Abstract
Although its use in daily practice is not common, optical coherence tomography (OCT) is a powerful research tool in invasive cardiology. This report describes a hazy angiography image after percutaneous coronary intervention that has been assessed using OCT. Based on the results of the OCT, the patient underwent an elective coronary angioplasty with standard anticoagulation. After implantation of the stent, an intracoronary hazy image was seen on angiography. The use of OCT permitted a correct diagnosis and a successful treatment. This paper provides a discussion of the advantages and disadvantages of OCT, and a comparison with intravascular ultrasound.
Keywords: Tomography, optical coherence; Intravascular ultrasonography; Cardiac catheterization; Thrombosis; Stents
Introduction
During percutaneuos coronary intervention (PCI) procedures, several complications can occur, such as dissections, thrombi, ulcers or plaque, may appear as angiographic hazy or uncertain images. A proper diagnosis is important to guide the correct management in the catheterization laboratory and to improve patient outcomes. Angiography has important limitations to characterize those entities that, with contrast, can be diagnosed accurately thanks to intravascular imaging.1) Among the intracoronary imaging techniques available, intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are the most commonly used for research. Both techniques provide great value for characterization of coronary lesions and for guidance of PCI. IVUS is very useful to measure the vessel wall and to evaluate plaque burden, whereas the OCT (with almost microscopic resolution) is an excellent method for characterizing luminal and intimal lesions.2-4)
Herein, we present a case that illustrates the ability of OCT for accurate diagnosis of an intracoronary post-angioplasty that had an uncertain hazy image, and its usefulness as a guide for therapeutic strategy.
Case
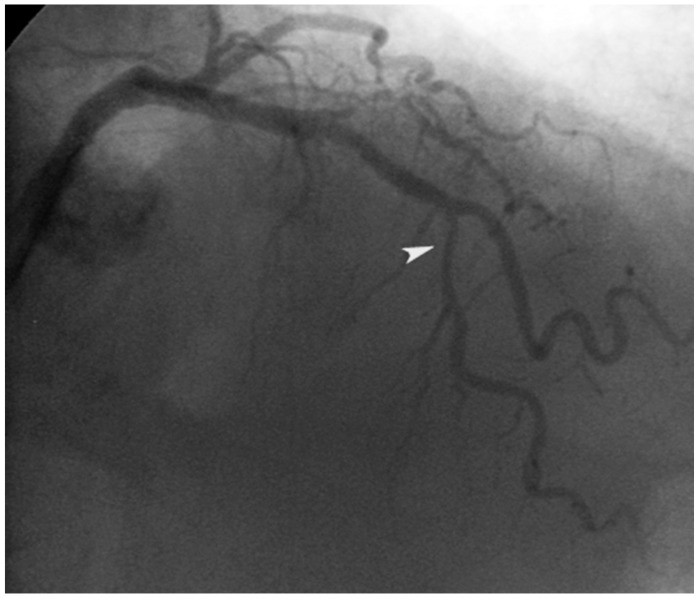
A 73-year-old female patient underwent coronary angiography after being admitted with a non-ST elevation myocardial infarction. We determined that the patient had 2 diseased coronary vessels, with the culprit lesion in the right coronary artery (RCA) and a severe lesion in the left anterior descending artery (LAD) (Fig. 1). We treated the RCA lesion with implantation with a bare metal stent (BMS). We deferred treatment of the LAD lesion for 3 weeks. The patient remained asymptomatic until the second procedure.
Fig. 1.
A severe lesion in the middle portion of the left anterior descendent coronary artery (arrow head).
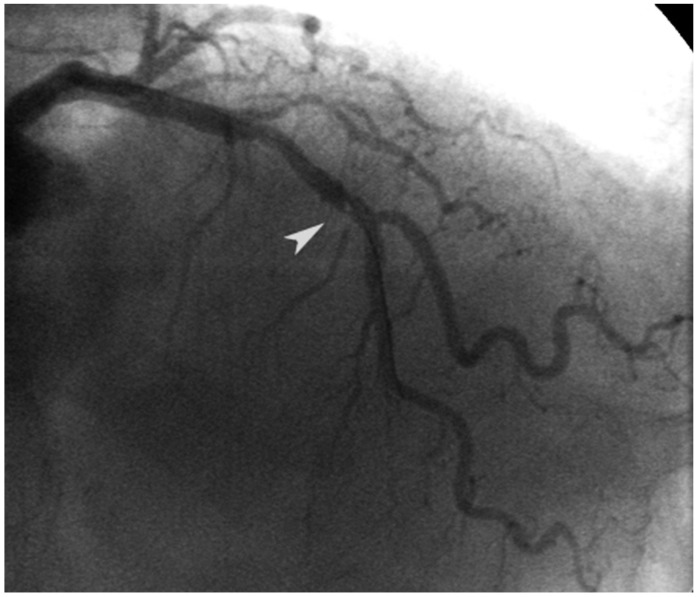
Ten hours before the second procedure, the patient was given a sub-cutaneous dose of 1 mg/kg enoxaparin. Following the current recommendation guidelines,5) we gave the patient a 0.3 mg/kg enoxaparine dose by intravenous administration immediately before the angioplasty procedure; we decided against giving the patient unfractionated heparin. We performed a predilatation procedure using a compliant balloon angioplasty in the LAD without angiographic complications, and after that we implanted a BMS of 2.5×23 mm. In the control post-PCI angiography, we found a focal, intra-stent repletion defect (Fig. 2), with distal Thrombolysis in Myocardial Infarction 3 flow. Because of the uncertain interpretation by angiography, we decided to perform OCT in order to ascertain the mechanism of the angiographic hazy image (dissection, thrombus, plaque prolapse). We used a C7-XR, Saint Jude OCT Frequency Domain System with a Dragon Fly Catheter (St. Jude Medical, St. Paul, MN, USA). The OCT study showed an intra-stent protruding signal rich mass with irregular borders and low-backscattering, a finding compatible with a white thrombus.
Fig. 2.
Intrastent luminal hazy image on the middle portion of the left anterior descendent coronary artery (arrow head).
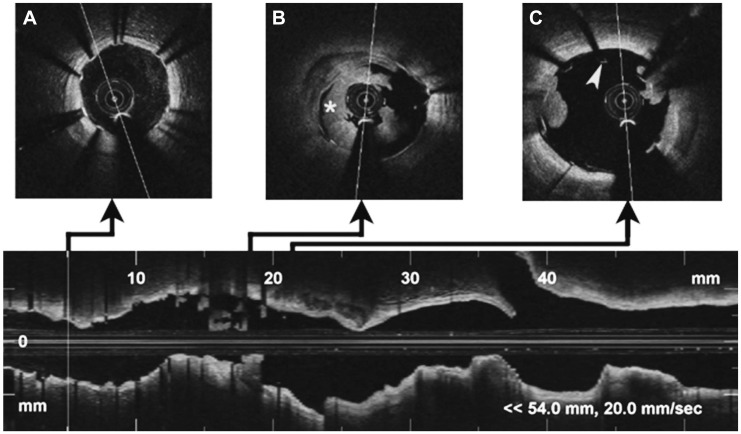
The stent was fully expanded but showed some strut malapposition at the proximal border (Fig. 3). We performed manual thrombus aspiration and obtained white thrombus material. We postdilated the proximal segment of the stent with a non-compliant balloon. The final result was significant reduction of the thrombus and correction of the stent malapposition (Fig. 4).
Fig. 3.
Optical coherence tomography imaging of left anterior descending artery. Distal (A): correct apposition of the stent without intraluminal content. Middle (B): a signal rich, low-backscattering protrusion image compatible with white thrombus (mark), which occupies the greater part of the vessel lumen. Proximal (C): stent malapposition (arrow head) in the proximal border, with small images of thrombus.
Fig. 4.
Final optical coherence tomography imaging of the left anterior descending artery. There is almost complete reduction of the thrombus (arrow head) and correct apposition of the stent.
The patient was discharged 3 days after the procedure and did not experience any additional clinical events. At 4 months follow-up, the patient had remained asymptomatic.
Discussion
Acute stent thrombosis may occur in patients, with or without acute coronary syndrome.4),6-12) Rinaldi et al.6) identified 4 independent predictors of stent thrombosis during PCI: higher baseline platelet count, acute myocardial infarction indication, use of a coil or self-expanding stent, and preprocedural thrombus. In this particular case, a great thrombus burden was found during the first acute PCI performed in the RCA, but no angiographic thrombotic images that were found suggested LAD thrombosis prior to PCI. We had not performed an initial OCT, so a possible previous thrombus could be present, but had remained undetected by angiography. However, we consider that a high-risk patient, the stent malapposition and possibly an inadequate anticoagulation could explain the acute stent thrombosis. An alternate explanation, such as prolapsed thrombus, could not be dismissed without a previous OCT.
Hazy angiographic images are a real issue to the invasive cardiologist. An incorrect diagnostic leads to an incorrect choice in therapeutics and potential damage to the patient. The choice of IVUS or OCT in order to obtain a specific diagnosis of an intravascular lesion depends in most of the cases on the availability and or experience in the laboratory. The OCT has a spatial resolution of about 10-15 µm with a penetration depth of 2 to 3 mm. The frequency-domain OCT modality, allows for high-resolution imaging, with pullbacks of 50 mm in less than 3 seconds, with minimum use of contrast and without coronary occlusion requirement.2),3) OCT allows the characterization of histologic composition of the lesion and a precise identification of the luminal border, with high sensitivity to detect tiny dissections, plaque prolapse or intraluminal thrombus (even to distinguish white and red thrombus).2-4),7-12) In contrast, IVUS has a spatial resolution much lower, around 130 µm, and does not allow the accurate characterization of some intravascular images.4),8),9)
Comparing both intravascular image techniques and after performing PCI in their study, Kawamori et al.8) could identify thrombus in 15% with OCT, and 5% with IVUS. In the setting of PCI for acute coronary syndrome Kubo et al.9) could detect thrombus in all patients with OCT, but only in 33% of patients with IVUS.
In this case, discrimination between dissections, plaque prolapsed, or thrombus was crucial to the choice of treatment. A dissection would have required a new stent implantation, and it would certainly have contraindicated thrombus aspiration. A plaque prolapse generally does not require additional treatment.
Optical coherence tomography is a straightforward and useful method to assess misleading angiographic lesions, probably with higher accuracy than IVUS when scanning intravascular or intra-stent images, and may be useful during PCI procedures when angiography is uncertain.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Grewal J, Ganz P, Selwyn A, Kinlay S. Usefulness of intravascular ultrasound in preventing stenting of hazy areas adjacent to coronary stents and its support of support spot-stenting. Am J Cardiol. 2001;87:1246–1249. doi: 10.1016/s0002-9149(01)01513-2. [DOI] [PubMed] [Google Scholar]
- 2.Raffel OC, Akasaka T, Jang IK. Cardiac optical coherence tomography. Heart. 2008;94:1200–1210. doi: 10.1136/hrt.2007.130765. [DOI] [PubMed] [Google Scholar]
- 3.Stamper D, Weissman NJ, Brezinski M. Plaque characterization with optical coherence tomography. J Am Coll Cardiol. 2006;47(8 Suppl):C69–C79. doi: 10.1016/j.jacc.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 4.Bourantas CV, Garg S, Naka KK, Thury A, Hoye A, Michalis LK. Focus on the research utility of intravascular ultrasound - comparison with other invasive modalities. Cardiovasc Ultrasound. 2011;9:2. doi: 10.1186/1476-7120-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popma JJ, Berger P, Ohman EM, Harrington RA, Grines C, Weitz JI. Antithrombotic therapy during percutaneous coronary intervention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):576S–599S. doi: 10.1378/chest.126.3_suppl.576S. [DOI] [PubMed] [Google Scholar]
- 6.Rinaldi MJ, Kirtane AJ, Piana RN, et al. Clinical, procedural, and pharmacologic correlates of acute and subacute stent thrombosis: results of a multicenter case-control study with 145 thrombosis events. Am Heart J. 2008;155:654–660. doi: 10.1016/j.ahj.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias D, Salinas P, Jiménez-Valero S. Spontaneous coronary artery dissection evaluated by optical coherence tomography. J Cardiovasc Med (Hagerstown) 2011;12:743–744. doi: 10.2459/JCM.0b013e32834a138c. [DOI] [PubMed] [Google Scholar]
- 8.Kawamori H, Shite J, Shinke T, et al. The ability of optical coherence tomography to monitor percutaneous coronary intervention: detailed comparison with intravascular ultrasound. J Invasive Cardiol. 2010;22:541–545. [PubMed] [Google Scholar]
- 9.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki M, Bouma BE, Bressner J, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. 2006;48:81–88. doi: 10.1016/j.jacc.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 11.Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 12.Prati F, Stazi F, Dutary J, et al. Detection of very early stent healing after primary angioplasty: an optical coherence tomographic observational study of chromium cobaltum and first-generation drug-eluting stents. The DETECTIVE study. Heart. 2011;97:1841–1846. doi: 10.1136/heartjnl-2011-300782. [DOI] [PubMed] [Google Scholar]