Abstract
Background
Endometrial carcinoma is a highly prevalent gynecologic malignancy. The International Federation of Gynecology and Obstetrics (FIGO) staging system underwent significant revision on 2009. Key changes in the FIGO staging system include simplification of stage I endometrial cancer and removal of cervical mucosal invasion as a separate stage. MRI is a noninvasive diagnostic method for preoperative staging of endometrial cancer.
Objectives
The main purpose of this study was to investigate the diagnostic efficacy of pelvic MRI in determining the depth of myometrial invasion and cervical involvement in endometrial carcinoma. The other aim was to compare the accuracy of pelvic MRI using the old and new FIGO staging systems in endometrial carcinoma.
Patients and Methods
Between November 2010 and January 2012, 54 patients underwent primary surgical staging in our department due to endometrial adenocarcinoma. Pre-operative pelvic MRI was performed and MRI staging was done according to old and new FIGO staging, separately. The sensitivity, specificity, positive and negative predictive values as well as the accuracy of MRI for deep myometrial invasion and cervical infiltration were calculated. MRI accuracy was also compared for old and new FIGO staging. Pathological staging was the standard of reference.
Results
The mean age was 53.31 (SD = 11.52) and the most common histological subtype was the endometrioid type of endometrial adenocarcinoma (90.8%). In the evaluation of deep tumoral invasion of the myometrium (> 50%), sensitivity, specificity, diagnostic accuracy and positive and negative predictive values of MRI were 82.35%, 94.59%, 90.74%, 87.5% and 92.1%, respectively. For cervical stromal involvement, these values were 54.54%, 100%, 90.74%, 100% and 89.58%, respectively. In case of cervical mucosal involvement (in old FIGO staging), the positive predictive value was only 50% and the accuracy decreased to 74.07%. Agreement between MRI and the final histology using the old and new FIGO classification was appropriate with Kappa = 0.62 and 0.72, respectively (P < 0.001).
Conclusion
Using 2009 FIGO classification increases the accuracy of pelvic MR imaging for preoperative staging of patients with early stages of endometrial cancer.
Keywords: Endometrial Neoplasms, Uterus, Magnetic Resonance Imaging, Myometrium, Cancer Staging
1. Background
Endometrial cancer is the most common gynecological cancer. The incidence is growing because of increasing obesity and age (1). One of the most important aspects of successful patient management with endometrial cancer is the accurate staging of the disease at the time of diagnosis and initiating a right treatment plan without causing any more morbidity. Before 1988, staging was performed by examination under anesthesia and D & C. In this method, at least 13-22% of the patients underwent inaccurate staging before treatment planning, which results in a decreased survival (2). In 1988, surgicopathologic International Federation of Gynecology and Obstetrics (FIGO) staging was introduced (Table 1) (3). With reported similar prognosis in some of the FIGO substages (stage Ia and IIa) in 2009, some precise changes occurred in staging (Table 2) (4-6). In the new staging system for stage I, there are two substages Ia (< 50% myometrial invasion) and Ib (≥ 50% myometrial invasion) in comparison with the old staging system in which there are three substages; Ia (confirmed to endometrium), Ib (< 50% myometrial invasion) and Ic (≥ 50% myometrial invasion). There are no more substages for stage II and only cervical stromal involvement (stage IIb, old system) is considered and endocervical glandular invasion (stage IIa, old system) is now diagnosed as stage I endometrial cancer. Differentiation between stage Ia and Ib in the old staging system and also glandular involvement of the cervix had some challenges. It is believed that with the new 2009 FIGO staging, a better accuracy in MRI staging is obtained (7, 8). The information about the role of MRI in endometrial cancer in our country is limited. In this study, we assessed the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of MRI in recognizing the depth of myometrial invasion and cervical involvement. Furthermore, its accuracy in old (1988) and new (2009) FIGO staging classifications is compared.
Table 1. 1988 FIGO Staging Classification for Endometrial Cancer.
| Stage | Characteristics |
|---|---|
| Ia G1, 2, 3 | Confined to endometrium |
| Ib G1, 2, 3 | < 50% myometrial involvement |
| Ic G1,2,3 | ≥ 50% myometrial involvement |
| IIa | Endocervical glandular involvement |
| IIb | Cervical stromal invasion |
| IIIa | Invasion to uterine serosa, adnexa and/or positive peritoneal cytology |
| IIIb | Parametrium involvement, vaginal metastasis |
| IIIc | Pelvic or paraaortic lymph nodes involvement |
| Iva | Bladder and/or bowel mucosal metastasis |
| IVb | Distant metastasis including intraabdominal or inguinal nodes |
Table 2. 2009 FIGO Staging System for Endometrial Cancer (New Staging).
| FIGO Stage | Pathologic Finding | MRI Finding |
|---|---|---|
| Stage Iac | Tumor confined to the uterus, < 50% myometrial invasion | The endometrial mass is confirmed to the endometrium or invades < 50% of the myometrial wall with disruption of the JZ |
| Stage Ibc | Tumor confined to the uterus, ≥ 50% myometrial invasion | Mass invades ≥50% of the myometrium with a preserved stripe enhancing outer myometrial wall |
| Stage IIc | Cervical stromal invasion | Disruption of the low-signal intensity inner cervical stroma (T2). |
| Disruption of the cervical epithelium enhancement (CE T1) | ||
| Stage IIIac | Tumor invasion into serosa or adnexa | Disruption of continuity of the outer myometrium or presence of nodules on the peritoneal surface or adnexa |
| Stage IIIbc | Vaginal or parametrial involvement | Tumor extension into the upper vagina and/or parametrium |
| Stage IIIc1c | Pelvic node involvement | Enlarged pelvic lymph nodes (cut-off value: > 10mm for short-axis diameter) |
| Stage IIIc2c | Para-aortic node involvement | Enlarged para-aortic lymph node |
| Stage IVac | Tumor invasion into the bladder or bowel mucosa | Tumor extension into the bladder/rectum with disruption of the hypointense signal of the bladder or rectal wall |
| Stage IVbc | Distant metastases including abdominal or inguinal lymph node involvement | Intra-peritoneal metastases in the peritoneum or omentum |
| Distant metastases to the lung, liver or bones. Distant lymph node metastases |
aExclusively endometrial glandular involvement should be considered stage I
bPositive cytology should be reported separately without changing the stage
cEither G1, G2 or G3
Abbreviations: CE; contrast enhanced, FIGO; International Federation of Gynecology and Obstetrics, JZ; junctional zone, T1; T1-weighted imaging, T2; T2-weighted imaging
2. Objectives
The aim of this study was to assess the diagnostic accuracy of MR imaging for presumed myometrial and cervical involvement. Meanwhile, we compared the new and old FIGO staging and their agreement with MRI diagnosis.
3. Patients and Methods
Our study was performed between November 2010 and January 2012 on 65 women with endometrial cancer. Eleven patients were excluded from the study; six of whom were due to medical contraindications for surgery or advanced disease that needed chemotherapy or radiotherapy before surgery and five patients due to the final carcinosarcoma diagnosis. For MR imaging, the patients fasted for 4-6 hours before the procedure. They voided before MRI to avoid bladder fullness. MR imaging was acquired with 1.5 T system and phased-array pelvic coil. In our protocol, T2-weighted sequences in three planes (axial, axial oblique and sagittal sections of the uterus body) were obtained. The obtained axial oblique images were perpendicular to the endometrial cavity. Native T1-weighted sequences in the sagittal plane were taken too. The later sequence was repeated in axial oblique and sagittal planes with fat suppression before and after IV-contrast administration. Gadolinium was used as the intravenous-contrast in a dose of 0.1 mmol/kg of body weight. Contrast-enhanced MR imaging was not performed in patients with renal impairment (GFR < 30ml/min). MRI findings were reviewed by a radiologist that was aware of endometrial cancer diagnosis, but without knowledge about the surgical and pathological stage of the patients. On MRI images, when the low signal intensity of the junctional zone was preserved, the tumor was limited to the endometrium, so myometrial infiltration was excluded. Concerning the cervix, involvement of endocervical glands and stroma were assessed. Radiological staging was performed according to both new and old FIGO staging systems (Table 2). Surgical specimens were sectioned across the longitudinal plane of the uterus. The depth of myometrial invasion was estimated macroscopically and microscopically without awareness of MR findings and was staged according to FIGO classification. Ultimately, MRI findings were compared with the pathological reports. All patients underwent staging surgery including total hysterectomy and bilateral salpingo-oophorectomy. For suspicious cases, lymphadenectomy was performed. If the cervix was involved, a radical hysterectomy would be done. Statistical analysis was performed by SPSS for Windows 17 (SPSS Inc., Chicago, Illinois, USA). Descriptive statistics, chi-square and Kappa index were used for analysis. The sensitivity, specificity, positive and negative predictive values and accuracy for deep myometrial and cervical infiltration were calculated.
4. Results
Among 54 patients included in this study, the mean age was 53.31 years (range 24-73years). The most common chief complaint was post-menopausal bleeding (57.4%) and abnormal bleeding before menopause (33.3%). Pelvic mass, ascites and inguinal lymph node were the first presentation in the other patients. The most common histological subtype was the endometrioid type detected in 90.8% (49) of the patients. Papillary serous, clear cell and adenosquamous types were the other subtypes. The most common grade was well differentiated type (G1) 53.7% (Table 3). In the surgicopathological report, in 51.9% (28/54), the myometrial depth of invasion was less than 50% and in 31.5% (17/54), there was an equal or greater than 50% involvement and nine patients (16.75%) had no involvement of the myometrium. Seven out of 16 patients (43.8%) who did not have any myometrial invasion in the MRI report, had lower than 50% myometrial involvement in the final pathological findings (Figure 1). Three out of 22 patients (13.6%) with less than 50% myometrial involvement in MRI ultimately had more than 50% myometrial involvement. Fourteen out of 16 patients (87.5%) with an MRI report of more than 50% myometrial invasion had the same findings in final pathology reports. In the evaluation of the deep myometrial invasion (more than 50%), the sensitivity, specificity, diagnostic accuracy, positive and negative predictive values and positive and negative likelihood ratios of MRI (calculated with 95% confidence intervals) were 82.35%, 94.59%, 90.74%, 87.5% ,92.1% ,15.22 and 0.1865, respectively. Table 4 shows the correlation of myometrial involvement in MRI and pathology.
Table 3. Patients’ Characteristics.
| Number of women | 54 |
|---|---|
| Mean age in years, range | 53.31(24-73, SD = 11.52) |
| BMI, No. (range) | 31.1(17-48, SD = 6.44) |
| Chief complaint, No. (%) | |
| Post-menopausal bleeding | 31(54.7) |
| Abnormal bleeding before menopause | 18(33.3) |
| Pelvic mass | 3(5.6) |
| Ascites | 1(1.9) |
| Inguinal node | 1(1.9) |
| Time between MRI and surgery, mean in days (ranges) | 7.64(2-15) |
| Tumor type, No. (%) | |
| Endometrioid | 49(90.8) |
| Papillary serous | 3(5.6) |
| Clear cell | 1(1.9) |
| Adenosquamous | 1(1.9) |
| Tumor grade, No. (%) | |
| G1 | 29(53.7) |
| G2 | 18(33.35) |
| G3 | 7(13) |
Abbreviations: BMI; Body Mass Index, SD; Standard Deviation
Figure 1. A 40-year-old woman with vaginal bleeding.
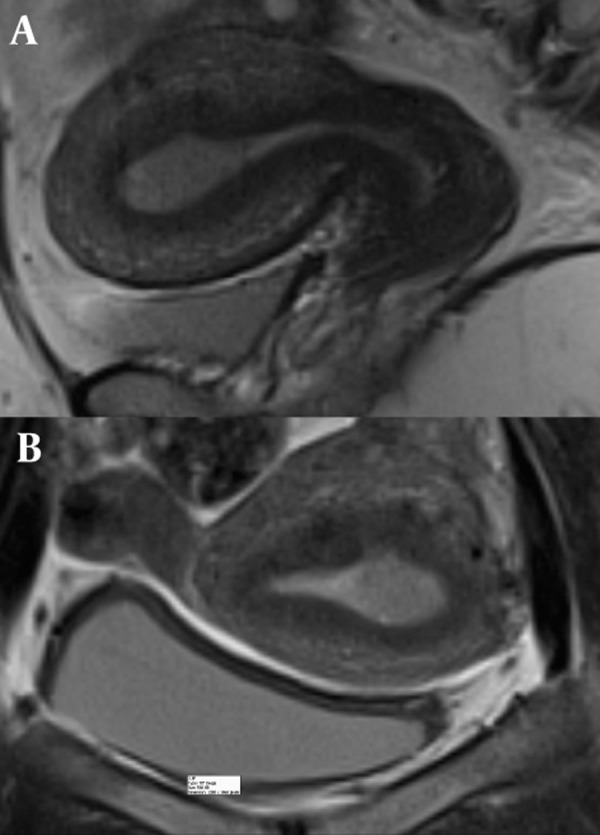
Sagittal T2-weighted (A) and coronal T2-weighted (B) images show an intermediate signal lesion within the endometrial cavity in A and B. The junctional zone is intact. Histopathology revealed stage Іa endometrial carcinoma.
Table 4. Correlation of MR Imaging with Histopathologic Results in 54 Patients.
| Myometrial Invasion in Pathology | Total | |||
|---|---|---|---|---|
| none | < 50% | ≥ 50% | ||
| No myometrial invasion in MRI | 9 | 7 | 0 | 16 |
| < 50% myometrial invasion in MRI | 0 | 19 | 3 | 22 |
| ≥ 50% myometrial invasion in MRI | 0 | 2 | 14 | 16 |
| Total | 9 | 28 | 17 | 54 |
Kappa test = 0.656
According to the correlation of cervical invasion in MRI and the final pathology report, 29 out of 46 patients (63%) without cervical involvement in MRI did not have any cervical invasion in the surgicopthological report, 13 patients (28.3%) had only mucosal and four patients (8.7%) had stromal involvement. One out of two patients (50%) with mucosal involvement in MRI had mucosal and another one had stromal involvement (Figure 2). In case of cervical stromal involvement in the MRI report, all of them had stromal invasion in the pathology report (100% correlation between MRI and pathology). For cervical stromal involvement, the sensitivity, specificity, diagnostic accuracy, positive and negative predictive values and positive and negative likelihood ratios of MRI were 54.54%, 100%, 90.74%, 100%, 89.58%, 2.85 and 0.95, respectively (calculated with 95% confidence intervals). In the case of mucosal involvement, the positive predictive value was only 50% and the accuracy decreased to 74.07%. For agreement between MRI report and pathology of cervix, the Kappa was 0.347. According to old and new FIGO staging classifications, among the patients with stage Ia in MRI based on the old FIGO staging system, eight patients (57.1%) had the same pathological stage, two patients (14.3%) had Ib, one (7.1%) had IIa, one patient (7.1%) had IIb and two patients (14.3%) had IIIa. Six (85.7%) of the patients with Ic,had the same stage and one patient had stage IIa. Two (66.7 %) of the patients with stage IIb in MRI, had the same report and one (33.3%) had IIIa. The Kappa for agreement of old staging between MRI and pathology results was 0.628.
Figure 2. A 46-year-old woman with biopsy-proven endometrial carcinoma. Sagittal T2-weighted MRI shows a large mass within the endometrial cavity invading the deep myometrium and cervical stroma. Histopathology confirmed stage ІІ endometrial carcinoma.
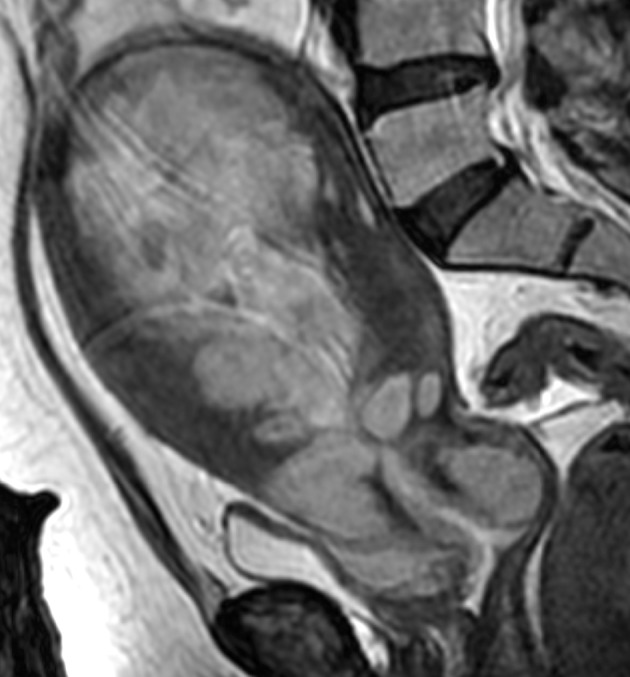
Based on the new FIGO staging, among patients who had stage Ia in MRI, 78.8% (26 patients) had the same staging, 6.1% (two patients) had Ib, 9.1% (three patients) had stage II and 6.1% (two patients) were in stage IIIa. All patients with stage Ib according to MRI had the same pathological stage. Two out of three patients with stage II according to revised FIGO staging in MRI had the same pathological staging and one had invasion to uterine serosa on pathology (IIIa). The Kappa for new staging between MRI and pathology was 0.72 (Table 5).
Table 5. Comparison of Diagnostic Performance Regarding Detection of Myometrial and Cervical Invasion in 54 Enrolled Patients.
| N | TP | FP | FN | TN | Sen, % | Spe, % | Accuracy, % | PPV, % | NPV, % | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Cervical Invasion | 54 | 29 | 17 | 0 | 8 | 100 | 32 | 68.5 | 63.0 | 100 | 1.4 | 0 |
| Cervical Mucus Invasion | 54 | 1 | 1 | 13 | 39 | 71.4 | 97.5 | 74.0 | 50 | 75 | 2.8 | 0.95 |
| Cervical Stromal Invasion | 54 | 6 | 0 | 5 | 43 | 54.5 | 100 | 90.7 | 100 | 89.5 | _ | 0.45 |
| No Myometrial Invasion | 54 | 9 | 7 | 0 | 38 | 100 | 84.4 | 87.0 | 56.2 | 100 | 6.4 | 0 |
| < 50% Myometrial Invasion | 54 | 19 | 3 | 9 | 23 | 67.8 | 88.4 | 77.7 | 86.3 | 71.8 | 5.8 | 0.36 |
| ≥ 50% Myometrial Invasion | 54 | 14 | 2 | 3 | 35 | 82.3 | 94.5 | 90.7 | 87.5 | 92.1 | 15.2 | 0.18 |
Abbreviations: FN; false negative, FP; false positive, NLR; negative likelihood ratio NPV; negative predictive value, PLR; positive likelihood ratio, PPV; positive predictive value, Sen; sensitivity, Spe; specificity, TN; true negative, TP; true positive
5. Discussion
Although modalities such as CT scan and MRI are not a part of FIGO staging for endometrial carcinoma, MRI can provide valuable data to estimate staging and choose the best treatment planning (9-11). The treatment of choice for endometrial cancer is surgery. Based on staging, cervical stromal involvement and the depth of myometrial invasion, the type of surgery can differ from a simple hysterectomy without lymphadenectomy or only a lymph node sampling to radical surgery with systematic lymphadenectomy (12, 13). MRI does not have any ionizing rays while it provides a high soft tissue contrast that makes it an optimal imaging technique for the female pelvis (14).
Moreover, with MRI we can decide about conservative management for patients who want fertility preservation or have medical problems that are high risk for surgery. It can be used for radiotherapy mapping too. The accuracy and sensitivity of MRI in the preoperation staging of endometrial cancer have been shown in several studies (15-17). Hirano et al. found that the accuracy, sensitivity, specificity, PPV and NPV in postcontrast MRI were 91%, 91%, 89%, 94% and 84%, respectively (15). Similar to other studies, in this analysis, the endometrioid type was the most common histology (18, 19). In our study, the majority of patients were in the early stage according to the final surgicopathology report (74.1% for stage I and II). The reported sensitivity of MRI in the diagnosis of cervical invasion for endometrial carcinoma had a range of 19-100% (20-28). A very low sensitivity (19%) reported in some studies may be due to the undetectable endocervical glandular involvement (stage IIA in the old system). In our study, the sensitivity and specificity in stromal invasion was higher in contrast to mucosal invasion diagnosis, 54.54% and 100% vs. 71.4% and 97.5%, respectively. These findings indicate the higher correlation of the new FIGO staging with MRI in the diagnosis of cervical involvement and its use in choosing the best treatment options. If there is a stromal cervical involvement, a radical surgery is the choice of treatment. The accuracy of MRI for diagnosing cervical mucosal involvement was very lower than cervical stromal involvement, 74.07% in contrast to 90.74%. The overall accuracy and specificity for cervical invasion ranged from 46-98% and 87-100 %, respectively (20-28). It has been proved that the depth of myometrial invasion has a direct relation with lymph node involvement and prognosis. If involvement of the myometrium is equal to 50% and more, the risk of lymph node involvement increases to more than 6-7 fold (13). Avoidance of unnecessary lymphadenectomy is important in low risk patients based on the depth of myometrial invasion, grading and histological subtype before surgery. So, knowledge about myometrial invasion is important for the extension and type of surgery (29, 30). It is indicated that MRI has the highest accuracy among the other imaging techniques in predicting the myometrial invasion (21-26, 28, 31, same as other studies 32). In a study performed by Riek et al. (31), there was a low sensitivity, accuracy and specificity, (47%, 36% and 50%, respectively) due to using an unenhanced MRI protocol. A meta-analysis revealed enhanced MRI is a better protocol for assessing myometrial invasion (9).We found an accuracy of 90.74% in our study for deep myometrial invasion. On the other hand, with a negative predictive value of 92.1%, this study indicates that MRI can predict deep invasion. This is in agreement with the results of other studies which found the accuracy of MRI for determining the depth of myometrial invasion ranging from 83% to 91% (17, 21-26, 28, 31, 33). Regarding staging and comparing the old and new FIGO staging in MRI and its correlation with surgicopathological findings, our results indicate a higher accuracy for the new staging classification. With a Kappa agreement of 0.72 for new and 0.62 for old staging, our results are as the same as other studies (21-26, 28). There are few works regarding the comparison of new and old staging. One of them indicates that the accuracy of MRI in new staging is more than the old system, but their study did not address myometrial and cervical involvement in detail (7). Thus, the present study could give useful data about MRI and endometrial cancer staging which does not have the limitation of such studies that have only assessed early stages of the disease. Furthermore, this work has all stages of endometrial cancer which results in lower sensitivity in contrast to the other studies.
Our study has some limitations. For example, uterine curettage could change the appearance of endometrial-myometrial interface on MRI. In addition, the interobserver agreement between the radiologists was not assessed in this study. Moreover, in certain conditions, it is difficult to assess the depth of myometrial invasion by MRI properly, for example in case of existence of the polypoid feature of the tumor, existence of a large uterine fibroma or adenomyosis, and when there is an isointense junctional zone (JZ) compared to the myometrium. In conclusion, MRI is a valuable imaging modality for presurgical staging of endometrial cancer, especially based on revised FIGO staging.
Acknowledgments
None declared.
Footnotes
Implication for health policy/practice/research/medical education: This research deals with the diagnostic accuracy of pelvic MRI for endometrial cancer. Accurate staging of endometrial cancer is crucial for treatment planning and the patient’s prognosis.
Please cite this paper as: Zamani F, Goodarzi S, Hallaji F, Zamiri A, Deilami T, Malek M, et al. Diagnostic Value of Pelvic MRI for Assessment of the Depth of Myometrial Invasion and Cervical Involvement in Endometrial Cancer: Comparison of New Versus Old FIGO Staging. Iran J Radiol. 2012; 9 (4): 202-8. DOI: 10.5812/iranjradiol.5276
Authors’ Contribution: All authors contributed extensively to the study.
Financial Disclosure: None declared.
Funding/Support: None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lee EJ, Byun JY, Kim BS, Koong SE, Shinn KS. Staging of early endometrial carcinoma: assessment with T2-weighted and gadolinium-enhanced T1-weighted MR imaging. Radiographics. 1999;19(4):937–45. doi: 10.1148/radiographics.19.4.g99jl06937. discussion 46-7. [DOI] [PubMed] [Google Scholar]
- 3.Mikuta JJ. International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer. 1993;71(4 Suppl):1460–3. doi: 10.1002/cncr.2820710409. [DOI] [PubMed] [Google Scholar]
- 4.Alexiou VG, Ierodiakonou V, Peppas G, Falagas ME. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect (Larchmt). 2010;11(4):343–8. doi: 10.1089/sur.2009.023. [DOI] [PubMed] [Google Scholar]
- 5.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105(2):109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–43. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 7.Ballester M, Koskas M, Coutant C, Chereau E, Seror J, Rouzier R, et al. Does the use of the 2009 FIGO classification of endometrial cancer impact on indications of the sentinel node biopsy? BMC Cancer. 2010;10:465. doi: 10.1186/1471-2407-10-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddy P, O'Neill AC, Yamamoto AK, Addley HC, Reinhold C, Sala E. FIGO staging system for endometrial cancer: added benefits of MR imaging. Radiographics. 2012;32(1):241–54. doi: 10.1148/rg.321115045. [DOI] [PubMed] [Google Scholar]
- 9.Kinkel K, Kaji Y, Yu KK, Segal MR, Lu Y, Powell CB, et al. Radiologic staging in patients with endometrial cancer: a meta-analysis. Radiology. 1999;212(3):711–8. doi: 10.1148/radiology.212.3.r99au29711. [DOI] [PubMed] [Google Scholar]
- 10.Barwick TD, Rockall AG, Barton DP, Sohaib SA. Imaging of endometrial adenocarcinoma. Clin Radiol. 2006;61(7):545–55. doi: 10.1016/j.crad.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Frei KA, Kinkel K. Staging endometrial cancer: role of magnetic resonance imaging. J Magn Reson Imaging. 2001;13(6):850–5. doi: 10.1002/jmri.1121. [DOI] [PubMed] [Google Scholar]
- 12.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 13.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitao MM, Jr., Chi DS. Fertility-sparing options for patients with gynecologic malignancies. Oncologist. 2005;10(8):613–22. doi: 10.1634/theoncologist.10-8-613. [DOI] [PubMed] [Google Scholar]
- 15.Hirano Y, Kubo K, Hirai Y, Okada S, Yamada K, Sawano S, et al. Preliminary experience with gadolinium-enhanced dynamic MR imaging for uterine neoplasms. Radiographics. 1992;12(2):243–56. doi: 10.1148/radiographics.12.2.1561414. [DOI] [PubMed] [Google Scholar]
- 16.Hricak H, Rubinstein LV, Gherman GM, Karstaedt N. MR imaging evaluation of endometrial carcinoma: results of an NCI cooperative study. Radiology. 1991;179(3):829–32. doi: 10.1148/radiology.179.3.2028000. [DOI] [PubMed] [Google Scholar]
- 17.Lien HH, Blomlie V, Trope C, Kaern J, Abeler VM. Cancer of the endometrium: value of MR imaging in determining depth of invasion into the myometrium. AJR Am J Roentgenol. 1991;157(6):1221–3. doi: 10.2214/ajr.157.6.1950869. [DOI] [PubMed] [Google Scholar]
- 18.Rose PG. Endometrial carcinoma. N Engl J Med. 1996;335(9):640–9. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 19.Creasman WT, Rock J, Jones HW. Malignant tumors of the uterine corpus. Operative gynecology. 9th ed. Philadelphia: Lippincott Williams & Wilkins; pp. 919–59. [Google Scholar]
- 20.Toki T, Oka K, Nakayama K, Oguchi O, Fujii S. A comparative study of pre-operative procedures to assess cervical invasion by endometrial carcinoma. Br J Obstet Gynaecol. 1998;105(5):512–6. doi: 10.1111/j.1471-0528.1998.tb10151.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanjuan A, Escaramis G, Ayuso JR, Roman SM, Torne A, Ordi J, et al. Role of magnetic resonance imaging and cause of pitfalls in detecting myometrial invasion and cervical involvement in endometrial cancer. Arch Gynecol Obstet. 2008;278(6):535–9. doi: 10.1007/s00404-008-0636-1. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos C, Felix A, Cunha TM. Preoperative assessment of deep myometrial and cervical invasion in endometrial carcinoma: comparison of magnetic resonance imaging and histopathologic evaluation. J Obstet Gynaecol. 2007;27(1):65–70. doi: 10.1080/01443610601056418. [DOI] [PubMed] [Google Scholar]
- 23.Ortashi O, Jain S, Emannuel O, Henry R, Wood A, Evans J. Evaluation of the sensitivity, specificity, positive and negative predictive values of preoperative magnetic resonance imaging for staging endometrial cancer. A prospective study of 100 cases at the Dorset Cancer Centre. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):232–5. doi: 10.1016/j.ejogrb.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Sala E, Crawford R, Senior E, Shaw A, Simcock B, Vrotsou K, et al. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int J Gynecol Cancer. 2009;19(1):141–6. doi: 10.1111/IGC.0b013e3181995fd9. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi R, Mirk P, Maresca G, Margariti PA, Testa A, Zannoni GF, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology. 2004;231(2):372–8. doi: 10.1148/radiol.2312021184. [DOI] [PubMed] [Google Scholar]
- 26.Cunha TM, Felix A, Cabral I. Preoperative assessment of deep myometrial and cervical invasion in endometrial carcinoma: comparison of magnetic resonance imaging and gross visual inspection. Int J Gynecol Cancer. 2001;11(2):130–6. doi: 10.1046/j.1525-1438.2001.011002130.x. [DOI] [PubMed] [Google Scholar]
- 27.Cabrita S, Rodrigues H, Abreu R, Martins M, Teixeira L, Marques C, et al. Magnetic resonance imaging in the preoperative staging of endometrial carcinoma. Eur J Gynaecol Oncol. 2008;29(2):135–7. [PubMed] [Google Scholar]
- 28.Savelli L, Ceccarini M, Ludovisi M, Fruscella E, De Iaco PA, Salizzoni E, et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet Gynecol. 2008;31(5):560–6. doi: 10.1002/uog.5295. [DOI] [PubMed] [Google Scholar]
- 29.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 30.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375(9721):1165–72. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 31.Rieck GC, Bulman J, Whitaker R, Leeson SC. A retrospective review of magnetic resonance imaging in assessing the extent of myometrial infiltration for patients with endometrial carcinoma. J Obstet Gynaecol. 2005;25(8):765–8. doi: 10.1080/01443610500327951. [DOI] [PubMed] [Google Scholar]
- 32.Sanjuan A, Cobo T, Pahisa J, Escaramis G, Ordi J, Ayuso JR, et al. Preoperative and intraoperative assessment of myometrial invasion and histologic grade in endometrial cancer: role of magnetic resonance imaging and frozen section. Int J Gynecol Cancer. 2006;16(1):385–90. doi: 10.1111/j.1525-1438.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 33.Sironi S, Colombo E, Villa G, Taccagni G, Belloni C, Garancini P, et al. Myometrial invasion by endometrial carcinoma: assessment with plain and gadolinium-enhanced MR imaging. Radiology. 1992;185(1):207–12. doi: 10.1148/radiology.185.1.1523309. [DOI] [PubMed] [Google Scholar]


