Abstract
Evidence has been accumulating that some estrogen-dependent human breast cancers require estrogen for not only proliferation but also survival. To obtain insights into the molecular mechanisms of apoptosis of breast cancer cells subjected to estrogen starvation or exposed to antiestrogens, we characterized changes in the gene expression profile of MCF-7/BUS human breast cancer cells and revealed a strong induction of Bik, a member of the BH3-only proapoptotic proteins. The Bik mRNA transcript and protein were strongly induced by estrogen starvation or exposure to fulvestrant, a pure antiestrogen that competes with the natural estrogens for binding to the estrogen receptors. This Bik induction preceded apoptotic cell death, which was blocked by zVAD-fmk, a pancaspase inhibitor. Amounts of the Bcl-2-related proteins, such as Bcl-2, Bcl-XL, or Bax, showed only marginal changes in the presence or absence of estrogens or antiestrogens. Suppression of Bik expression by using the small interfering RNA effectively blocked the fulvestrant-induced breast cancer cell apoptosis. These results indicate that Bik is induced in MCF-7/BUS cells in the absence of estrogen signaling and plays a critical role in the antiestrogen-provoked breast cancer cell apoptosis.
At least 60% of human breast cancers express estrogen receptor α (ERα), and about half of these ERα-positive tumors require estrogen for their growth (1). Although the requirement of estrogen for proliferation of such hormone-dependent tumors has been widely accepted, evidence is accumulating that this steroid hormone is also necessary for survival of breast cancer cells (2–5). The importance of the mitochondria-dependent apoptotic pathway involving the Bcl-2 family apoptosis regulator proteins in estrogen-regulated breast cancer cell apoptosis has been supported by a number of studies (6–16). The antiapoptotic members of the Bcl-2 family, such as Bcl-2, share three or four conserved domains known as Bcl-2 Homology (BH) regions. Proapoptotic members such as Bax share two or three BH regions. The antiapoptotic members suppress the release of cytochrome c from mitochondria, whereas the proapoptotic members facilitate this process. When released to the cytosol, cytochrome c activates Apaf-1, which in turn activates the apoptosis-initiator caspase (caspase-9). Another group of apoptosis regulators, often referred as BH3-only proteins, only share the 9-aa BH3 region and, through this domain, bind directly to the antiapoptotic members of the Bcl-2 family to inhibit their apoptosis-suppressing function. It has been reported that expression of Bcl-2 in MCF-7 human breast cancer cells is enhanced by 17β-estradiol (E2) and decreased by antiestrogens, whereas expression of Bax is not affected by E2 or antiestrogens (7–14). However, reported changes in the amount of Bcl-2 with estrogen starvation or exposure to antiestrogens have been relatively small, typically <2-fold, suggesting possible involvement of other hormonally controlled factors that coordinately regulate apoptosis with Bcl-2.
The MCF-7 human breast cancer cell line has been commonly used as a conventional in vitro model of breast cancer. However, since its original isolation in the early 1970s (6), in the past three decades a number of MCF-7 cell “stocks” have been established and maintained independently by a number of laboratories. Because the estrogen responsiveness, the degree of oncogene amplification, and the karyotype of such MCF-7 stocks differ significantly (17–20), it is no longer possible to precisely define the original MCF-7 culture. Among these stocks, the MCF-7/BUS stock has been characterized by robust and highly reproducible estrogen dose-dependent growth (17, 21). Taking advantage of these features, we have recently determined detailed dose-dependent aspects of induction or suppression of the estrogen-regulated genes in MCF-7/BUS cells (21). Thus, MCF-7/BUS is an ideal MCF-7 cell stock for studying quantitative aspects of estrogen actions on breast cancer cells.
In the present study, we describe the induction of the Bik BH3-only protein by estrogen starvation and exposure to the antiestrogen fulvestrant in human breast cancer cells. Low concentrations of E2 effectively blocked Bik expression as well as apoptosis of MCF-7/BUS cells, and inhibition of Bik protein expression by small interfering RNA (siRNA) suppressed the fulvestrant-provoked apoptosis of this cell line. Bik protein expression was not detected in breast cancer cell lines that are resistant to the fulvestrant-provoked apoptosis, whereas some of these cell lines expressed the Bik mRNA transcripts constitutively or in a fulvestrant-dependent manner. These results suggest a critical role of Bik in antiestrogen-provoked breast cancer cell apoptosis.
Materials and Methods
Cell Culture. MCF-7/BUS and MCF-7/E8CASS cells were provided by C. Sonnenschein and A. M. Soto (Tufts University). T47D, ZR75, SK-BR3, and MDA435 human breast cancer cells were purchased from American Type Culture Collection. All cells were maintained in DMEM supplemented with 5% FCS (HyClone, DEFINED grade); this medium contained ≈60 pM serum-derived E2. To examine effects of estrogen starvation, cells at the subconfluent density were washed three times with phenol red-free medium (containing no serum) and incubated in the wash medium for 60 min at 37°C. The medium was then replaced by phenol red-free DMEM supplemented with 5% charcoal/dextran-stripped FCS (HyClone) and 0–100 pM E2 (Calbiochem) with or without 50 μM zVAD-fmk (Sigma), and the cells were cultured for 0–72 h. To examine the effects of fulvestrant, cells were cultured in the regular medium supplemented with 5% DEFINED grade FCS and 0–100 nM fulvestrant (Tocris) for 0–72 h.
DNA Microarray, RT-PCR, and Western Blotting. RNA isolation and the Affymetrix DNA microarray experiments were performed as described (21). cDNA was synthesized from 100 ng of total RNA by using SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen) according to the manufacturer's protocol. Semiquantitative RT-PCR under unsaturated amplification was performed as described (22); detailed conditions for each gene will be provided on request. Primer sequences were the following: Bik, forward 5′-GCCAGAGGAGAAATGTCTGA-3′, reverse 5′-AGTGTGGTGAAACCGTCCAT-3′; Bax, forward 5′-ATGGACGGGTCCGGGGAGCAGCCC-3′, reverse 5′-GGTGAGCACTCCCGCCACAAAGAT–3′; Bcl-XL, forward 5′-TTGGACAATGGACTGGTGGA-3′, reverse 5′-GTAGAGTGGATGGTCAGTG-3′; Bcl-2, forward 5′-CGACGACTTCTCCTCCCGCCGCTACCG C-3′, reverse 5′-CCGCATGCTGGGGCCGTACAGTTCC-3′; GAPDH, forward 5′-CCACCCATGGCA A AT TCCATGGCA-3′, reverse 5′-TCTAGACGGCAGGTCAGGTCCACC-3′. Western blotting was performed following the standard protocol. All primary antibodies were purchased from Santa Cruz Biotechnology: Anti-Bik (N-19, FL-160), anti-actin (I-19), anti-Bcl-2 (C-2), anti-Bcl-XL (L-19), anti-Bax (B-9), anti-Bid (N-19), and anti-poly(ADP-ribose) polymerase (PARP) (F-2).
siRNA Suppression of Bik Expression. To suppress expression of Bik by siRNA, subconfluent MCF-7/BUS cells were transfected with oligoduplex Bik siRNA (sense 5′-AAGACCCCUCUCCAGAGACAU-3′, antisense 5′-AAAUGUCUCUGGAGAGGGGUC-3′) and control, sequence-scrambled siRNA (Dharmacon, Lafayette, CO) by using Oligofectamine reagent (Invitrogen) according to the manufacturer's protocol.
Results
Estrogen Starvation and Exposure to Antiestrogens Induce Apoptosis of MCF-7/BUS Human Breast Cancer Cells. To determine whether MCF-7/BUS cells undergo apoptotic cell death in the absence of estrogen, cells were cultured in the presence of varying low concentrations of E2 (0 ∼ 30 pM). The growth of MCF-7/BUS cells depends on the dose of estrogen between 2 and 80 pM (21). After 72 h of incubation, the E2-free cultures showed marked cell death, whereas cultures containing as little as 10 pM E2 showed only marginal evidence of cell death (Fig. 1 A and C). The dead cells detached from the dish and showed a condensed, spherical shape that implied the apoptotic mechanism of cell death versus necrosis, and these detached cells were unable to reattach to the culture dish even when transferred to a new dish with fresh medium containing 30 pM E2 (data not shown). This cell death was effectively suppressed by z-VAD-fmk, a specific pan-caspase inhibitor that permeates the plasma membrane of living cells and suppresses apoptosis (23), indicating that cells died by apoptosis. Consistent with this observation, the pure antiestrogen fulvestrant also induced massive cell death (Fig. 1 B and C), which was also suppressed by zVAD-fmk (data not shown). Tamoxifen, a partial antiestrogen, also induced MCF-7/BUS cell death (data not shown). Whereas the fulvestrant-induced cell death was observed after 48 h of exposure (Fig. 1C), the tamoxifen-induced cell death required at least 72 h of exposure (data not shown).
Fig. 1.
Estrogen depletion provokes MCF-7/BUS cell apoptosis. (A and C) Estrogen starvation induces apoptosis. Cells were cultured in the presence of indicated concentrations of E2 for 72 h with or without 50 μM zVAD-fmk. The appearance of the culture was photographed (A), and numbers of apoptotic cells (round-shaped and detached form the dish) and live cells were counted (C Left; mean ± SEM, n = 3). (B and C) Fulvestrant-induced apoptosis. Cells were cultured in the presence or absence of 100 nM fulvestrant for 24–72hin the medium containing ≈60 pM serum-derived E2. The culture was photographed (B), and numbers of apoptotic cells and live cells were counted (C Right; mean ± SEM, n = 3).
The Bik BH3-Only Protein Is Induced in Human Breast Cancer Cells by Estrogen Starvation and Antiestrogen Exposure. To characterize roles of the Bcl-2 family apoptosis regulators in the estrogen-regulated breast cancer cell apoptosis, we determined their mRNA expression profiles in MCF-7/BUS cells cultured in the presence or absence of E2 (Figs. 2 A and B). Cells were cultured for 48 h in the presence of varying low concentrations of E2 (0 ∼ 100 pM), and their total RNA was subjected to DNA microarray analysis. In separate experiments, cells were cultured in the absence of E2 for 0, 24, and 48 h, and total RNA preparations were subjected to DNA microarray analysis (Fig. 2B). To our surprise, of the 13 members of the Bcl-2 family evaluated by these experiments, only the mRNA transcripts for the Bik proapoptotic BH3-only protein showed strong reduction in the presence of low concentrations of E2 (Fig. 2 A), whereas expression of the mRNA transcripts for the other 12 members (including Bcl-2) showed only modest changes in either the presence or absence of E2 (Fig. 2 A). During the 48 h of estrogen starvation, the mRNA transcripts for Bik increased >40-fold, whereas the mRNA transcripts for other Bcl-2-family proteins showed relatively modest changes (Fig. 2B). These DNA microarray data were confirmed by semiquantitative RT-PCR, in which the mRNA transcripts for Bik, Bax, Bcl-XL, and Bcl-2 were amplified at subsaturated PCR conditions. As shown in Fig. 2C, expression of the Bik mRNA transcripts was remarkably suppressed with increasing E2 concentrations, whereas it was enhanced dramatically during estrogen starvation. Amounts of the transcripts for other Bcl-2-family proteins showed only marginal changes, if any.
Fig. 2.
Effects of E2 and antiestrogen on expression of the mRNA transcripts for Bcl-2 family proteins in breast cancer cells. (A and B) DNA microarray data. (C) RT-PCR. MCF-7/BUS cells were cultured in the presence of indicated concentrations of E2 for 48 h (A and C) or in the absence of E2 for indicated periods of time (B and C), and the relative amounts of the mRNA transcripts were determined by the Affymetrix DNA microarray. Each datum point represents mean ± SEM (n = 5).
The induction of Bik protein in MCF-7/BUS cells by estrogen starvation and antiestrogen exposure was confirmed by Western blotting. As shown in Fig. 3A, the 22.5-kDa Bik protein was strongly expressed in the absence of E2 (i.e., 48 h estrogen starvation) and only weakly in the presence of 10 pM E2; it was undetectable in the presence of 30 pM and higher concentrations of E2. A dramatic accumulation of Bik during 2 days of hormone starvation was also confirmed (Fig. 3B). The pan-caspase inhibitor zVAD-fmk did not affect this Bik induction (Fig. 3B), while it effectively suppressed MCF-7/BUS cell apoptosis (data not shown; see Fig. 1 A). Therefore, the induction of Bik is not a downstream event of caspase activation. When cells were cultured in medium containing serum-derived estrogens (equivalent to ≈60 pM E2) in the absence of fulvestrant, there was no Bik detectable until 24 h of culture, and a very weak Bik expression was observed after 48 ∼ 72 h (Fig. 3C). However, in the presence of 10 nM or 100 nM fulvestrant, Bik was strongly induced after 48 ∼ 72 h; a weak expression could also be observed at 24 h. Although fulvestrant is usually used at 100 nM concentration for cell culture-based experiments, as low as 10 nM effectively induced Bik, provoking apoptosis of this cell line (data not shown).
Fig. 3.
Effects of E2 and antiestrogen on expression of the Bcl-2 family proteins in breast cancer cells. (A–E) Western blotting. Actin or GAPDH was visualized on the same blot as control. (A) Suppression of Bik expression by E2. MCF-7/BUS cells were cultured in the presence of indicated concentrations of E2 for 48 h. (B) Induction of Bik by estrogen starvation. MCF-7/BUS cells were subjected to estrogen starvation for up to 2 days in the presence or absence of 50 μM zVAD-fmk. (C) Induction of Bik by fulvestrant. MCF-7/BUS cells were cultured in the presence of indicated concentrations of fulvestrant for 24–72 h. The medium contained ≈60 pM serum-derived E2. (D) Effects of E2 on expression of other Bcl-2 family proteins. MCF-7/BUS cells were cultured in the presence (+) or absence (–) of 30 pM E2 for 48 h. (E) Diminished induction of Bik in E8CASS, an estrogen-independent MCF-7 derivative. Cells were cultured in the presence or absence of E2 for 48 h. (F) Expression of the Bik mRNA transcripts (RT-PCR) or protein (Western blotting) in human breast cancer cells. Cells were cultured for 48 h in the medium containing ≈60 pM serum-derived E2 with or without 100 nM fulvestrant.
To determine whether or not the induction of Bik in apoptotic MCF-7/BUS cells is specific among the Bcl-2 family members, we determined expression of Bcl-2 and Bcl-XL (antiapoptotic members), Bax (a proapoptotic, Bcl-2-like member), and Bid (BH3-only proapoptotic members) in the presence or absence of 30 pM E2 by Western blotting (Fig. 3D). Only marginal changes were observed, if any, for any of these proteins. In separate Western blotting experiments, we also observed no significant changes in expression of three other BH3-only proteins, namely, Puma, Noxa, and Bim, in the presence or absence of fulvestrant (data not shown). These results suggested a unique role for Bik among the Bcl-2 family members in the estrogen-regulated apoptosis of human breast cancer cells.
Lack of Bik Protein Expression in Breast Cancer Cells That Are Resistant to Estrogen-Regulated Apoptosis. MCF-7/E8CASS cell line was isolated from the original MCF-7 culture as a subclone that grows in an estrogen-independent manner (24). E8CASS cells express functional ERα, and their estrogen-induced transactivation of the pS2 gene is normal (24). Interestingly, as shown in Fig. 3E, the estrogen-starved E8CASS cells did not express Bik, suggesting that the inability of E8CASS cells to induce Bik in the absence of estrogen might contribute to their estrogen-independent growth.
We then determined whether fulvestrant induces Bik mRNA transcripts and protein in other human breast cancer cell lines. For this purpose, we used two ERα-positive lines (T47D and ZR75) and two ERα-negative lines (SK-BR3 and MDA-MB-435), all of which are commonly used in studies of breast cancer cell biology. These four cell lines were all resistant to antiestrogen-provoked cell death, but the antiestrogens did effectively inhibit proliferation of T47D and ZR75 cells (data not shown). As shown in Fig. 3F, fulvestrant strongly induced the Bik mRNA transcripts in T47D cells. A fulvestrant-dependent increase in the amount of the Bik mRNA transcripts was also observed with ZR75 cells, although a significant amount of the Bik mRNA was expressed even in the absence of the antiestrogen. MDA-435 did not express the Bik mRNA transcripts at all in the presence or absence of fulvestrant. Unexpectedly, SK-BR3 cells expressed the Bik mRNA transcripts very strongly with or without fulvestrant. In contrast to MCF-7/BUS cells, which express both the Bik mRNA transcripts and protein and undergo apoptosis in the presence of fulvestrant, the expression of the Bik mRNA transcripts in these apoptosis-resistant cells was not associated with any detectable expression of the Bik protein. Although the mechanisms of this lack of Bik protein expression even in the presence of its mRNA transcripts are unclear, these observations are consistent with the resistance of these cell lines to the antiestrogen-provoked apoptosis.
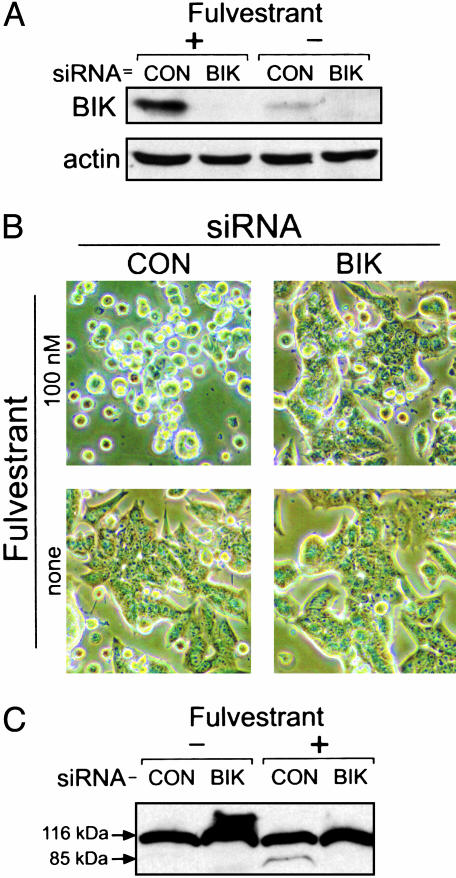
Suppression of Bik Expression by siRNA Inhibited Fulvestrant-Induced Apoptosis of MCF-7/BUS Cells. To test whether the Bik induction is required for the fulvestrant-provoked breast cancer cell apoptosis, we suppressed expression of Bik in MCF-7/BUS cells by the siRNA. As shown in Fig. 4A, transient transfection of a Bik-suppressing siRNA (before fulvestrant treatment) completely blocked fulvestrant-induced expression of Bik. A control siRNA did not affect the fulvestrant-induced Bik expression. Strikingly, cells transfected with the Bik-suppressing siRNA showed remarkable resistance to the fulvestrant-provoked apoptosis; in contrast, the culture transfected with the control siRNA showed the expected, marked apoptosis (Fig. 4B). Without fulvestrant, neither the Bik-suppressing nor the control siRNA affected cell viability. Thus, Bik induction is required for the fulvestrant-provoked apoptosis of MCF-7/BUS cells.
Fig. 4.
Bik is necessary for the fulvestrant-induced MCF-7/BUS cell apoptosis. (A) Suppression of Bik expression by siRNA. Cells were transiently transfected with Bik-specific (BIK) or sequence-scrambled control (CON) siRNA and then cultured in the presence or absence of 100 nM fulvestrant for 48 h, followed by evaluation of expression of Bik and actin (as control) by Western blotting. (B) Suppression of fulvestrant-induced cell death by Bik-specific siRNA. Cells were transiently transfected with Bik-specific (BIK) or sequence-scrambled control (CON) siRNA and then cultured in the presence or absence of 100 nM fulvestrant for 72 h. The phase-contrast images of the cultures show apoptotic cells that are detached from the dish and round-shaped. (C) Suppression of fulvestrant-induced PARP cleavage by Bik-specific siRNA. Cells were transiently transfected with Bik-specific (BIK) or sequence-scrambled control (CON) siRNA and then cultured in the presence or absence of 100 nM fulvestrant for 72 h. The full-length (116 kDa) and apoptosis-signature fragment (85 kDa) of PARP were detected by Western blotting.
Because MCF-7 cells lack the CASP-3 gene that encodes caspase-3 (25), an executioner caspase required for effective DNA cleavage, DNA fragmentation is not typically observed very strongly with apoptotic MCF-7 cells (26, 27). Concomitant with this, our attempts to demonstrate MCF-7/BUS cell apoptosis by assays dependent on DNA fragmentation were not successful (data not shown). Therefore, to further confirm that Bik is required for the fulvestrant-provoked cell apoptosis, effects of the siRNAs on fulvestrant-induced cleavage of the endogenous PARP in MCF-7/BUS cells was evaluated. In nonapoptotic cells, PARP is observed in its uncleaved form (116-kDa); in apoptotic cells, PARP is characteristically cleaved by activated caspases to an 85-kDa fragment (28). As shown in Fig. 4C, the 85-kDa apoptosis-indicating PARP fragment was observed in fulvestrant-exposed cells transfected with the control siRNA. This fulvestrant-induced PARP cleavage was not observed in cells transfected with the Bik-suppressing siRNA. These results provided further evidence that Bik is involved in the fulvestrant-induced apoptosis of human breast cancer cells.
Discussion
It has been widely accepted that many ERα-positive human breast cancer cells require estrogen for their proliferation (1). However, a number of published studies have demonstrated that estrogen is also required for survival of certain breast cancer cells. When subjected to estrogen starvation, exposure to antiestrogens, or treatment with aromatase inhibitors, significant apoptosis of breast cancer cells (including MCF-7 cells) have been observed in vivo and in vitro (2–5), although the mechanisms of regulation of apoptosis by estrogen is understood only poorly. In the present study, we have found an unexpected induction of the Bik proapoptotic BH3-only protein in human breast cancer cells during estrogen starvation and exposure to antiestrogens. Experiments using siRNA demonstrated that the induction of Bik is necessary for the antiestrogen-provoked apoptosis of MCF-7/BUS cells. However, in other breast cancer cell lines resistant to the antiestrogen-provoked apoptosis, expression of the Bik protein was not detected in the presence or absence of fulvestrant, although some expressed the Bik mRNA transcripts constitutively or in a fulvestrant-inducible manner. These findings point to the importance of Bik in estrogen-regulated apoptosis of breast cancer cells. Although several previous studies reported that expression of Bcl-2 in MCF-7 cells is enhanced by E2 and decreased by antiestrogens (5, 7–14), we observed only modest changes in Bcl-2 expression in MCF-7/BUS cells cultured in the presence of varying concentrations of E2. Although this may reflect differences in MCF-7 cell stocks used in these studies, it is important to note that changes in expression of Bcl-2 and Bcl-XL antiapoptotic proteins, which are Bik target proteins, will strongly affect the efficiency of the Bik-induced apoptosis.
Bik is a BH3-only, proapoptotic member of the Bcl-2 family of apoptosis regulators (29). Bik binds directly to Bcl-2 and Bcl-XL through its BH3 domain to inactivate their antiapoptotic functions and provokes apoptosis in a Bax-dependent fashion (30). Bik is reported to play a critical role in the apoptotic selection of mature B lymphocytes (31, 32), and the Bik mRNA transcripts are strongly expressed in several lymphoma cell lines (33). Interestingly, missense mutations of BIK gene are frequently found in human B-cell lymphomas (34). These studies suggest that Bik may function as an apoptosis-inducing tumor suppressor in B-cell lymphomas. The fact that a short chromosomal segment containing the BIK locus (22q13) is frequently lost in human colorectal cancers (35) and head-and-neck tumors (36) further suggests a potential role of Bik as a tumor suppressor. Our present observation that breast cancer cell lines resistant to the antiestrogen-provoked apoptosis did not express the Bik protein even when they strongly expressed the Bik mRNA transcripts (Fig. 3F) is also consistent with the notion that Bik acts as an apoptosis-inducing tumor suppressor. Although the mechanism of the suppression of Bik protein expression in the apoptosis-resistant cells is unknown, nucleotide sequences of the Bik cDNA isolated from them did not show any changes in the ORF or around the translational initiation site (J.H. and T.S., unpublished), eliminating a possibility that mutations in the BIK gene suppressed protein expression. Whether there is increased Bik protein degradation in the apoptosis-resistant cells is currently being studied. Several laboratories have proposed the in vivo delivery of Bik to cancer cells as a novel strategy of gene therapy (37–40). Authors of these studies suspected that Bik may not only kill the cancer cells by apoptosis but also enhance sensitivity of the cancer cells to the apoptosis-inducing actions of chemotherapeutic agents (33, 41, 42). Our finding that Bik is inducible in breast cancer cells by antiestrogens may imply that the antiestrogen therapy might enhance the efficacy of chemotherapy by inducing Bik. Further studies are necessary to explore this attracting possibility.
The molecular mechanisms leading to the increased expression of the Bik mRNA transcripts by estrogen starvation and antiestrogen exposure are unknown but probably involve the transcriptional activation of the BIK gene. The reported human BIK gene promoter sequence (up to –1.7 kb upstream from the transcriptional initiation site) does not contain canonical estrogen responsive elements (43). Mathai et al. (44) reported that expression of the Bik mRNA transcripts in the KB human oral epithelial cells depends on the p53 tumor suppressor, but the authors did not identify functional p53-interacting elements in the BIK promoter. Whether or not the estrogen-regulated expression of the Bik mRNA transcripts observed in the present study involves p53 remains to be determined. Interestingly, using knockout mice, Villunger et al. (45) recently demonstrated that two other BH3-only proteins, Puma and Noxa, are induced in a p53-dependent manner in lymphocytes and fibroblasts and mediate the apoptosis provoked by DNA damage, suggesting their roles as tumor suppressors. It will be important to determine whether Bik is also involved in the p53-dependent, DNA damage-provoked apoptosis.
Because mice lacking Blk (the rodent homologue of Bik) have not yet been reported, it is not known whether Blk/Bik is involved in physiological growth, differentiation, and/or involution of mammary epithelial cells. Interestingly, Reginato et al. (46) recently demonstrated that Bim, another BH3-only proapoptotic protein, induced anoikis-induced apoptosis of MCF-10A nontumorigenic human mammary epithelial cells and that Bim expression was strongly induced when cells were detached from the extracellular matrix. Bmf is yet another BH3-only protein that is expressed constitutively in MCF-7 cells but sequestered to myosin V motors by association with dynein light chain 2. Loss of cell attachment (anoikis) unleashes Bmf, which then translocates to mitochondria and induces apoptosis (47). It is therefore tempting to speculate that a number of different members of the BH-3 only proteins may play important roles in regulating the death of normal and malignant mammary epithelial cells at multiple physiological or pathological steps.
In summary, we have demonstrated a strong induction of the Bik proapoptotic BH3-only protein in MCF-7/BUS human breast cancer cells by estrogen starvation and antiestrogen exposure. Suppression of Bik protein expression by siRNA revealed that Bik induction was necessary for the antiestrogen-induced apoptosis of this cell line. Breast cancer cell lines that are resistant to the antiestrogen-provoked apoptosis did not express Bik protein even when they strongly express the Bik mRNA transcripts constitutively or in an antiestrogen-inducible manner. These findings suggest that Bik, via its role in inducing apoptosis, may function as a tumor suppressor in breast cancer.
Acknowledgments
We thank Ana Soto and Carlos Sonnenschein (Tufts University) for providing us with MCF-7/BUS cells and technical advice. This work was supported by the AVON Project for Breast Cancer Research and National Institutes of Health Grant R01 CA82230 (to T.S.).
Abbreviations: BH, Bcl-2 homology; E2, 17β-estradiol; ERα, estrogen receptor α; PARP, poly(ADP-ribose) polymerase; siRNA, small interfering RNA.
References
- 1.Sommer, S. & Fuqua, S. A. (2001) Semin. Cancer Biol. 11, 339–352. [DOI] [PubMed] [Google Scholar]
- 2.Thiantanawat, A., Long, B. J. & Brodie, A. M. (2003) Cancer Res. 63, 8037–8050. [PubMed] [Google Scholar]
- 3.Bursch, W., Ellinger, A., Kienzl, H., Torok, L., Pandey, S., Sikorska, M., Walker, R. & Hermann, R. S. (1996) Carcinogenesis 17, 1595–1607. [DOI] [PubMed] [Google Scholar]
- 4.Ellis, P. A., Saccani-Jotti, G., Clarke, R., Johnston, S. R., Anderson, E., Howell, A., A'Hern, R., Salter, J., Detre, S., Nicholson, R., et al. (1997) Int. J. Cancer 72, 608–613. [DOI] [PubMed] [Google Scholar]
- 5.Kandouz, M., Lombet, A., Perrot, J. Y., Jacob, D., Carvajal, S., Kazem, A., Rostene, W., Therwath, A. & Gompel, A. (1999) J. Steroid Biochem. Mol. Biol. 69, 463–471. [DOI] [PubMed] [Google Scholar]
- 6.Simstein, R., Burow, M., Parker, A., Weldon, C. & Beckman, B. (2003) Exp. Biol. Med. (Maywood) 228, 995–1003. [DOI] [PubMed] [Google Scholar]
- 7.Burow, M. E., Weldon, C. B., Tang, Y., McLachlan, J. A. & Beckman, B. S. (2001) J. Steroid Biochem. Mol. Biol. 78, 409–418. [DOI] [PubMed] [Google Scholar]
- 8.Pratt, M. A., Bishop, T. E., White, D., Yasvinski, G., Menard, M., Niu, M. Y. & Clarke, R. (2003) Mol. Cell. Biol. 23, 6887–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, G. J., Kimijima, I., Onda, M., Kanno, M., Sato, H., Watanabe, T., Tsuchiya, A., Abe, R. & Takenoshita, S. (1999) Clin. Cancer Res. 5, 2971–2977. [PubMed] [Google Scholar]
- 10.Leung, L. K. & Wang, T. T. (1999) Br. J. Cancer 81, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandouz, M., Siromachkova, M., Jacob, D., Chretien Marquet, B., Therwath, A. & Gompel, A. (1996) Int. J. Cancer 68, 120–125. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira, C., Reed, J. C. & Pratt, M. A. (1995) Cancer Res. 55, 3902–3907. [PubMed] [Google Scholar]
- 13.Wang, T. T. & Phang, J. M. (1995) Cancer Res. 55, 2487–2489. [PubMed] [Google Scholar]
- 14.Diel, P., Smolnikar, K. & Michna, H. (1999) Breast Cancer Res. Treat. 58, 87–97. [DOI] [PubMed] [Google Scholar]
- 15.Dong, L., Wang, W., Wang, F., Stoner, M., Reed, J. C., Harigai, M., Samudio, I., Kladde, M. P., Vyhlidal, C. & Safe, S. (1999) J. Biol. Chem. 274, 32099–32107. [DOI] [PubMed] [Google Scholar]
- 16.Perillo, B., Sasso, A., Abbondanza, C. & Palumbo, G. (2000) Mol. Cell. Biol. 20, 2890–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne, J., Jones, C., Lakhani, S. & Kortenkamp, A. (2000) Sci. Total Environ. 248, 51–62. [DOI] [PubMed] [Google Scholar]
- 18.Bahia, H., Ashman, J. N., Cawkwell, L., Lind, M., Monson, J. R., Drew, P. J. & Greenman, J. (2002) Int. J. Oncol. 20, 489–494. [DOI] [PubMed] [Google Scholar]
- 19.Graham, K. A., Richardson, C. L., Minden, M. D., Trent, J. M. & Buick, R. N. (1985) Cancer Res. 45, 2201–2205. [PubMed] [Google Scholar]
- 20.Sullivan, J. A., Spriggs, L. L. & Hill, S. M. (2000) Cancer Lett. 148, 87–93. [DOI] [PubMed] [Google Scholar]
- 21.Coser, K. R., Chesnes, J., Hur, J., Ray, S., Isselbacher, K. J. & Shioda, T. (2003) Proc. Natl. Acad. Sci. USA 100, 13994–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shioda, T., Munn, L. L., Fenner, M. H., Jain, R. K. & Isselbacher, K. J. (1997) Am. J. Pathol. 150, 2099–2112. [PMC free article] [PubMed] [Google Scholar]
- 23.Van Noorden, C. J. (2001) Acta Histochem. 103, 241–251. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenschein, C., Szelei, J., Nye, T. L. & Soto, A. M. (1994) Oncol. Res. 6, 373–381. [PubMed] [Google Scholar]
- 25.Liang, Y., Yan, C. & Schor, N. F. (2001) Oncogene 20, 6570–6578. [DOI] [PubMed] [Google Scholar]
- 26.Janicke, R. U., Sprengart, M. L., Wati, M. R. & Porter, A. G. (1998) J. Biol. Chem. 273, 9357–9360. [DOI] [PubMed] [Google Scholar]
- 27.Wolf, B. B., Schuler, M., Echeverri, F. & Green, D. R. (1999) J. Biol. Chem. 274, 30651–30656. [DOI] [PubMed] [Google Scholar]
- 28.Tewari, M., Quan, L. T., O'Rourke, K., Desnoyers, S., Zeng, Z., Beidler, D. R., Poirier, G. G., Salvesen, G. S. & Dixit, V. M. (1995) Cell 81, 801–809. [DOI] [PubMed] [Google Scholar]
- 29.Puthalakath, H. & Strasser, A. (2002) Cell Death Differ. 9, 505–512. [DOI] [PubMed] [Google Scholar]
- 30.Gillissen, B., Essmann, F., Graupner, V., Starck, L., Radetzki, S., Dorken, B., Schulze-Osthoff, K. & Daniel, P. T. (2003) EMBO J. 22, 3580–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, A. & Clark, E. A. (2001) J. Immunol. 166, 6025–6033. [DOI] [PubMed] [Google Scholar]
- 32.Klein, U., Tu, Y., Stolovitzky, G. A., Keller, J. L., Haddad, J., Jr., Miljkovic, V., Cattoretti, G., Califano, A. & Dalla-Favera, R. (2003) Proc. Natl. Acad. Sci. USA 100, 2639–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel, P. T., Pun, K. T., Ritschel, S., Sturm, I., Holler, J., Dorken, B. & Brown, R. (1999) Blood 94, 1100–1107. [PubMed] [Google Scholar]
- 34.Arena, V., Martini, M., Luongo, M., Capelli, A. & Larocca, L. M. (2003) Genes Chromosomes Cancer 38, 91–96. [DOI] [PubMed] [Google Scholar]
- 35.Castells, A., Ino, Y., Louis, D. N., Ramesh, V., Gusella, J. F. & Rustgi, A. K. (1999) Gastroenterology 117, 831–837. [DOI] [PubMed] [Google Scholar]
- 36.Reis, P. P., Rogatto, S. R., Kowalski, L. P., Nishimoto, I. N., Montovani, J. C., Corpus, G., Squire, J. A. & Kamel-Reid, S. (2002) Oncogene 21, 6480–6487. [DOI] [PubMed] [Google Scholar]
- 37.Azar, Y. & Lorberboum-Galski, H. (2000) Apoptosis 5, 531–542. [DOI] [PubMed] [Google Scholar]
- 38.Tong, Y., Yang, Q., Vater, C., Venkatesh, L. K., Custeau, D., Chittenden, T., Chinnadurai, G. & Gourdeau, H. (2001) Mol. Cancer Ther. 1, 95–102. [PubMed] [Google Scholar]
- 39.Zou, Y., Peng, H., Zhou, B., Wen, Y., Wang, S. C., Tsai, E. M. & Hung, M. C. (2002) Cancer Res. 62, 8–12. [PubMed] [Google Scholar]
- 40.Naumann, U., Schmidt, F., Wick, W., Frank, B., Weit, S., Gillissen, B., Daniel, P. & Weller, M. (2003) Hum. Gene Ther. 14, 1235–1246. [DOI] [PubMed] [Google Scholar]
- 41.Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S. & Korsmeyer, S. J. (2002) Cancer Cell 2, 183–192. [DOI] [PubMed] [Google Scholar]
- 42.Radetzki, S., Kohne, C. H., von Haefen, C., Gillissen, B., Sturm, I., Dorken, B. & Daniel, P. T. (2002) Oncogene 21, 227–238. [DOI] [PubMed] [Google Scholar]
- 43.Verma, S., Budarf, M. L., Emanuel, B. S. & Chinnadurai, G. (2000) Gene 254, 157–162. [DOI] [PubMed] [Google Scholar]
- 44.Mathai, J. P., Germain, M., Marcellus, R. C. & Shore, G. C. (2002) Oncogene 21, 2534–2544. [DOI] [PubMed] [Google Scholar]
- 45.Villunger, A., Michalak, E. M., Coultas, L., Mullauer, F., Bock, G., Ausserlechner, M. J., Adams, J. M. & Strasser, A. (2003) Science 302, 1036–1038. [DOI] [PubMed] [Google Scholar]
- 46.Reginato, M. J., Mills, K. R., Paulus, J. K., Lynch, D. K., Sgroi, D. C., Debnath, J., Muthuswamy, S. K. & Brugge, J. S. (2003) Nat. Cell Biol. 5, 733–740. [DOI] [PubMed] [Google Scholar]
- 47.Puthalakath, H., Villunger, A., O'Reilly, L. A., Beaumont, J. G., Coultas, L., Cheney, R. E., Huang, D. C. & Strasser, A. (2001) Science 293, 1829–1832. [DOI] [PubMed] [Google Scholar]