Abstract
Background and Objectives
Hypercholesterolemia is a key factor in the development of atherosclerosis. We sought to evaluate the relation between hypercholesterolemia and plaque composition in patients with coronary artery disease.
Subjects and Methods
Study subjects consisted of 323 patients (mean 61.5 years, 226 males) who underwent coronary angiography and virtual histology-intravascular ultrasound examination. Patients were divided into two groups according to total cholesterol level: hypercholesterolemic group (≥200 mg/dL, n=114) and normocholesterolemic group (<200 mg/dL, n=209).
Results
Hypercholesterolemic patients were younger (59.7±13.3 years vs. 62.6±11.5 years, p=0.036), than normocholesterolemic patients, whereas there were no significant differences in other demographics. Hypercholesterolemic patients had higher corrected necrotic core volume (1.23±0.85 mm3/mm vs. 1.02±0.80 mm3/mm, p=0.029) as well as percent necrotic core volume (20.5±8.5% vs. 18.0±9.2%, p=0.016) than normocholesterolemic patients. At the minimal lumen area site, percent necrotic core area (21.4±10.5% vs. 18.4±11.3%, p=0.019) and necrotic core area (1.63±1.09 mm2 vs. 1.40±1.20 mm2, p=0.088) were also higher than normocholesterolemic patients. Multivariate linear regression analysis showed that total cholesterol level was an independent factor of percent necrotic core volume in the culprit lesion after being adjusted with age, high density lipoprotein-cholesterol , hypertension, diabetes mellitus, smoking and acute coronary syndrome (beta 0.027, 95% confidence interval 0.02-0.053, p=0.037).
Conclusion
Hypercholesterolemia was associated with increased necrotic core volume in coronary artery plaque. This study suggests that hypercholesterolemia plays a role in making plaque more complex, which is characterized by a large necrotic core, in coronary artery disease.
Keywords: Hypercholesterolemia, Coronary artery disease, Ultrasonography, Interventional plaque, Atherosclerotic
Introduction
Atherosclerosis is one of leading causes of death in developed countries. Rupture of vulnerable plaque characterized by large lipid contents is a main cause of acute coronary syndrome (ACS) and sudden cardiac death.1-4) Accumulation of low density lipoprotein (LDL) particles is the initial step in atherogenesis and cholesterol is a major component of atherosclerotic plaque.5),6) Inflammation and vascular smooth muscle cell proliferation are also important in atherogenesis.7),8) Although hypercholesterolemia promotes accumulation of LDL particles in intima, tissue characterization of coronary plaque according to cholesterol level has not been reported.
A recently developed virtual histology-intravascular ultrasound (VH-IVUS) provides a method of accurate in vivo analysis regarding coronary plaque using radiofrequency spectral analysis identifying the fibrous, fibro-fatty, dense calcium and necrotic cores in the coronary plaque in coronary artery.9),10) It has been shown to have a 93-97% ex vivo and 87-92% in vivo accuracy for specific tissue composition.11-13) We sought to evaluate the relation between hypercholesterolemia and in vivo plaque composition in patients with coronary artery disease using VH-IVUS analysis.
Subjects and Methods
Study population
A total of 331 consecutive patients who underwent coronary angiography (CAG) and VH-IVUS for evaluating ischemic heart disease were enrolled in the study. Eight patients whose cholesterol values were not available were excluded. Finally, the study population consisted of 323 patients; 178 patients with stable angina pectoris, 31 patients with unstable angina pectoris, and 114 patients with myocardial infarction. This study was approved by the ethics committee of Konyang University Hospital.
Clinical information on patients was obtained by analyzing their medical records and blood glucose and lipid analyses were based on the fasting values measured before the CAG. In cases regarding primary percutaneous coronary intervention (PCI), fasting blood was obtained during the morning of the next day for blood chemistry. Subjects were divided into two groups according to total cholesterol values14-16): hypercholesterolemic group (≥200 mg/dL, n=114) and normocholesterolemic group (<200 mg/dL, n=209).
Coronary angiography
All patients received aspirin 300 mg and clopidogrel 300-600 mg, and an additional 120 IU/kg of unfractionated heparin intravenously before CAG and VH-IVUS examination. CAG was done by femoral or radial approach using a 5 Fr catheter with a conventional method. The culprit lesion was identified by the ventricle wall motion abnormalities and the appearance of the angiographic lesion.
Out of 323 study patients, 299 patients underwent PCI whereas the remaining 24 patients were treated by medication. PCI was performed by usual pattern via the transradial or transfemoral approach.
Intravascular ultrasound-examination and analysis
Virtual histology-intravascular ultrasound examination was performed with a dedicated VH-IVUS console (Volcano Therapeutics, Rancho Cordova, CA, USA) and 20-MHz, 2.9 Fr monorail, electronic Eagle Eye Gold IVUS catheter (Volcano Therapeutics, Rancho Cordova, CA, USA) during CAG after intracoronary administration of 100 to 200 µg nitroglycerin. The IVUS catheter was advanced into the target lesion after wiring (n=159, 49.2%) or ballooning (n=164, 50.8%), and automatic pullback at 0.5 mm/s was done. Later one, the VH-IVUS image was recorded on a DVD-ROM for off-line analysis.
Both qualitative and quantitative analyses of gray scale IVUS were performed according to the criteria of the American College of Cardiology's Clinical Expert Consensus Document on IVUS.17) The proximal and distal reference were defined as the site with the largest lumen proximal and distal to a stenosis but within the same segment (usually within 10 mm of the stenosis with no major intervening branches), respectively.
Spectral analysis of intravascular ultrasound radiofrequency data
These analyses were done on the target lesion with customized software (IVUS Lab.; Volcano Therapeutics, Rancho Cordova, CA, USA) by an examiner who was unaware of the gray scale IVUS results. For both the lumen and the media-adventitia interface, automatic border detection was done at the predefined lesion segment. Then, the border detection was manually corrected again in the lesion after automatic border detection. The border was determined only when the two circulatory doctor's judgments agreed. When their opinions differed, another doctor was asked to make a judgment. After confirming the border detection, the software automatically calculates and shows the results. For each frame, histologic findings were expressed in colors (green for fibrous, green-yellow for fibrofatty, white for dense calcified, and red for necrotic core area). The predictive accuracy of this method with tissue mapping has been validated.11-13) The area (mm2) and percent area of each tissue component of plaque were analyzed at the minimal lumen area (MLA) site, and the volume (mm3) and percent volume of each tissue component of plaque were evaluated at the full segment of the culprit lesion. Volume was divided by lesion length to adjust for the different lesion length of each patient and described as corrected volume (mm3/mm).
Statistical analysis
All statistical data were indicated as means and standard deviations for continuous variables and as percentage ratio for categorical variables. The statistical data for the subject groups were processed using Statistical Package for the Social Sciences (SPSS) Statistics Program (version 18.0; SPSS Inc., Chicago, IL, USA). The independent t-test in continuous variables and the chi-square test in discontinuous variables were performed to compare differences in the two groups. Pearson correlation coefficient was used to measure the association between cholesterol values and tissue compositions. Multiple regression analysis with either method was performed to evaluate independent predictors of necrotic core volume. A p<0.05 was considered statistically significant.
Results
Clinical characteristics
Study subjects were divided into two groups; hypercholesterolemic and normocholesterolemic group. The hypercholesterolemic group was younger than the normocholesterolemic group (59.7±13.3 years vs. 62.6±11.5 years, p=0.036). There were no significant differences in gender, prevalence of hypertension, diabetes, smoking and prior myocardial infarction, diagnosis, multivessel disease and the location of the lesion. In the hypercholesterolemic group, total cholesterol (226±64 mg/dL vs. 168±30 mg/dL, p<0.001), triglyceride (202±185 mg/dL vs. 145±77 mg/dL, p<0.001), high density lipoprotein-cholesterol (HDL-C) (46.5±11.0 mg/dL vs. 42.5±10.5 mg/dL, p=0.002), and low density lipoprotein-cholesterol (LDL-C) (146±34 mg/dL vs. 109±23 mg/dL, p<0.001) was significantly higher than the normocholesterolemic group. Blood glucose, creatinine, and highsensitivity C-reactive protein showed no significant differences between the two groups (Table 1).
Table 1.
Clinical characteristics of the study subjects
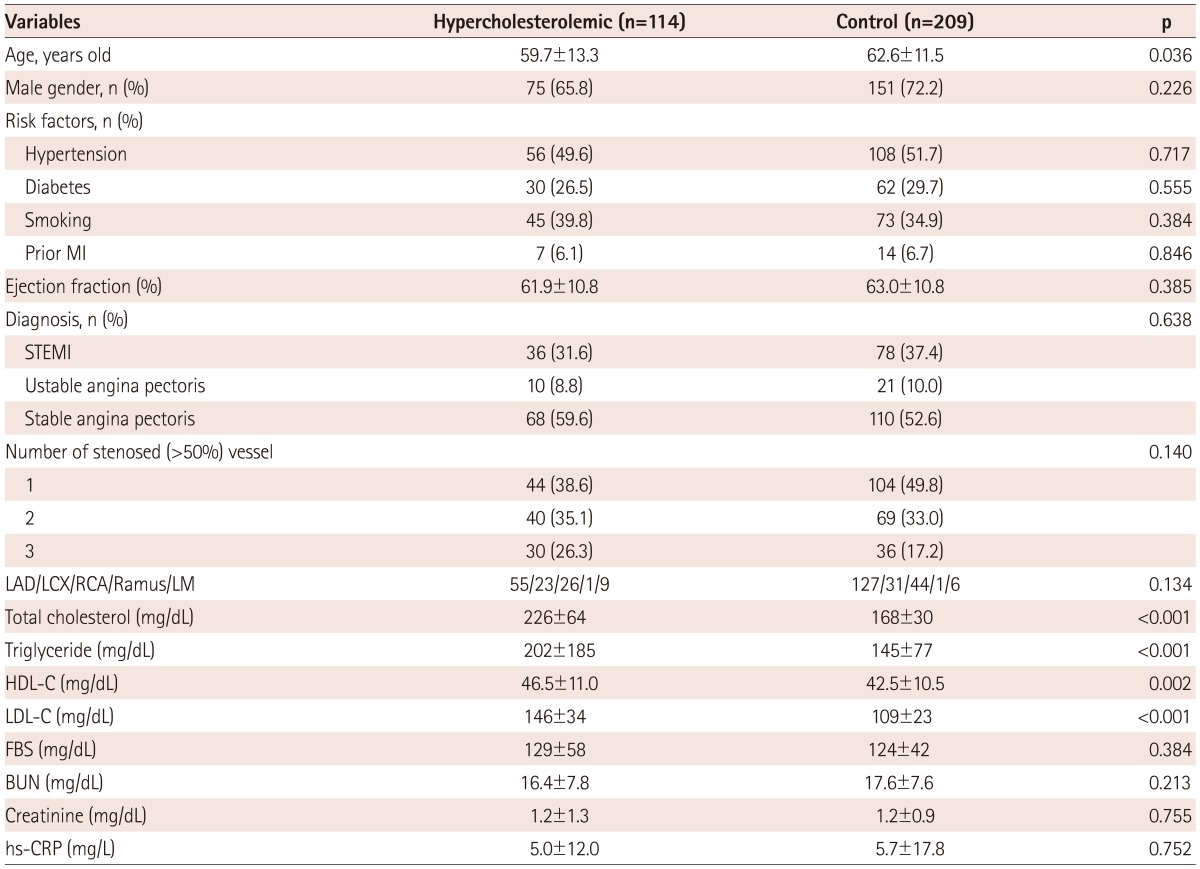
MI: myocardial infarction, STEMI: ST-segment elevation myocardial infarction, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, LM: left main artery, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, FBS: fasting blood sugar, BUN: blood urea nitrogen, hs-CRP: high sensitivity C-reactive protein
Virtual histology-intravascular ultrasound findings
In the gray-scale IVUS finding, there were no significant differences in lesion length, remodeling index, and volumetric analysis adjusted by the lesion length in two groups, but also plaque and lumen area at the MLA site (Table 2).
Table 2.
Gray-scale intravascular ultrasound finding
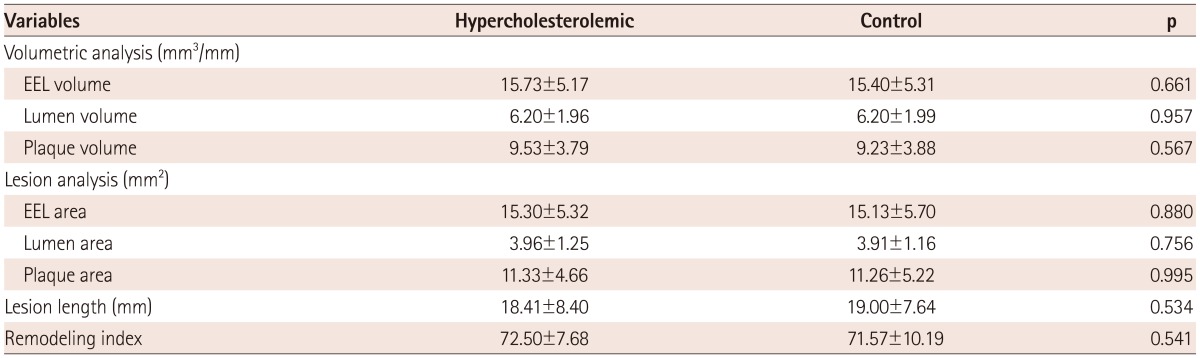
Volumetric data was divided by lesion length to adjust for different lesion lengths. EEL: external elastic lumen
In VH-IVUS analysis over the entire segment of the culprit lesion, necrotic core volume was significantly larger in the hypercholesterolemic group (1.23±0.85 mm3/mm vs. 1.02±0.80 mm3/mm, p=0.029), and percent dense calcified (10.2±7.0% vs. 8.6±6.2%, p=0.041) and percent necrotic core volume (20.5±8.5% vs. 18.0±9.2%, p=0.016) were significantly higher in the hypercholesterolemic group (Table 3, Fig. 1).
Table 3.
Virtual-histology intravascular ultrasound finding
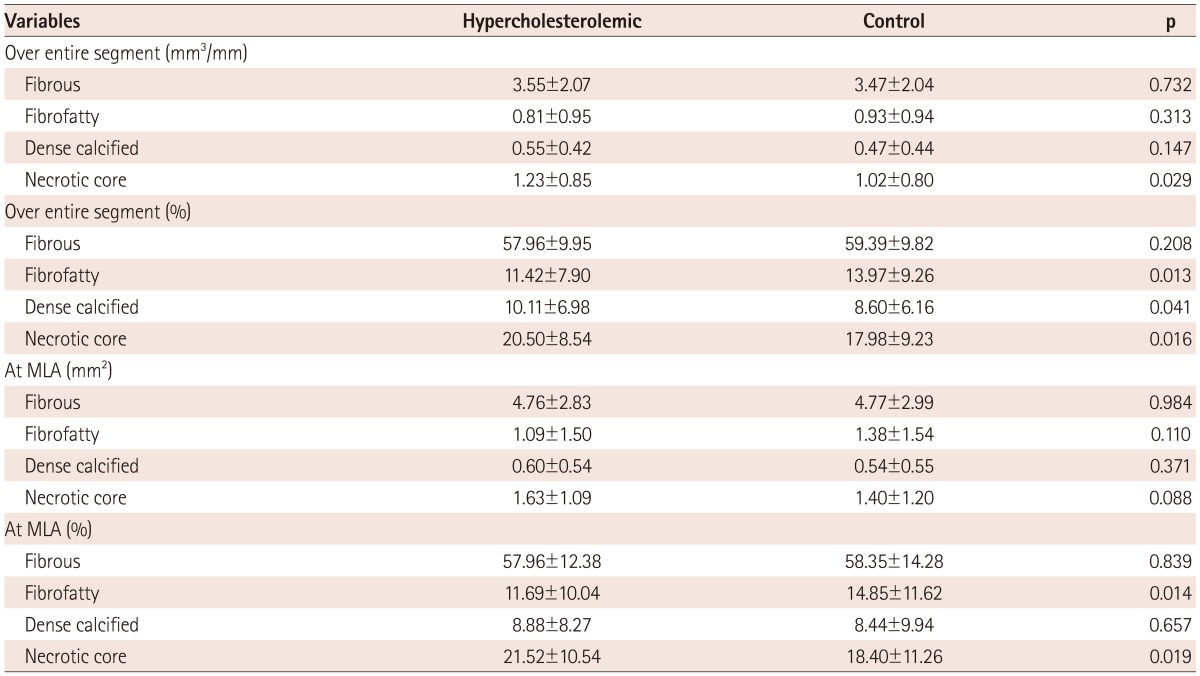
Volumetric data was divided by lesion length to adjust for different lesion lengths. MLA: minimal lumen area
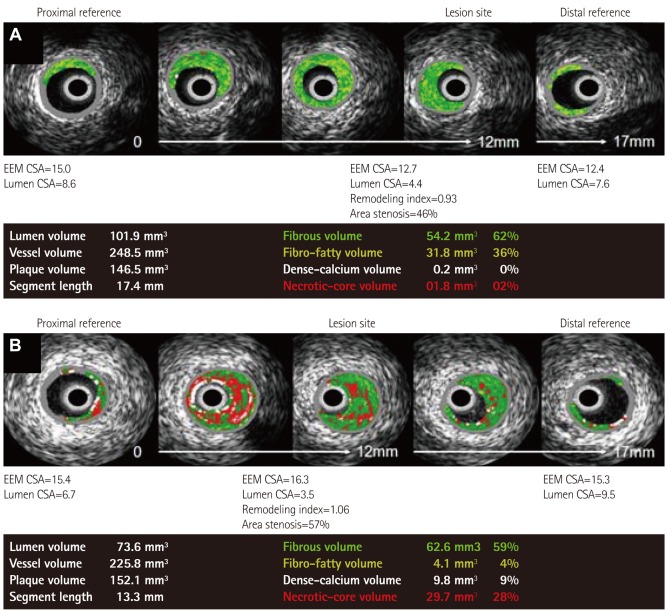
Fig. 1.
Representative VH-IVUS findings in both groups. A: normocholesterolemic patient (42 years old man with stable angina pectoris) showed small amounts of necrotic core volume. B: hypercholesterolemic patient (55 years old man with ST-segment elevation myocardial infarction) showed large amounts of necrotic core volume. VH-IVUS: virtual histology-intravascular ultrasound, EEM: external elastic membrane, CSA: cross sectional area.
In VH-IVUS analysis at the MLA site, the necrotic core area was larger in the hypercholesterolemic group (1.63±1.09 mm2 vs. 1.40±1.20 mm2, p=0.088), but has no statistical significance. However, percent necrotic core area was significantly higher in the hypercholesterolemic group (21.4±10.5% vs. 18.4±11.3%, p=0.019) (Table 3).
Correlation analysis between the serum lipid parameter and tissue compositions of the culprit lesion revealed that total cholesterol was correlated with percent fibro-fatty volume (r=-0.149, p=0.007) and percent necrotic core volume (r=0.132, p=0.018) (Table 4).
Table 4.
Correlation between lipid profiles and tissue composition on culprit lesion
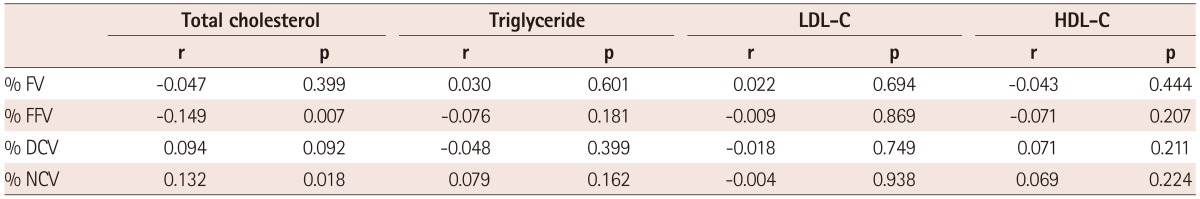
LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, FV: fibrous volume, FFV: fibrofatty volume, DCV: dense calcified volume, NCV: necrotic core volume
Multivariate linear regression analysis revealed that the total cholesterol level was an independent factor of percent necrotic core volume in the culprit lesion after being adjusted with age, HDL-C, hypertension, diabetes mellitus, smoking and ACS (beta 0.027, 95% confidence interval 0.02-0.053, p=0.037).
Discussion
The main finding of the present study is that hypercholesterolemia was associated with necrotic core volume and percent necrotic core volume in the culprit lesion in patients with coronary artery disease.
A recent study showed that diabetes mellitus,18) ACS,19-22) and positive remodeling23) is related with a high percentage of necrotic volume. Also, it is well known that hypercholesterolemia is one of the major coronary risk factors. However, the role of hypercholesterolemia in the progression of atherosclerosis is uncertain. This study showed that necrotic core volume and percent necrotic core volume in the culprit lesion were higher in hypercholesterolemic patients and total cholesterol level was an independent predictor of percent necrotic core volume in the culprit lesion. Our study suggests that hypercholesterolemia may be associated with the progression of atherosclerosis, especially with regards to lipid accumulation in plaque.
Large amounts of lipid in plaque are a characteristic of vulnerable plaque that causes ACS. Previous studies showed that patients with ACS have a high prevalence of plaque rupture and a large amount of necrotic core volume in the culprit lesion.19-22) In this study, the prevalence of ACS did not show a significant difference, so the relationship between hypercholesterolemia and ACS was not proved. This was mainly because we analyzed the study population according to the total cholesterol level, not according to the diagnosis.
High density lipoprotein-cholesterol was higher in the hypercholesterolemic group in this study. HDL-C has antiatherogenic properties24) and a low level of HDL-C is an independent predictor of the risk of coronary heart disease.25) But in the present study, total cholesterol was an independent predictor of necrotic core volume even after being adjusted with HDL-C. Our study revealed that both LDL-C and HDL-C levels were higher in the hypercholesterolemic group, but the difference of the LDL-C level between the two groups was greater than those of the HDL-C level. This difference may explain why total cholesterol level, not HDL-C level, was independently associated with necrotic core volume.
This study has some limitation. First, this study is a single center, cross-sectional study. However, a relatively large number of study subjects were prospectively enrolled. Second, medication of the lipid lowering agent and other risk factors including diabetes, atherosclerotic vascular disease and hypertension were not considered in this analysis. However, there was no significant difference between the two groups in terms of those variables. Finally, 164 lesions (50.8%) from the study population required small sized ballooning in order to advance the VH-IVUS catheter, which could impact on plaque components. However, it is real clinical practice and it would not significantly affect the plaque components unless there was significant plaque embolization.
In conclusion, hypercholesterolemia was associated with increased necrotic core content in coronary artery plaque. This study suggests that hypercholesterolemia plays a role in making plaque more complex, which is characterized by a large necrotic core, in coronary artery disease.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D, Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 6.Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. 2005;353:9–11. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 9.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 10.Raichlin E, Bae JH, Kushwaha SS, et al. Inflammatory burden of cardiac allograft coronary atherosclerotic plaque is associated with early recurrent cellular rejection and predicts a higher risk of vasculopathy progression. J Am Coll Cardiol. 2009;53:1279–1286. doi: 10.1016/j.jacc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 11.Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Granillo GA, McFadden EP, Valgimigli M, et al. Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J. 2006;151:1020–1024. doi: 10.1016/j.ahj.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007;3:113–120. [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger PS, Smith DA, Mehta AE, et al. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18(Suppl 1):1–78. doi: 10.4158/ep.18.s1.1. [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Oh DJ, Kim EJ, et al. The comparison of serum lipid levels and risk factors according to the status of coronary atherosclerosis in Koreans. Korean Circ J. 2003;33:465–474. [Google Scholar]
- 17.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 18.Marso SP, Mercado N, Maehara A, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S42–S52. doi: 10.1016/j.jcmg.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Bae JH, Kwon TG, Kim KH, et al. In-vivo coronary plaque composition in patients with acute coronary syndrome: a virtual histology intravascular ultrasound study. Korean Circ J. 2007;37:437–442. doi: 10.4070/kcj.2013.43.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi R, Oshima S, Jingu M, et al. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2007;50:1641–1646. doi: 10.1016/j.jacc.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Missel E, Mintz GS, Carlier SG, et al. Necrotic core and its ratio to dense calcium are predictors of high-risk non-ST-elevation acute coronary syndrome. Am J Cardiol. 2008;101:573–578. doi: 10.1016/j.amjcard.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Hong MK, Mintz GS, Lee CW, et al. Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am J Cardiol. 2007;100:953–959. doi: 10.1016/j.amjcard.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Kröner ES, van Velzen JE, Boogers MJ, et al. Positive remodeling on coronary computed tomography as a marker for plaque vulnerability on virtual histology intravascular ultrasound. Am J Cardiol. 2011;107:1725–1729. doi: 10.1016/j.amjcard.2011.02.337. [DOI] [PubMed] [Google Scholar]
- 24.Barter P. CETP and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2029–2031. doi: 10.1161/01.atv.20.9.2029. [DOI] [PubMed] [Google Scholar]
- 25.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124 Suppl:S11–S20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]