more than 400 million people around the world suffering from persistent HBV infection [2]. Of these, 10–20% will develop liver cirrhosis and 1–5% will progress to hepatocellular carcinomas. As a highly endemic area for HBV infection, it is estimated that 93 million people are infected with HBV in China [3–5]. Approximately 90% adults with chronic infection were acquired from infancy or in early childhood [6]. It is well known that once the HBV infection is established, clearance of the virus is determined by the interactions between the virus and host factors [7,8]. A great deal of evidence has suggested that the cellular and humoral immune responses are essential for viral clearance, and that the non-HBV-specific immune response appears to be responsible for liver damage [9–12].
CD4+ T helper type (Th) cells have been described as playing a critical role in the pathogenesis of HBV infectious disease by providing help for cytotoxic T cells and producing cytokines [13]. Recently, a novel subset of interleukin (IL)-17-producing CD4+ Th17 cells has been reported, which is distinct from Th1 and Th2 cells [14,15]. A wealth of data have supported the importance of the Th17 cytokines in the development and progression of autoimmune, allergic and infectious disease [16–19]. More recently, it has been shown that Th17 cells are highly enriched in livers of chronic hepatitis B (CHB) patients and potentially exacerbate liver damage during chronic HBV infection [20]. However, its role in paediatric patients with CHB remains unknown. Therefore, in this study, we characterized the frequency of Th17 cells, quantified the expression of Th17 cytokines and analysed the correlation between IL-17 and clinical characters in paediatric patients with CHB from Beijing 302 Hospital.
Materials and methods
Sixty-five paediatric patients with CHB who visited Beijing 302 Hospital from July 2011 to April 2012 were enrolled into the study. All patients were anti-HCV- and anti-HIV-negative (45 male patients and 20 female patients, mean age 8·6 ± 4·7 years, range 1–18 years). The diagnostic criteria were based on the Management Scheme of Diagnostic and Therapy Criteria of Viral Hepatitis, issued by the Chinese Society of Infectious Diseases and Parasitology and the Chinese Society of Hepatology, which have been described in detail in our previous studies [21,22]. The inclusion criteria were: all patients who were serum hepatitis B surface antigen (HBsAg)-positive for at least 6 months, but there was no evidence for hepatocellular carcinoma (HCC), or concomitant of HCV, HDV, HIV infection and autoimmune liver disease. The exclusion criteria of HCV infection were based on the American Association for the Study of Liver Diseases (AASLD) practice guidelines [23].The exclusion criteria of HDV were based on the management scheme of diagnostic and therapy criteria of viral hepatitis [24]. The exclusion criteria of autoimmune liver disease were based on the European Association for the Diseases of the Liver (EASL) autoimmune hepatitis guidelines [25]. The exclusion criteria of HIV were based on China-CDC [26]. No patients received anti-HBV agent or steroid 6 months before sampling. Nine healthy subjects were selected as controls. The study protocol was approved by the Beijing 302 Hospital Research Ethics Committee, and written informed consent was obtained from each patient's parents. The basic characteristics of these subjects are listed in Table 1.
Table 1.
Clinical characteristics of the patients enrolled in the study
| Group | HC | CHB |
|---|---|---|
| Case | 9 | 65 |
| Sex (male/female) | 6/3 | 45/20 |
| Age (years) | 7·7 (3–15) | 8·6 (1–18) |
| ALT (U/l) | 12·4 (8–19) | 129·5 (49–732) |
| HBV DNA (IU/ml) | n.d. | 1·78 × 108 (5·05 × 105–1·37 × 109) |
Data are shown as median and range. ALT: alanine aminotransferase; CHB: chronic hepatitis B; HC: healthy control; n.d.: not determined.
Detection of serological markers
The virological assay was performed according to our previous description [27]. Serological markers and quantitation of HBV DNA, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL) and other biochemical parameters were measured by standard procedures. HBeAg/anti-HBe, HBsAg/anti-HBs and anti-HBc were detected by enzyme-linked immunosorbent (Kewei Diagnostic Ltd, Beijing, China) or chemiluminescent assay (Abbott Laboratories, North Chicago, IL, USA). HBV DNA loads were determined by a real-time–polymerase chain reaction (RT–PCR) kit (Fosun Pharmaceutical Co., Shanghai, China) with a lower limit of detection of 500 copies/ml (about 100 IU/ml).
Flow cytometric analysis
For intracellular IL-17 staining, fresh heparinized peripheral blood (200 μl) was incubated with phorbol 12-myristate 13-acetate (PMA, 300 ng/ml; Sigma, St Louis, MO, USA) and ionomycin (1 μg/ml; Sigma-Aldrich, St Louis, MO, USA) in 800 μl RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) for 6 h. Monensin (0·4 μM; BD PharMingen, San Jose, CA, USA) was added during the first hour of incubation. The blood was incubated with phycoerythrin (PE) anti-human CD8 and fluorescein isothiocyanate (FITC) anti-human CD3 (BDIS). After surface staining, the blood was then lysed with fluorescence activated cell sorting (FACS) lysing solution (BD PharMingen) and further permeabilized, stained with anti-human IL-17 (eBioscience, San Jose, CA, USA), fixed and analysed using FACSCalibur and FlowJo software (Tristar, San Carlos, CA, USA), as described by Zhang [20].
Gene expression analysis by RT–PCR
Peripheral blood mononuclear cells (PBMCs) were isolated and total RNA was prepared using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The RNA was reverse-transcribed to complementary DNA (cDNA) using oligo (dT) primers at 37°C for 15 min and at 85°C for 5 s. Relative quantitative RT–PCR was performed using SYBR-green I Premix ExTaq on the ABI Prism 7500 (Applied Biosystems, Foster City, CA, USA), following the manufacturer's instructions. The specific primers were as follows: RAR-related orphan receptor (ROR)γt sense: 5′-GTG CTG GTT AGG ATG TGC CG-3′, RORγt anti-sense: 5′-GTG GGA GAA GTC AAA GAT GAA-3′, β-actin sense: 5′-GAG CTA CGA GCT GCC TGA CG-3′, β-actin anti-sense 5′-GTA GTT TCG TGG ATG CCA CAG-3′ [28]. Quantitative expressions of RORγt transcripts were determined by staining with the fluorogenic dye SYBR Green (Takara, Beijing, China).
Thermal cycling consisted of 95°C for 30 s, 40 cycles at 95°C for 3 s and 60°C for 30 s. β-actin was used to normalize the samples in each PCR reaction. Results were expressed in terms of relative mRNA quantification calculated by using the arithmetical formula 2ΔCt.
Analysis of histopathological score
The degree of hepatic inflammation was graded using Scheuer's scoring scheme. Formalin-fixed paraffin-embedded liver tissues were cut into 4-μm-thick sections placed onto polylysine-coated slides and stained with haematoxylin and eosin for histology analysis.
Statistical analysis
Data were analysed with spss version 12·0 for Windows (SPSS, Chicago, IL, USA). Statistical analysis was performed using the Kruskal–Wallis H-test and Mann–Whitney non-parametric U-test to assess the differences between groups. Spearman's correlation analysis was performed between the frequency of circulating Th17 cells and other parameters. A probable value of P < 0·05 was considered to be statistically significant.
Results
The frequency of circulating Th17 cells in peripheral blood from paediatric patients with CHB
We examined the frequency of circulating Th17 cells in peripheral blood by flow cytometry analyses from paediatric patients with CHB (n = 65) and healthy controls (n = 9). As illustrated in Fig. 1, the frequency of Th17 cells in paediatric patients with CHB was significantly higher than that of healthy subjects (P < 0·01).
Figure 1.
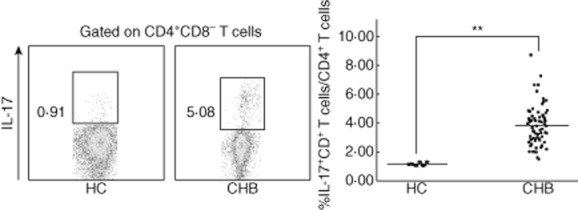
The frequency of CD4+ T helper type 17 (Th17) cells in peripheral blood is significantly higher in paediatric patients with chronic hepatitis B (CHB) compared with healthy controls. The frequencies of Th17 in peripheral blood were examined by flow cytometry from paediatric patients with CHB (n = 65) and healthy controls (n = 9). Horizontal bars represent the median percentages of Th17 frequency. **P < 0·01.
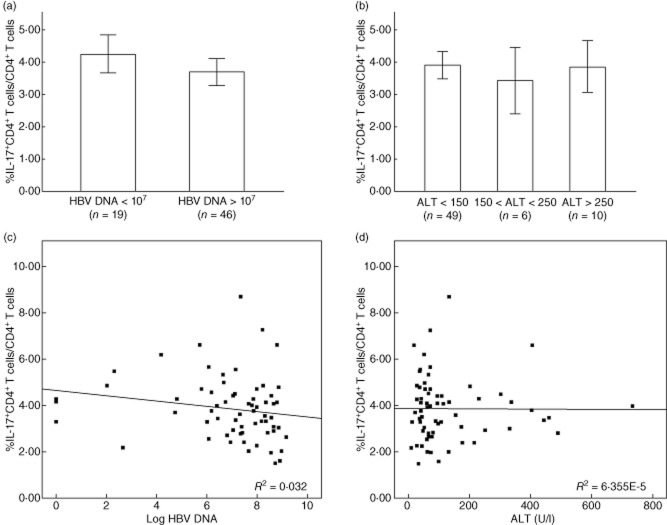
The frequency of circulating Th17 cells is not associated with serum ALT levels and serum HBV DNA loads in paediatric patients with CHB
All paediatric patients with CHB were divided into different groups based on their serum HBV DNA loads and ALT levels, respectively. An HBV DNA load of 107 copies/ml was taken as the cut-off of HBV DNA (Fig. 2a), while 150 U/l and 250 U/l were taken as the cut-offs of ALT level (Fig. 2b). There was no correlation between circulating Th17 cells and HBV DNA loads (r = −0·01548, P = 0·9051) (Fig. 2c) or serum ALT levels (r = −0·00790, P = 0·9502) (Fig. 2d) in these subjects.
Figure 2.
Association between the frequency of circulating T helper type 17 (Th17) cells and serum hepatitis B virus (HBV) DNA loads or alanine aminotransferase (ALT) levels in paediatric patients with chronic hepatitis B (CHB). Patients were divided into different groups based on serum ALT levels (a) or HBV DNA loads (b). The bars represent the (95% confidence interval) Th17 cell frequency for each group, and the significance of differences was calculated using the Kruskal–Wallis H-test. (c) The frequency of circulating Th17 cells had no correlation with serum ALT levels (r = −0·00790, P = 0·9502). (d) The frequency of circulating Th17 cells had no correlation with serum HBV DNA loads in CHB patients (r = −0·01548, P = 0·9051). The correlation between circulating Th17 cell frequency and serum ALT levels or HBV DNA loads was analysed using Spearman's correlation analysis.
The expression of RORγt mRNA in PBMC
RORγt is an important transcription factor for the differentiation of Th17 [29]; we investigated the expression of RORγt in PBMC. The level of mean relative RORγt expression in paediatric patients with CHB was significantly higher than those in healthy subjects (Fig. 3).
Figure 3.
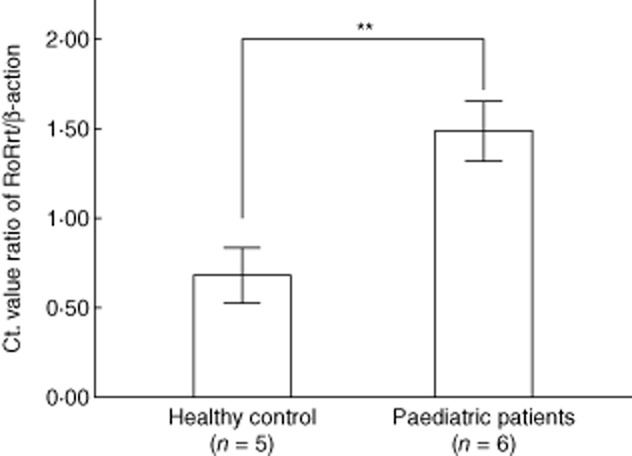
The relative quantitative expression of RAR-related orphan receptor (ROR)γt in peripheral blood mononuclear cells (PBMC). Thermal cycling consisted of 95°C for 30 s, 40 cycles at 95°C for 5 s and 58°C for 30 s. Relative expression levels of RORγt in the subjects were presented as the Ct value ratio of RORγt/β-actin from PBMC in paediatric patients and healthy controls. **P < 0·01, which was calculated using the Kruskal–Wallis H-test.
The correlation between patient ages and Th17 cell frequencies
The correlation between patient ages and Th17 cell frequencies was examined. As shown in Fig. 4, Th17 cell frequencies were higher in the older age group (age 7–18, n = 38) compared with the younger age group (age 1–6, n = 27).
Figure 4.
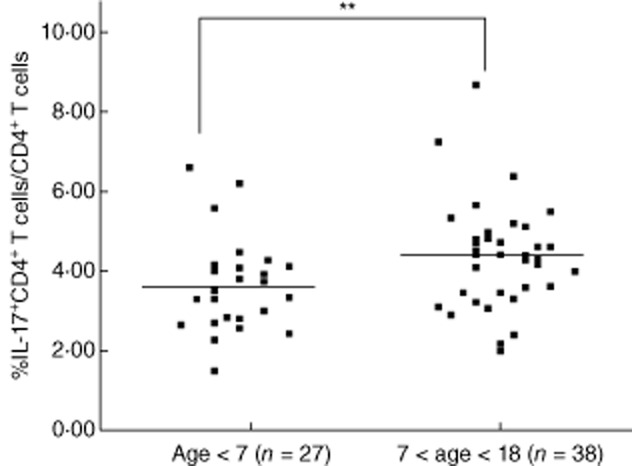
There was a significant correlation between patient ages and Th17 cell frequencies. The patients with older age (age 7–18, n = 38) had a higher frequency of T helper type 17 (Th17) cells in their peripheral blood compared with patients with younger age (age 1–6, n = 27, **P < 0·001).
Increased Th17 population is correlated positively with liver inflammation in paediatric patients with CHB
To address whether IL-17 contributes to liver damage in paediatric patients with CHB, we analysed the association between the density of IL-17-producing cells and the inflammatory activity grades of 65 paediatric patients with CHB. The inflammation was assessed using Scheuer's scoring scheme [30]. The paediatric patients with CHB with higher HAI scores (G2–G3) (n = 31) had a greater proportion of Th17 cells than those with lower HAI scores (G0–G1) (n = 34) (Fig. 5). These data suggested that peripheral Th17 cell frequency was associated closely with liver injury in paediatric patients with CHB (r = 0·29788, P = 0·0168).
Figure 5.
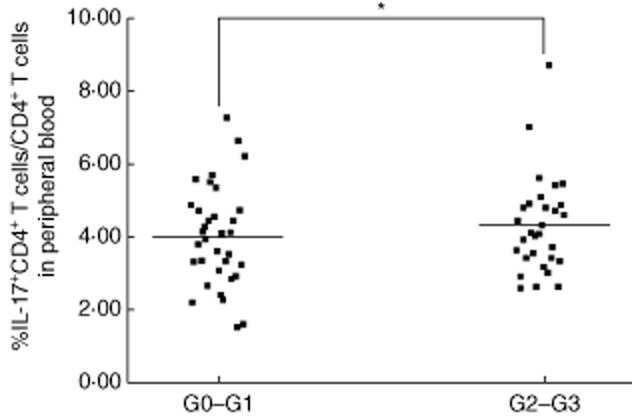
Peripheral T helper type 17 (Th17) frequency was correlated significantly with liver inflammation. Significant correlations were observed between the Th17 frequency and inflammatory activity grades. Patients with higher histological activity index (HAI) scores (G2–G3, n = 31) had a higher frequency of Th17 cells in their peripheral blood compared with patients with lower HAI scores (G0–G1, n = 34). *P < 0·05; horizontal bars represent the median percentages of Th17 frequency.
Discussion
In the present study, we analysed the frequency of Th17 cells of paediatric patients with CHB in peripheral blood, and also evaluated the association between the frequency of Th17 cells and the inflammation grades of liver tissue as well as clinical characters.
Our results showed that the frequency of Th17 cells in paediatric patients with CHB was significantly higher in peripheral blood compared with healthy controls. No obvious correlation was observed between the frequency of Th17 cells and serum HBV DNA loads, which was consistent with the previous report in adults [15]. Although HBV DNA is considered to be one of the important indicators of viral replication, its expression level was affected by many factors. No significant correlation was found between IL-17 expression and the serum HBV DNA loads in our study, which could possibly explain why HBV DNA was not a significant abnormality in some patients with liver histopathological activity [31]. Additionally, Wu et al. [15] reported that elevated prevalence of Th17 cells is associated positively with increased serum ALT levels in CHB adults, but this result was not supported in our paediatric patients. The difference between adult and paediatric patients in this respect may be explained by the following reasons: the immune system of paediatric patients developed incompletely; and the immune response to infection might be different compared to that of adults [32]. Compared with adult patients, paediatric patients were more susceptible to immunological tolerance. Therefore, ALT levels may not really reflect liver inflammation activity in paediatric patients.
Interestingly, there was a close relationship between Th17 cell frequency and age in the current study. Compared with the younger age group (1–6 years), the older age group (7–12 years) had a higher Th17 cell frequency. According to the description of Listeria monocytogenes in a model of L. monocytogenes infection in the liver, IL-17 produced by γδ T cells was critical for protective immunity in the early stages of infection [33]. The authors thought that IL-17-producing γδT cells took part in protective immunity before adaptive Th17 cells appear, and IL-17 can have opposing effects on different infection stages due to the different time-points of IL-17 appearance, which led to different functions. Our studies agreed with the above views. We presumed that IL-17 is produced by innate immune cells at a very early stage of paediatric patient infection (1–6 years), whereas in the older age group (7–18 years) who developed adaptive immune responses, the Th17 was produced by adaptive immune cells, and the fulfilled IL-17 functions lead to immunopathology. This means that IL-17 did not contribute greatly to inflammation if released in an early innate environment, but it exerts more aggravating actions in the context of adaptive immune reactions.
We also found that the patients with a higher HAI score had a greater proportion of Th17 cells than those patients with a lower HAI score, suggesting that Th17 cells might participate actively in the immunopathogenesis of paediatric patients with CHB, which is consistent with the observation in adult patients reported by Zhang et al. [20] and Ye et al. [12].
In conclusion, our results demonstrated that Th17 cell frequency was associated closely with grades of liver inflammation as well as age in paediatric patients with CHB, which showed a possible role of Th17 in the occurrence and development of chronic inflammation in paediatric patients with HBV infection.
Acknowledgments
We thank Dr Fusheng Wang, Zheng Zhang, Jiyuan Zhang (Institute of Infectious Disease, 302 Hospital, Beijing, China) for technical assistance and Dr Bin Gao (Section on Liver Biology, Laboratory of Physiologic Studies, NIAAA/NIH, 5625 Fishers Lane, Bethesda, MD 20892, USA) for critically reading the manuscript. This study was supported by the Capital Medical Development Study Foundation (no. 2009-3057), Wujieping Medical Foundation (no. LDWMF-SY-2011A004), the Military Subject of the Twelfth Five-year Plan for Science and Technology Research of China (CWS11J161) and Beijing 302 Hospital Research Foundation (YNKT2012004). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Li X, Wang L, Zhong Y. Hepatitis B virus (HBV) subgenotypes C2 and B2 differ in lamivudine and adefovir-resistance-associated mutational patterns in HBV-infected Chinese patients. J Clin Microbio. 2010;48:4363–4369. doi: 10.1128/JCM.01518-10. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu W, Shi Y, Li SP. Blockade of Tim-3 signaling restores the virus-specific CD8+ T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42:1180–1191. doi: 10.1002/eji.201141852. et al. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (EASL). EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2009;3:334–342. doi: 10.1007/s12072-008-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong YW, Han JQ, Zou ZS. Quantitation of HBV covalently closed circular DNA in micro formalin fixed paraffin-embedded liver tissue using rolling circle amplification in combination with real-time PCR. Clin Chim Acta. 2011;412:1905–1911. doi: 10.1016/j.cca.2011.06.031. et al. [DOI] [PubMed] [Google Scholar]
- 6.Jonas MM, Block JM, Haber BA. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology. 2010;52:2192–2205. doi: 10.1002/hep.23934. et al. [DOI] [PubMed] [Google Scholar]
- 7.Nebbia G, Peppa D, Maini MK. Hepatitis B infection: current concepts and future challenges. Q J Med. 2012;105:109–113. doi: 10.1093/qjmed/hcr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther. 2010;15:133–143. doi: 10.3851/IMP1497. [DOI] [PubMed] [Google Scholar]
- 9.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;15:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park O, Jeong WI, Wang L. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011;26:173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 12.Ye YF, Xie XJ, Yu JW. Involvement of Th17 and Th1 effector responses in patients with hepatitis B. J Clin Immunol. 2010;30:546–555. doi: 10.1007/s10875-010-9416-3. et al. [DOI] [PubMed] [Google Scholar]
- 13.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver. Dis Clin Dev Immunol. 2011 doi: 10.1155/2011/345803. ; doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–334. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 15.Wu W, Li J, Chen F. Circulating Th17 cells frequency is associated with the disease progression in HBV infected patients. J Gastroenterol Hepatol. 2010;25:750–757. doi: 10.1111/j.1440-1746.2009.06154.x. et al. [DOI] [PubMed] [Google Scholar]
- 16.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;7:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–968. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Kristine K, Liu L, Song QN, Jonathon D. The IL-23/Th17 axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JY, Zhang Z, Lin F. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. et al. [DOI] [PubMed] [Google Scholar]
- 21.Zhong YW, Li J, Song HB. Virologic and clinical characteristics of HBV genotypes/subgenotypes in 487 Chinese pediatric patients with CHB. BMC Infect. 2011;11:262–267. doi: 10.1186/1471-2334-11-262. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wang C, Zhong Y. Genotypic resistance profile of hepatitis B virus (HBV) in a large cohort of nucleos(t)ide analogue-experienced Chinese patients with chronic HBV infection. J Viral Hepat. 2011;18:29–39. doi: 10.1111/j.1365-2893.2010.01360.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinese Society of Infectious Diseases and Parasitology, Chinese Society of Hepatology. Management scheme of diagnostic and therapy criteria of viral hepatitis. Chin J Hepatol. 2000;6:324–329. [Google Scholar]
- 25.Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol. 2011;55:171–182. doi: 10.1016/j.jhep.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 26.China-CDC, China Ministry of Health. Beijing: China-CDC, China Ministry of Health; 2003. UN theme group on HIV/AIDS in China. [Google Scholar]
- 27.Zhong YW, Lv JY, Li J. Virology and antiviral drugs susceptibility of hepatitis B virus rtN238H polymerase mutation from 1865 Chinese patients with chronic hepatitis B. Antivir Res. 2012;93:185–190. doi: 10.1016/j.antiviral.2011.11.012. et al. [DOI] [PubMed] [Google Scholar]
- 28.Jia S, Li C, Wang G, Yang J, Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol. 2010;162:131–137. doi: 10.1111/j.1365-2249.2010.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, McKenzie BS, Zhou L. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17-T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. et al. [DOI] [PubMed] [Google Scholar]
- 30.Tonetto PA, Gonçales NS, Fais VC. Hepatitis B virus: molecular genotypes and HBeAg serological status among HBV-infected patients in the southeast of Brazil. BMC Infect. 2009;9:149–155. doi: 10.1186/1471-2334-9-149. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang LY, Chen SJ, Xu KS. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int J Mol Med. 2011;27:385–392. doi: 10.3892/ijmm.2011.594. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Chen D, Yao J. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol. 2007;122:173–180. doi: 10.1016/j.clim.2006.09.006. et al. [DOI] [PubMed] [Google Scholar]
- 33.Hamada S, Umemura M, Shiono T. IL-17 a produced by γδT cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]