Summary
Acute graft-versus-host disease (GVHD) following allogeneic bone marrow transplantation (BMT) is initiated by donor T lymphocytes that recognize histocompatibility antigens presented by recipient dendritic cells (DCs). Current approaches to reduce GVHD are focused on suppressing donor T lymphocyte responses to alloantigens. However, these strategies may be inadequate in the setting of allogeneic transplants (particularly histoincompatible transplants), may increase the risk of tumour relapse and are associated with high rates of opportunistic infections. We hypothesized that inhibition of recipient DCs might suppress GVHD. We recently demonstrated in vitro that azithromycin, a macrolide antibiotic, also acts as a nuclear factor (NF)-κB inhibitor of murine DCs and inhibits their maturation and functions, including allogeneic responses. We investigated whether azithromycin could prevent alloreactions in a murine histoincompatibility model. Oral administration of azithromycin to recipient mice for 5 days during major-histoincompatible BMT suppressed lethal GVHD significantly, whereas ex-vivo lymphocyte function was not affected by the drug. These data suggest that azithromycin has potential as a novel prophylactic drug for lethal GVHD.
Keywords: azithromycin, dendritic cells, graft-versus-host disease, nuclear factor (NF)-κB, transplantation
Introduction
Haematopoietic stem cell transplantation from an allogeneic donor provides curative therapy for patients with malignant and non-malignant haematological diseases. However, acute graft-versus-host disease (GVHD) is still a major cause of morbidity and mortality after allogeneic bone marrow transplantation (BMT). GVHD is initiated by donor T lymphocytes that recognize host histocompatibility antigens that distinguish host from donor. To date, most therapeutic approaches designed to attenuate GVHD have focused on suppressing donor T lymphocytes [1–5]. These approaches, however, often result in incomplete GVHD attenuation, especially in histoincompatible transplants. Recent murine studies have shown that interactions between donor T lymphocytes and host antigen-presenting cells (APCs) are essential for triggering GVHD [6–11].
Dendritic cells (DCs) derived from haematopoietic stem cells are distributed ubiquitously in blood, lymphoid and peripheral tissues and play important roles in the immune system by stimulating naive T lymphocytes and secreting cytokines that initiate the immune response [12]. The state of DC maturation influences their functions. Various factors, including bacteria-derived antigens such as lipopolysaccharide (LPS), viral products, inflammatory cytokines and conditioning regimens such as total body irradiation (TBI) can induce maturation of DCs, which is characterized by up-regulation of major histocompatibility complex (MHC) class II, co-stimulatory molecules and essential chemokine receptors. Mature DCs (mDCs) promote antigen-specific T cell activation and proliferation. Moreover, following CD40 ligation or Toll-like receptor ligation, mDCs secrete interleukin (IL)-12 p70, which induces interferon (IFN)-γ-producing T helper type 1 (Th1) cells that are considered a pivotal pathogenic factor in acute GVHD [12–15].
Nuclear factor (NF)-κB is a rapid response transcription factor in various cells involved in immune and inflammatory reactions and exerts its effect by inducing expression of cytokines, chemokines, cell adhesion molecules and growth factors [16,17]. NF-κB is sequestered normally in the cytoplasm of non-stimulated cells and is translocated into the nucleus in response to a variety of stimuli, such as bacterial lipopolysaccharide (LPS) and tumour necrosis factor (TNF)-α. Because it also plays a crucial role in DC maturation [18,19], NF-κB in DCs might be a rational target for preventing GVHD. Recently, early administration of bortezomib, a proteasome inhibitor that is effective against multiple myeloma, was shown to attenuate acute GVHD in a murine histoincompatibility model through suppression of NF-κB activity in DCs [20–22]. Moreover, a Phase I clinical trial was conducted of human leucocyte antigen (HLA)-mismatched reduced-intensity conditioning for unrelated donor allogeneic BMT using bortezomib, tacrolimus and methotrexate for GVHD prophylaxis. It was reported that bortezomib appeared safe, was well tolerated and might be a novel immunomodulatory agent in allogeneic transplantation [23].
We reported recently in this journal that azithromycin (AZM), a macrolide antibiotic, blocked LPS-induced nuclear translocation of NF-κB in murine bone marrow-derived DCs and inhibited significantly their immunophenotypic and functional maturation [24]. Therefore, we hypothesize that AZM, being not only an antibiotic but also a NF-κB inhibitor, has potential as a novel drug for manipulation of allogeneic responses such as acute GVHD after BMT. In support of that, we report here, for the first time, that AZM attenuated acute GVHD in a fully allogeneic murine GVHD model.
Materials and methods
Mice
Female C57BL/6 (H-2 Kb) donor mice and BALB/c (H-2 Kd) recipient mice aged 6–12 weeks were purchased from Japan SLC, Inc. (Shizuoka, Japan). Institutional approval was obtained for all animal experimentation.
Antibodies and media
Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) used to detect cell surface expression of CD3, CD4, CD11c, CD40, CD69, CD80, CD86, I-Ab, H-2Kb and H-2Kd by flow cytometry, as well as isotype-matched control mAbs, were purchased from BD Pharmingen and eBioscience (San Diego, CA, USA). RPMI-1640 supplemented with 10% fetal calf serum (FCS), 5 × 10−5M 2-mercaptoethanol (ME) and 10 mM HEPES was used as the culture medium.
BMT and induction of lethal acute GVHD
Mice underwent allo-BMT, as described elsewhere [25]. Briefly, recipient BALB/c mice (H-2d, 11 animals in each group) received 7·5 Gy total total body irradiation (TBI). On the day of transplantation (day 0), within 24 h of irradiation recipients received a single injection of BM cells (2 × 106) and spleen cells (2 × 106) obtained from donor C57BL/6 mice (H-2b) for allogeneic BMT or BALB/c mice for syngeneic BMT through the tail vein. Recipients in each group received 100 mg/kg of azithromycin (AZM) (Pfizer Inc., Groton, CT, USA) or vehicle orally once a day from day −2 to day 2, respectively (see Fig. 1a).
Figure 1.
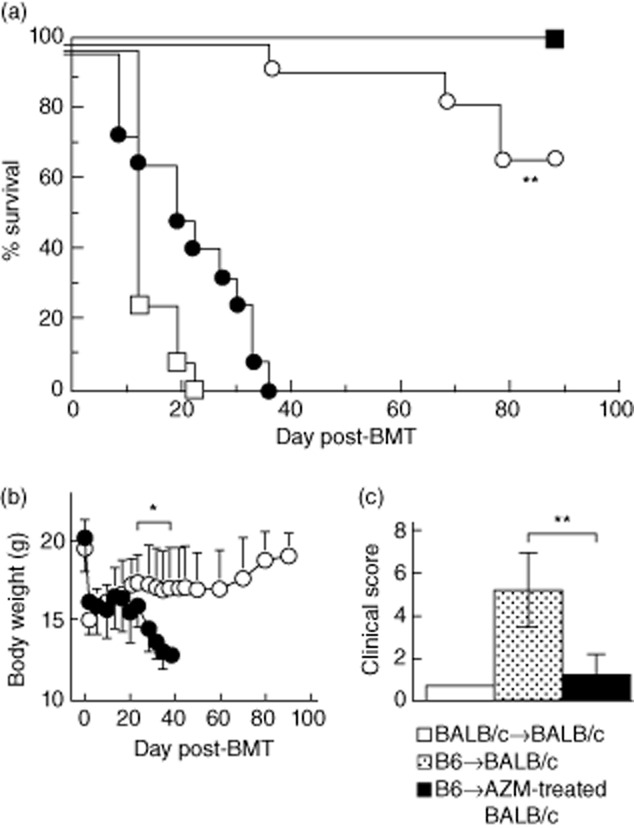
Azithromycin treatment confers protection from lethal acute graft-versus-host disease (GVHD). Lethally irradiated BALB/c recipients treated with or without oral azithromycin (AZM) as a nuclear factor (NF)-κB inhibitor, were injected intravenously with bone marrow (BM) and spleen cells from C57BL/6 (B6; allogeneic) or BALB/c (syngeneic) donors. Survival (a) and serial change in body weight (b) from the day of transplantation to 90 days after BM transplantation (BMT) were monitored. □: Lethally irradiated BALB/c; ■: BALB/c→BALB/c; •: B6→BALB/c; ○: B6→AZM-treated BALB/c (a)*P < 0·01 (• versus ○) (b) **P < 0·05 (• versus ○). (c) Clinical score of acute GVHD on day 14 after BMT. The degree of clinical GVHD was assessed by a scoring system, which sums the changes in five clinical parameters: weight loss, posture, activity, fur texture and skin integrity (maximum index = 10), as described in Materials and methods. B6→BALB/c recipients developed significant acute GVHD as determined by the clinical score, compared with B6→AZM-treated BALB/c recipients. ***P < 0·01. All data are expressed as the mean ± standard deviation (n = 11 per group).
Systemic and histopathological analysis of GVHD
Survival and the degree of clinical GVHD by a scoring system as described [7,26] were monitored once every 3 days after BMT. Skin, small intestine and liver tissues, as primary GVHD target organs, were obtained from recipients on day 7 after BMT. Sections were stained with haematoxylin and eosin. Slides were examined systematically by two of the authors (T.Y. and S.I.) using a semiquantitative scoring system, as described elsewhere [27].
Flow cytometric analysis
Spleen cells suspended in phosphate-buffered saline (PBS) were preincubated with FcγR blocking antibody (anti-mouse CD16/CD32; BD Pharmingen) and then incubated with FITC- or PE-labelled mAbs at 4°C for 20 min. After staining, the cells were washed twice with PBS, incubated with propidium iodide at room temperature for 5 min and then subjected to fluorescence activated cell sorter (FACS) analysis. Flow cytometry was performed on a FACScan or FACSCantoII with CellQuest or Diva software (Becton Dickinson, Franklin Lakes, NJ, USA).
Preparation of BM-derived DCs
Bone marrow (BM)-derived DCs were generated as described previously [24]. Briefly, BM cells were flushed from tibias and femurs of BALB/c mice and seeded at 2 × 106 cells onto six-well culture plates in culture medium supplemented with 20 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (Kirin Brewery Co., Gunma, Japan). The culture medium, containing 20 ng/ml murine GM-CSF, was changed every 2 days. Loosely adherent cells were used on day 6 as immature DCs (imDCs). The purity of imDCs was routinely > 85%, as confirmed by dual positivity for major histocompatibility complex (MHC) class II (I-Ab) and CD11c. ImDCs were stimulated with 1 μg/ml LPS from Escherichia coli (serotype 055:B5) (Sigma, St Louis, MO, USA) for 24 h for maturation.
Mixed lymphocyte reaction (MLR)
Allogeneic MLR assay was performed as described, with minor modifications [28]. Splenic CD4+ T lymphocytes from C57BL/6 mice treated with or without oral AZM (100 mg/kg) once a day for 3 days were enriched using an EasySepTM-Murine CD4+ T cell enrichment kit (Stem Cell Technologies Inc., Vancouver, Canada) and used as responders. BALB/c BM-derived mDCs, as stimulator (2 × 104 cells), were irradiated with 30 Gy, added to responders (2 × 105 cells) in 96-well round-bottomed plates (Falcon, Tokyo, Japan) and then incubated for 3 days. CD4+ T cells were labelled with a cell tracer, carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA, USA), for proliferation assay. At the end of culture, cells were harvested and stained for flow cytometric analysis of CD4+ T cell proliferation by CFSE dilution.
Proliferation assay
[3H]-thymidine (Amersham Biosciences, Piscataway, NJ, USA) incorporation was measured to evaluate the mitogenic response of spleen cells from C57BL/6 mice treated with or without oral AZM (100 mg/kg) once a day for 3 or 5 days, as described previously [29]. Mitogens were used at the following concentrations: 10 μg/ml concanavalin A (ConA) (Sigma), 5 μg/ml pokeweed mitogen (PWM) (Sigma) and 10 μg/ml LPS (Sigma).
Statistical analysis
Survival curves were plotted using Kaplan–Meier estimates. Analysis of variance (anova) and unpaired two-tailed t-tests were used to determine the statistical significance of in-vitro data and clinical scores. P < 0·05 was considered statistically significant.
Results
AZM attenuates lethal GVHD
Interactions between recipient DCs and donor T lymphocytes are critical for triggering the induction of GVHD [7,10,30]. Interestingly, MacDonald et al. [31] reported that lack of the NF-κB/Rel family in DCs, using Rel knock-out (KO) mice, suppressed initiation and maintenance of GVHD due to the failure of donor Th1 expansion after transplantation. We postulated that administration of AZM, as a NF-κB inhibitor, to recipient mice during transplantation might inhibit alloreactions in vivo, i.e. the development of lethal GVHD in a MHC-incompatible BMT model (B6→BALB/c). All BALB/c recipient mice receiving 7·5 Gy of irradiation alone died, but syngeneic BALB/c BM graft rescued all mice. Meanwhile, irradiated mice injected intravenously with B6 BM and spleen cells all died of lethal GVHD by day 24. In contrast, 73% of similarly treated mice survived when they were placed on oral AZM (Fig. 1a). The changes in body weight (Fig. 1b) and clinical score (Fig. 1c) following transplantation were compatible with the clinical course of lethal GVHD [7,26]. Flow cytometric analysis found that more than 95% of BM cells at 6 months post-transplantation expressed donor-type H-2b (data not shown). Thus, AZM did not inhibit engraftment. These findings indicate that AZM attenuates lethal GVHD significantly while permitting long-term engraftment of histoincompatible donor marrow cells.
AZM treatment is effective in reducing GVHD-associated histopathology
Tissue samples from GVHD target organs were taken from representative acute GVHD-positive control mice, AZM-treated mice and GVHD-negative syngeneic control mice on day 7 after BMT. Recipients of syngeneic BMT showed no signs of GVHD in their tissues (Fig. 2d,g). Skin from control mice with GVHD [32–34] showed epidermal hyperplasia, basal layer cell injury, severe inflammatory infiltrates with intraepidermal lymphocytes, acidophilic bodies and loss of hair follicles (Fig. 2b). Such changes were not observed in mice administered AZM (Fig. 2c).
Figure 2.
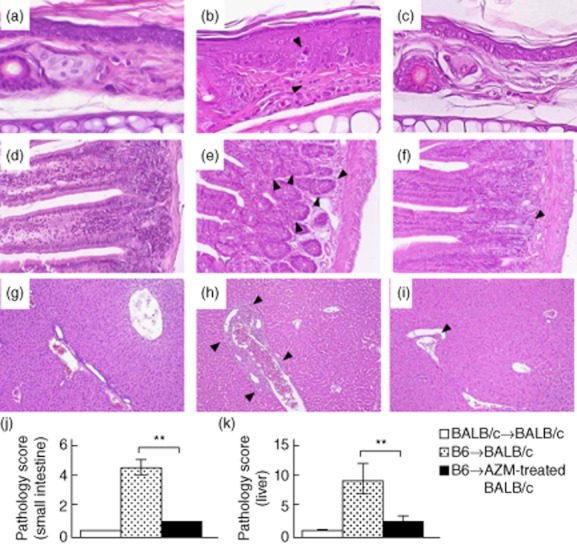
Administration of azithromycin attenuates development of acute graft-versus-host disease (GVHD) histopathological features. Skin, small intestine and liver samples were obtained on day 7 post-bone marrow transplantation (BMT) for pathological analysis (n = 3 per group). Paraffin sections were stained with haematoxylin and eosin (original magnification: skin, ×600; small intestine, ×100 liver; ×200). (a–i) Histology of the skin (a–c), small intestine (d–f) and liver (g–i) from BALB/c→BALB/c recipients (a,d,g), B6→BALB/c recipients (b,e,h), and B6→AZM-treated BALB/c recipients (c,f,i). Black arrowheads: inflammatory response associated with acute GVHD. (j,k) Pathology scores of small intestine and liver from the same recipients. Coded slides were scored semiquantitatively as described in Materials and methods. All data are expressed as the mean ± standard deviation (n = 3 per group). **P < 0·001.
The small intestine of control mice with GVHD [7,26,27] showed villous atrophy with epithelial apoptosis (Fig. 2e). The liver of those control mice with GVHD showed massive infiltration of mononuclear cells, mainly in the periportal areas (Fig. 2h). In contrast, such findings were hardly observed in the small intestine and liver of AZM-treated mice (Fig. 2f,i). The acute GVHD pathology scores [27] of the small intestine and liver of AZM-treated recipients were significantly lower than those of corresponding allogeneic control recipients (Fig. 2j,k). These results suggest that administration of AZM attenuates the development of acute GVHD-associated histopathological features in recipients of allogeneic BMT.
AZM does not inhibit donor lymphocyte functions
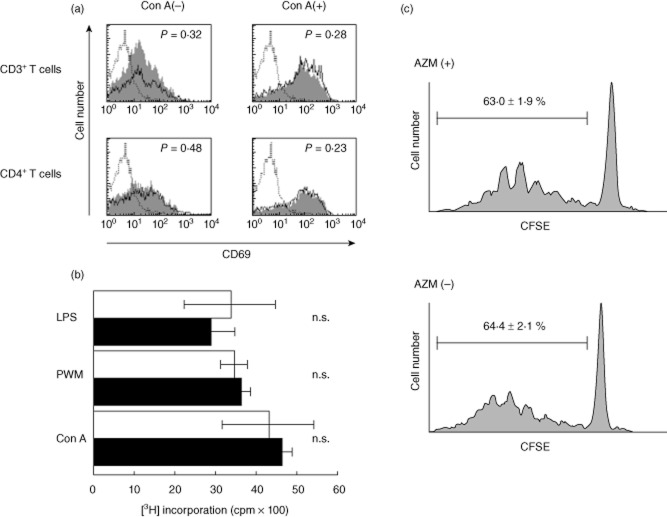
It has not been known whether AZM affects lymphocyte functions. AZM was administered orally to C57BL/6 (B6) mice, which we used as donors in the murine BMT model, for 3 days. B6 splenic T lymphocytes were examined for their cell numbers and expression of CD69, an early activation marker of T lymphocytes. The number of splenic T lymphocytes was not affected by the AZM treatment (data not shown). After in-vitro stimulation with ConA, CD69 expression by both CD3+ and CD4+ T lymphocytes was up-regulated, but was not affected by AZM treatment (Fig. 3a). Furthermore, AZM did not affect splenic T or B lymphocyte proliferation in response to stimulation with LPS, PWM or Con A (Fig. 3b). Similar results were generated in longer, 5-day administration of AZM (data not shown). Additionally, CFSE-labelled splenic CD4+ T lymphocytes from C57BL/6 mice treated with or without AZM for 3 days were cultured in MLR with allogeneic BALB/c BM-derived mDCs for 3 days. It was confirmed that expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules (CD40, CD80 and CD86) on LPS-induced mDCs was elevated in comparison with imDCs (data not shown). We did not observe any differences in the dividing CFSElow CD4+ population between AZM-treated and untreated C57BL/6 mice in the allogeneic MLR (Fig. 3c). These data indicate that AZM does not inhibit donor lymphocyte functions ex vivo at the tested doses.
Figure 3.
Azithromycin (AZM) does not inhibit donor lymphocyte functions. (a,b) Spleen cells from B6 mice treated with oral AZM or vehicle alone for 3 days were co-cultured with concanavalin A (ConA), pokeweed mitogen (PWM) or lipopolysaccharide (LPS). Flow cytometric analysis of the expression of CD69 on B6 splenic CD3+ or CD4+ T lymphocytes after 4 h stimulation with ConA. The staining profiles of splenic T lymphocytes from B6 mice treated with AZM (grey) or vehicle (thin line) are shown. Dotted lines refer to isotype controls. The results are representative of three independent experiments (a). B6 splenocyte proliferation was stimulated by the mitogens. [3H]-thymidine incorporation was measured after 48 h culture with Con A, PWM or LPS. □: Control B6 spleen cells; ■: AZM-treated B6 spleen cells. The results are expressed as the mean counts per minute (cpm) ± standard deviation of triplicate cultures. The results are representative of three independent experiments. n.s.: not significant (b). Carboxyfluorescein succinimidyl ester (CFSE)-labelled splenic CD4+ T lymphocytes from C57BL/6 mice treated with oral AZM or vehicle alone for 3 days were cultured with allogeneic BALB/c BM-derived mature DCs for 3 days. Cultured cells were harvested, and proliferation was quantified by flow cytometry. Data represent three independent experiments (c).
Discussion
Novel immunomodulatory agents focused on NF-κB in host DCs [6–11,20–22,31] instead of the conventional immunosuppressants targeted on donor T lymphocytes [1–5] have been reported to prevent or attenuate GVHD in allogeneic haematopoietic transplantation, including in the histoincompatible setting. In this study, we used AZM – a macrolide antibiotic and a NF-κB inhibitor of murine DC maturation – alone for GVHD prophylaxis and showed that it inhibited acute GVHD significantly in MHC-incompatible bone marrow transplantation (BMT) without interfering with donor engraftment.
AZM is active against a wide variety of bacteria and also acts as an anti-inflammatory agent by modulating the functions of DCs, monocytes and/or macrophages [24,35–37]. Previously, Sugiyama et al. [35] and our team [24] have reported that AZM inhibits the maturation and functions of murine bone marrow-derived DCs in vitro. We also showed that AZM, by inhibiting the NF-κB pathway in LPS-stimulated DCs and generating DCs with regulatory DC properties, blocks murine DC–T lymphocyte interaction in allogeneic immune systems [24]. In murine allogeneic BMT models, recipient-type regulatory DCs, characterized by low expression levels of co-stimulatory molecules, moderate levels of MHC molecules, low production of IL-12, high production of IL-10 and suppression of NF-κB activity even after stimulation with LPS, inhibited acute GVHD, mediated partly by IL-10, as a key regulator of anti-inflammatory responses [38,39]. Sato et al. [38] also found that recipient-type regulatory DCs increased donor-type regulatory T cells (Treg) which produced IL-10 and resulted in protection from lethal acute GVHD. Additionally, we reported significantly increased IL-10 levels in co-cultures of allogeneic T lymphocytes and AZM-treated DCs [24].
The precise mechanisms underlying the findings presented in this report are unknown, because we did not analyse induction of Treg and/or plasma IL-10 of recipient mice treated with AZM, or for immunophenotypic or functional changes in DCs derived from recipients treated with AZM due to a numerical problem without in-vivo expansion stimulated with Flt3 ligand and/or other cytokines [11,40,41]. In addition, these cytokines for in-vivo expansion of DCs reportedly might alter the proportion and function of DC subsets in some tissues [42,43]. Therefore, in-vivo DC expansion system using such cytokines might not be preferable to examine the essential function of AZM in the present report. However, our in-vivo data suggest that acute GVHD was clearly suppressed, clinically and pathologically, by oral AZM (Figs 1 and 2). It is tempting to speculate that AZM-treated DCs may be related functionally to regulatory DCs, not only in vitro but also in vivo, and might induce Treg in an allogeneic BMT setting. We are also interested in testing whether injection of AZM-treated DCs to recipients following allogeneic BMT could attenuate acute GVHD, as observed with regulatory DCs [38]. However, it might be difficult to develop and expand these DCs ex vivo. Simply administering AZM orally to recipients would be much more practical from the clinical viewpoint.
Next, we confirmed the effects of AZM on donor lymphocytes. Tomazic et al. [44] reported that the absence of impairment of T and B lymphocytes by AZM might be an important property of this drug, especially in immunocompromised individuals. Our data for C57BL/6 murine lymphocytes are compatible with their results (Fig. 3). The fact that AZM has no deleterious effects on T lymphocyte functions in this setting is important for preservation of the graft-versus-leukaemia (GVL) effect of AZM therapy. Conversely, commonly used immunosuppressants such as tacrolimus (a 23-membered ring-macrolide) and cyclosporin inhibit T lymphocyte functions strongly by blocking the phosphatase activity of calcineurin, resulting in susceptibility to infections and a decreased GVL effect. Moreover, potential concerns for the use of these calcineurin inhibitors include renal toxicity, veno-occlusive disease of the liver, hypertension, hyperglycaemia and neurological side effects [45]. In contrast, AZM has been used safely worldwide as an antibiotic. Nevertheless, AZM is not without its own safety issues: reversible hearing loss with high doses (600 mg daily for 1·5–20 weeks) [46] and long-term treatment (600 mg once weekly for 1 year) [47] and cardiovascular effects; specifically, prolongation of the QT interval that leads to torsades de pointes, an abnormal heart rhythm that can be fatal [48].
In addition to the immunoregulatory effects of AZM, its anti-microbial effect may also be important in BMT as bacteria and bacterial products, especially LPS, are associated with exacerbation of GVHD [49,50]. In the clinical setting, Gram-negative gut decontamination has actually been found to reduce the incidence of GVHD [51,52,53]. Interestingly, some investigators reported that changes in the microbial flora, due to intestinal inflammation caused by TBI as preconditioning for murine recipients of allogeneic BMT, influenced the severity of acute GVHD, and that manipulation of the intestinal flora enabled regulation of acute GVHD [53,54]. We reported that AZM attenuated the response of DCs to LPS due to reduced Toll-like receptor (TLR)-4 expression on their surface [24]. Therefore, the attenuating effect of AZM on GVHD might be due partly to its control of bacteria.
Concerning the timing and dose of oral AZM, we chose a regimen of 100 mg/kg orally for 5 days starting from day −2 to day 2. Amsden et al. [55] reported that the blood concentration of the drug in humans became stable (0·5–1·0 mg/ml) after 3 or 5 days of oral AZM. The 100 mg/kg/day dosage was used because it corresponds to the human dosage after size correction [56]. Accumulating evidence indicates that early interaction between allogeneic T lymphocytes and residual recipient APCs immediately after allo-BMT is critical for eliciting acute GVHD [6,10].
Zhang et al. [57] studied the kinetic window during which recipient APCs elicited acute GVHD in a murine model and demonstrated that recipient DCs were activated and aggregated rapidly in T lymphocyte-rich areas of the spleen within 6 h after lethal irradiation. By 5 days after irradiation, <1% of recipient DCs were detectable, but the activated donor CD8+ T lymphocytes had already undergone as many as seven divisions. This indicates that, although recipient DCs disappear rapidly after allo-BMT, they first prime donor T lymphocytes and play a critical role in triggering donor CD8+ T lymphocyte-mediated GVHD. In our transplantation model, AZM-treated recipients developed GVHD in the later phase. Although Zhang et al. [57] demonstrated the critical, early role of DCs in initiating acute GVHD, they also found that a small number of radio-resistant recipient DCs remained even at 4 months after allo-BMT and pointed out the possibility that they might be important in amplifying the GVHD response. Further studies are necessary to elucidate later events in the induction of acute GVHD.
Taken together, our results suggest that blockade of DC–T lymphocyte interaction by inactivating DCs with AZM, i.e. DC targeting, might require administration of the drug for a short period before and after BMT. It is this period that should be targeted in an attempt to attenuate acute GVHD. Moreover, this treatment might not be accompanied by suppression of the beneficial GVL effect, as oral AZM had no effect on the lymphocyte functions of mice. AZM already has a history of use in the treatment of bacterial infections, so its administration should also be safe in patients undergoing BMT for haematological disorders. Similarly to bortezomib [23], AZM could be used singly or in conjunction with immunosuppressants to prevent acute GVHD in various clinical settings. AZM also seems to have potential for use in treating already developed GVHD. Further studies of the in-vivo effects of AZM in allogeneic BMT are clearly warranted.
Acknowledgments
We thank Dr Takashi Iwamoto of Chubu College of Life and Health Sciences for technical advice and Miyuki Namikata for technical assistance. This work was supported by a Research Grant for Tissue Engineering (H17- 014) and a Research Grant for Allergic Disease and Immunology (H20-015) from the Japanese Ministry of Health, Labour, and Welfare.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Choi SW, Hildebrandt GC, Olkiewicz KM. CCR1/CCL5 (RANTES) receptor–ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–3455. doi: 10.1182/blood-2007-05-087403. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drobyski WR, Majewski D. Treatment of donor mice with an alpha beta T-cell receptor monoclonal antibody induces prolonged T-cell nonresponsiveness and effectively prevents lethal graft-versus-host disease in murine recipients of major histocompatibility complex (MHC)-matched and MHC-mismatched donor marrow grafts. Blood. 1996;87:5355–5369. [PubMed] [Google Scholar]
- 3.Stuehler C, Mielke S, Chatterjee M. Selective depletion of alloreactive T cells by targeted therapy of heat shock protein 90: a novel strategy for control of graft-versus-host disease. Blood. 2009;114:2829–2836. doi: 10.1182/blood-2009-06-224600. et al. [DOI] [PubMed] [Google Scholar]
- 4.Park HB, Oh K, Garmaa N. CP-690550, a Janus kinase inhibitor, suppresses CD4+ T-cell-mediated acute graft-versus-host disease by inhibiting the interferon-γ pathway. Transplantation. 2010;90:825–835. doi: 10.1097/TP.0b013e3181f24e59. et al. [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan P, Heaphy CE, Costinean S. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119:4786–4797. doi: 10.1182/blood-2011-10-387522. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlomchik WD, Couzens MS, Tang CB. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. et al. [DOI] [PubMed] [Google Scholar]
- 7.Teshima T, Ordemann R, Reddy P. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. et al. [DOI] [PubMed] [Google Scholar]
- 8.Duffner UA, Maeda Y, Cooke KR. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. et al. [DOI] [PubMed] [Google Scholar]
- 9.Matte CC, Liu J, Cormier J. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. et al. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Ehrhardt MJ, Lees CJ. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–3488. doi: 10.1182/blood-2007-05-087940. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama M, Hashimoto D, Aoyama K. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. et al. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Briere F, Caux C. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. et al. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 15.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falvo JV, Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 17.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K, Wilkins DE, Anver MR. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao Y, Zhang W, Fang Y. Bortezomib attenuates acute graft-vs.-host disease through interfering with host immature dendritic cells. Exp Hematol. 2011;39:710–720. doi: 10.1016/j.exphem.2011.03.001. et al. [DOI] [PubMed] [Google Scholar]
- 23.Koreth J, Stevenson KE, Kim HT. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–3959. doi: 10.1182/blood-2009-07-231092. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwamoto S, Kumamoto T, Azuma E. The effect of azithromycin on the maturation and function of murine bone marrow-derived dendritic cells. Clin Exp Immunol. 2011;166:385–392. doi: 10.1111/j.1365-2249.2011.04480.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azuma E, Yamamoto H, Kaplan J. Use of lymphokine-activated killer cells to prevent bone marrow graft rejection and lethal graft-vs-host disease. J Immunol. 1989;143:1524–1529. [PubMed] [Google Scholar]
- 26.Cooke KR, Kobzik L, Martin TR. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. et al. [PubMed] [Google Scholar]
- 27.Hill GR, Cooke KR, Teshima T. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J Clin Invest. 1998;102:115–123. doi: 10.1172/JCI3132. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umemoto M, Azuma E, Hirayama M. Cytokine-enhanced mixed lymphocyte reaction (MLR) in cord blood. Clin Exp Immunol. 1998;112:459–463. doi: 10.1046/j.1365-2249.1998.00607.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlomchik WD, Couzens MS, Tang CB. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. et al. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald KP, Kuns RD, Rowe V. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood. 2007;109:5049–5057. doi: 10.1182/blood-2007-01-067249. et al. [DOI] [PubMed] [Google Scholar]
- 32.Cetkovic-Cvrlje M, Roers BA, Waurzniak B, Liu XP, Uckun FM. Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood. 2001;98:1607–1613. doi: 10.1182/blood.v98.5.1607. [DOI] [PubMed] [Google Scholar]
- 33.Merad M, Hoffmann P, Ranheim E. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugerman PB, Faber SB, Willis LM. Kinetics of gene expression in murine cutaneous graft-versus-host disease. Am J Pathol. 2004;164:2189–2203. doi: 10.1016/S0002-9440(10)63776-5. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiyama K, Shirai R, Mukae H. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin Exp Immunol. 2007;147:540–546. doi: 10.1111/j.1365-2249.2007.03299.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan AA, Slifer TR, Araujo FG, Remington JS. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–132. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi K, Shibata Y, Kimura T. Azithromycin suppresses interleukin-12p40 expression in lipopolysaccharide and interferon-gamma stimulated macrophages. Int J Biol Sci. 2009;5:667–678. doi: 10.7150/ijbs.5.667. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+ CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Keeffe M, Hochrein H, Vremec D. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1precursors. Blood. 2003;101:1453–1459. doi: 10.1182/blood-2002-03-0974. et al. [DOI] [PubMed] [Google Scholar]
- 41.Maraskovsky E, Brasel K, Teepe M. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masten BJ, Olson GK, Kusewitt DF, Lipscomb MF. Flt3 ligand preferentially increases the number of functionally active myeloid dendritic cells in the lungs of mice. J Immunol. 2004;172:4077–4083. doi: 10.4049/jimmunol.172.7.4077. [DOI] [PubMed] [Google Scholar]
- 43.Shurin MR, Pandharipande PP, Zorina TD. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. et al. [DOI] [PubMed] [Google Scholar]
- 44.Tomazic J, Kotnik V, Wraber B. In vivo administration of azithromycin affects lymphocyte activity in vitro. Antimicrob Agents Chemother. 1993;37:1786–1789. doi: 10.1128/aac.37.9.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratanatharathorn V, Nash RA, Przepiorka D. Phase III study comparing methothrexate and tacrolimus (Prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-indentical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. et al. [PubMed] [Google Scholar]
- 46.Tseng AL, Dolovich L, Salit IE. Azithromycin-related ototoxicity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1997;24:76–77. doi: 10.1093/clinids/24.1.76. [DOI] [PubMed] [Google Scholar]
- 47.Grayston JT, Kronmal RA, Jackson LA. Azythromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. et al. [DOI] [PubMed] [Google Scholar]
- 48.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooke KR, Gerbitz A, Crawford JM. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–1589. doi: 10.1172/JCI12156. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 51.Storb R, Prentice RL, Buckner CD. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308:302–307. doi: 10.1056/NEJM198302103080602. et al. [DOI] [PubMed] [Google Scholar]
- 52.Guthery SL, Heubi JE, Filipovich A. Enteral metronidazole for the prevention of graft versus host disease in pediatric marrow transplant recipients: results of a pilot study. Bone Marrow Transplant. 2004;33:1235–1239. doi: 10.1038/sj.bmt.1704474. [DOI] [PubMed] [Google Scholar]
- 53.Eriguchi Y, Takashima S, Oka H. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120:223–231. doi: 10.1182/blood-2011-12-401166. et al. [DOI] [PubMed] [Google Scholar]
- 54.Jenq RR, Ubeda C, Taur Y. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amsden GW, Nafziger AN, Foulds G. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob Agents Chemother. 1999;43:163–165. doi: 10.1128/aac.43.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumas JL, Chang R, Mermillod B, Piguet PF, Comte R, Pechère JC. Evaluation of the efficacy of prolonged administration of azithromycin in a murine model of chronic toxoplasmosis. J Antimicrob Chemother. 1994;34:111–118. doi: 10.1093/jac/34.1.111. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–1344. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]