Abstract
Increased serotonergic activity has been shown to reduce motivation to ingest, which may involve, in part, gustatory processes. Here, we examined the effect of paroxetine, a selective serotonin reuptake inhibitor, on appetitive responding for a preferred and an avoided taste solution using a progressive ratio (PR) task in which licking was employed as the operant. Male Sprague-Dawley rats (n = 8/taste stimulus) were trained to respond for a concentration series of sucrose or quinine on fixed and PR schedules of reinforcement. Performance for sucrose was assessed while the rats were partially food- and water-restricted and nondeprived, and performance for water and quinine was assessed while the rats were water-deprived. Then, the rats were injected with vehicle (10% dimethyl sulfoxide, 1mL/kg intraperitoneal [ip], −1h) or paroxetine (5mg/kg), and their responding on a PR schedule for sucrose measured when the rats were nondeprived or for water and quinine when the rats were water-deprived. Paroxetine decreased breakpoint, which was defined as the number of operant (e.g., dry) licks in the final reinforced ratio, for water, quinine, and sucrose. This demonstrates that a general systemic increase in serotonergic activity decreases the appetitive-based responses to both preferred and nonpreferred fluids under different deprivation states.
Key words: appetitive, fixed ratio, gustatory, motivation, operant behavior, schedule of reinforcement
Introduction
Serotonin, as well as some of its receptor subtypes, has been found in taste bud cells, where it is postulated to act in a paracrine manner as a feedback signal to decrease further release of adenosine triphosphate (Kaya et al. 2004; Tomchik et al. 2007; Huang et al. 2009). Activity of serotonin at some of its receptor subtypes has been shown to decrease food and fluid intake in both long-term and short-term tests either when injected peripherally or centrally (see Halford et al. 2007; Garfield and Heisler 2009; Lam et al. 2010; Hayes and Greenshaw 2011). The behavioral processes through which serotonin influences ingestive behaviors are multifaceted (Halford and Blundell 2000), and given its recent identification in peripheral gustatory cells, it is plausible that serotonin impacts taste-guided responses. Indeed, administration of paroxetine, a selective serotonin reuptake inhibitor (SSRI), has been shown to decrease the threshold at which sucrose and quinine are recognized (i.e., increase sensitivity) by human subjects (Heath et al. 2006). If serotonergic activity influences basic taste detectability, it is quite plausible that it influences taste-based motivated behavior.
Motivated behavior can be segregated into 2 components (see Craig 1918): appetitive responses, which bring an animal into contact with ingestive stimuli, and consummatory responses, which are reflex-like acts triggered by the interaction of stimuli with appropriate receptors. The distinction between these 2 components of motivated behavior is blurred in many of the tasks previously used to assess the effect of serotonergic activity on intake. Some procedures, however, provide an opportunity to discretely assess the effect of a given manipulation, such as drug administration, on both appetitive and consummatory responses. For example, when nondeprived rats were injected with paroxetine (5mg/kg ip) prior to a brief-access test, they initiated fewer trials of a range of sucrose concentrations delivered in randomized blocks, but licked the sucrose solutions in a manner not different from when they were injected with vehicle (Mathes and Spector 2011). Accordingly, under those test conditions, paroxetine had an effect on the appetitive component of the behavior (i.e., trial initiation) toward sucrose, but not the consummatory component (i.e., licks per trial). In this example, the decrease in trials taken coupled with the lack of change in licking resulted in an overall decrease in total licks, and presumably intake, of sucrose during the session. However, the nature of the brief-access test poses a couple of limitations in the assessment of appetitive and consummatory responsiveness to taste solutions. First, the brief-access test does not allow for concentration-dependent analysis of appetitive behavior because test trials are presented in blocks, and a response is required on each trial prior to delivery of a different solution. Second, the brief-access test is also associated with less-than-trivial intake of the taste solutions over the course of a session, which allows postingestive events to potentially influence responding.
Thus, in this report, we sought to specifically characterize the effect of paroxetine on taste-guided appetitive behavior to a concentration series of sucrose and quinine using an operant task in which reinforcement is contingent on a progressive ratio (PR) schedule (e.g., Hodos 1961; Richardson and Roberts 1996). In a PR task, a rat must respond a progressively increasing number of times to gain a small reward. For example, on a PR3, a rat would be required to produce an operant behavior 3 times to receive the first reinforcer access, 6 times for the next, 9 for the next, and so on. At some point, the rat stops responding, providing a measure of the amount of work a rat is willing to perform for a given reinforcer. This measure is referred to as the breakpoint and is quantified as the number of responses emitted prior to the last reinforcer earned. Because the amount of work increases readily and the size of each individual reinforcer is small, any effect of satiation is nearly eliminated.
Only a few studies have focused on concentration-dependent responses to taste solutions in a PR task (Reilly 1999; Brennan et al. 2001; Sclafani and Ackroff 2003), and none of which we are aware have assessed the effect of SSRI administration on breakpoint. Here, we employed licking as the operant behavior as has been done in previous studies (e.g., Hulse 1967; McGregor et al. 1999; Sclafani and Ackroff 2003) to examine concentration-dependent changes in the responsiveness of rats working to receive sucrose and quinine solutions in various fixed ratio (FR) and PR schedules of reinforcement using a within-subjects design. We focused on sucrose and quinine because these compounds fall on opposite ends of the palatability spectrum, allowing us to explore a preferred and an avoided taste solution. We then assessed the effect of systemically administered paroxetine and vehicle on PR responding for sucrose in rats when they were nondeprived and for water and quinine when the rats were water deprived so as to quantify the influence of a general increase in serotonergic tone on the reward value of taste solutions using a measure of pure appetitive responding.
Materials and methods
Experiment 1
Subjects
Eight male Sprague-Dawley rats (Charles River) that were 278–324g at the time of testing were used in this experiment. The rats were singly housed in conventional polycarbonate tub cages in a temperature- and humidity-controlled vivarium room that had a 12-h light:dark cycle (lights on at 06:00h, lights off at 18:00h). Except when noted, the rats had ad libitum access to standard rodent chow (PMI 5001) and deionized water. All procedures were approved by the animal care and use committee of Florida State University.
Taste stimuli and drugs
A series of sucrose solutions (Mallinckrodt; 0.1, 0.3, and 1.0M) and reverse osmosis deionized water were used as the taste test stimuli and served as reinforcers in the operant contingency. Paroxetine maleate (Tocris) was dissolved in dimethyl sulfoxide (DMSO; Tocris), and then, deionized water was added such that the final volume of DMSO was 10%.
Apparatus
Testing and training took place in a modified version of an apparatus known as a gustometer (see Spector et al. 1990; Blonde et al. 2006). The gustometer was equipped with a vertically oriented sample spout (constructed from Kel-F polychlorotrifluoroethylene plastic) that was connected via a metal rod to the shaft of a rotating stepping motor so that the spout centrally positioned behind an access slot in the front wall of an adjacent animal testing chamber or rotated out of reach. A rat could contact the sample spout, when it was centrally positioned, by extending its tongue through the slot. A stainless steel ring surrounding the orifice of the sample spout was connected to an electrical circuit (≤50 nA) such that tongue contact could be detected and licks measured. A stainless steel hypodermic tube (18 G) was covered by a Teflon shell and inserted into the shaft of the sample spout stopping just short of the bottom opening. This tube was connected via Teflon tubing to a computer-controlled solenoid valve, which in turn was connected to a pressurized fluid reservoir. The open-time of the solenoid valve was calibrated to deliver a 5 µL drop of solution at the end of the spout upon each lick when required. The animal testing chamber, sample spout, and motor were enclosed within a sound-attenuating cubical, and a constant broadband masking noise was present during testing and training sessions.
Procedures for training and assessment of concentration-dependent performance across operant schedules
Water bottles were removed from the home cages of the rats ~23h prior to the initiation of training, which consisted of once-daily sessions across 5 consecutive days. In these 30-min sessions, water was freely available such that each lick to the sample spout produced 5 µL of water delivered through the same sample spout (i.e., no dry operant licks were required). Water bottles were returned to the home cages of the rats ~30min after completion of the 5th session and remained on for ~48h prior to assessment of responding for sucrose.
Sucrose solution delivered through the sample spout was made available to the rats contingent on the completion of an operant criterion of dry licks of the sample spout. Each completed ratio provided the opportunity for 15 licks (i.e., 75 µL) of the fluid reinforcer, which was also delivered from the sample spout. Sessions ended when the rats failed to respond for 5min, at which time the sample spout rotated away from the access slot, and no more responses could be made. The requirements for fluid access progressed in difficulty across sessions in the following order: free access (FA), FR3, FR10, PR3, and PR10. Daily sessions on each schedule lasted between 5 and 15 days for each of 3 sucrose concentrations (0.1, 0.3, and 1M). The next schedule was implemented when performance appeared stable based on visual inspection of the data. Typically, this was after 5 days, but during testing with 1.0M sucrose, we extended performance out to 10 days to ensure that performance did not fluctuate with further experience. Only once during training of responding for 1.0M sucrose on a PR3 was 15 days necessary to achieve stability, and this was a consequence of a problem with light cycle timing during the change to daylight savings time. Performance across all the schedules for 1.0M sucrose was assessed first, and then, responses across schedules for the other concentrations were evaluated in descending order. Across all the schedules and concentrations, the rats were tested 5 days a week alternating between a partially food- and water-restricted state (i.e., limited to a ration of 10g chow and 20mL water provided ~23h prior to testing), the sessions for which occurred on Monday, Wednesday, and Friday (MWF), or a nondeprived state (i.e., given ad libitum access to food and water ~30min after MWF sessions), the sessions for which occurred on Tuesday and Thursday.
Drug testing procedures
After performance across all schedules and concentrations was assessed, we measured the effect of vehicle and paroxetine injections on the breakpoint for water while the rats were water deprived. Water bottles were removed from the rats, and, ~23h later, the rats were allowed to freely lick water from the sample spout. On the following day, water was available on a PR3 schedule. The day after that, water was again available on a PR3 schedule, but the rats were injected with vehicle (1mL/kg ip) 1h prior to testing. One hour prior to the test session on the next day, the rats were injected with paroxetine (5mg/kg), which is a dose and time course previously shown to impact appetitive behavior in some conditions during a brief-access test (Mathes and Spector 2011). Finally, on the 5th day, the rats were allowed to lick 1.0M sucrose freely to prepare them for the following phase in which the effect of vehicle and paroxetine on breakpoint for sucrose was evaluated. Water bottles were returned to the rats ~30min after the 5th test and remained on for ~48h prior to further sessions.
The animals were then tested in 3 pairs of days with sucrose contingent on a PR3, 1 sucrose concentration per pair. The first session for each pair was conducted after vehicle injection and the second after paroxetine injection. Breakpoint for 1.0M sucrose was assessed first, with the other 2 concentrations following in descending order. No food or water restriction was implemented during these sessions in an attempt to minimize the influence of physiological drive state such that taste would be the primary factor driving responding.
Data analysis
For the phase in which the effects of concentration and operant schedules were assessed, the total number of operant licks from each rat during the last 5 days of testing at each concentration and in each state (e.g., 3 sessions when partially food- and water-restricted and 2 sessions when nondeprived) was averaged and used in analysis. Operant licks were defined as licks to the dry spout that were required prior to delivery of the fluid reinforcer. Intake (milliliter and kilocalories) was calculated based on the number of consummatory licks of sucrose each rat took during reinforcement delivery. When a PR schedule was used in both the first phase and during drug testing, the number of operant licks in the final reinforced ratio was defined as the breakpoint. Nonparametric statistics were used to analyze the data because of the noncontinuous nature of the response scales. The data from each measure for each schedule and state were analyzed using Friedman’s 2-way analysis of variance with concentration as the within-subject factor. Differences in behavior during paroxetine and vehicle PR testing at each concentration, as well as comparisons of behavior between ratios and deprivation states at each concentration, were assessed using the Wilcoxon signed-rank test. Comparisons were considered significantly different when P < 0.05.
Experiment 2
Subjects
Eight naïve male Sprague-Dawley rats (Charles River) that were 292–336g at the time of testing were maintained as in Experiment 1.
Taste stimuli and drugs
A series of quinine hydrochloride solutions (Sigma; 0.03, 0.1, 0.3, and 1.0mM) dissolved in reverse osmosis deionized water was used as test stimuli and served as reinforcers. Paroxetine maleate (Tocris) was prepared as described in Experiment 1.
Apparatus, training, and assessment of concentration-dependent performance across operant schedules
Testing and training took place in the gustometer as described in Experiment 1. Water bottles were removed from the home cages of the rats on Sunday, Tuesday, and Thursday ~23h prior to once-daily training sessions on MWF across 1 week during which water was delivered with each lick the rats took (i.e., FA). Water bottles were returned to the home cages of the rats ~30min after completion of each session.
The procedures used to assess concentration-dependent performance across operant schedules in Experiment 2 were similar to those used in Experiment 1 except that 1) testing on FR3 and PR10 schedules was not performed, 2) water or quinine solution served as the reinforcer, 3) sessions on each schedule were performed between 3 and 9 days for each of the concentrations tested, and 4) the concentrations were tested in ascending order. As in Experiment 1, stability was assessed by visual inspection of the data, and, typically, the rats were stable after 3 days of performance. Performance was assessed for 9 sessions only on the first set of PR sessions (i.e., PR3 for the lowest concentration of quinine) to ensure stability. In Experiment 2, testing only occurred on MWF, with water bottles removed on Sundays, Tuesdays, and Thursdays, so that testing would take place after the rats had been given ample opportunity to rehydrate between test sessions.
Drug testing procedures
Water bottles were removed from the home cages, and ~23 later, the rats were allowed to freely lick for water. Thirty minutes after the completion of this session, water bottles were returned to the rats and removed the next day ~23h prior to session in which water was available on a PR3 schedule. For the next 16 days, the rats were run in sessions every other day, with water bottles removed on the days in between, beginning with sessions in which water served as the reinforcer and then quinine (0.1, 0.3, and 1.0mM) in ascending order. One hour prior to the start of each session, the rats were injected ip with either vehicle (1mL/kg) or paroxetine (5mg/kg), beginning with vehicle as described in Experiment 1. After completion of testing with the quinine concentration series, the effect of paroxetine and vehicle on PR3 responding for water was retested due to high variability in the initial test.
Data analysis
The total number of operant licks and intake (milliliter) of each rat during the last week of testing at each concentration was averaged and analyzed as described in Experiment 1. For water testing after vehicle and paroxetine administration, the operant responses were averaged across the 2 series of sessions. One rat did not respond at all during PR3 testing at 0.3mM quinine or across any of the schedules when 1.0mM was available and so was excluded from all analysis and not used in the subsequent drug testing phase.
Results
Experiment 1
Concentration-dependent performance for sucrose across reinforcement schedules
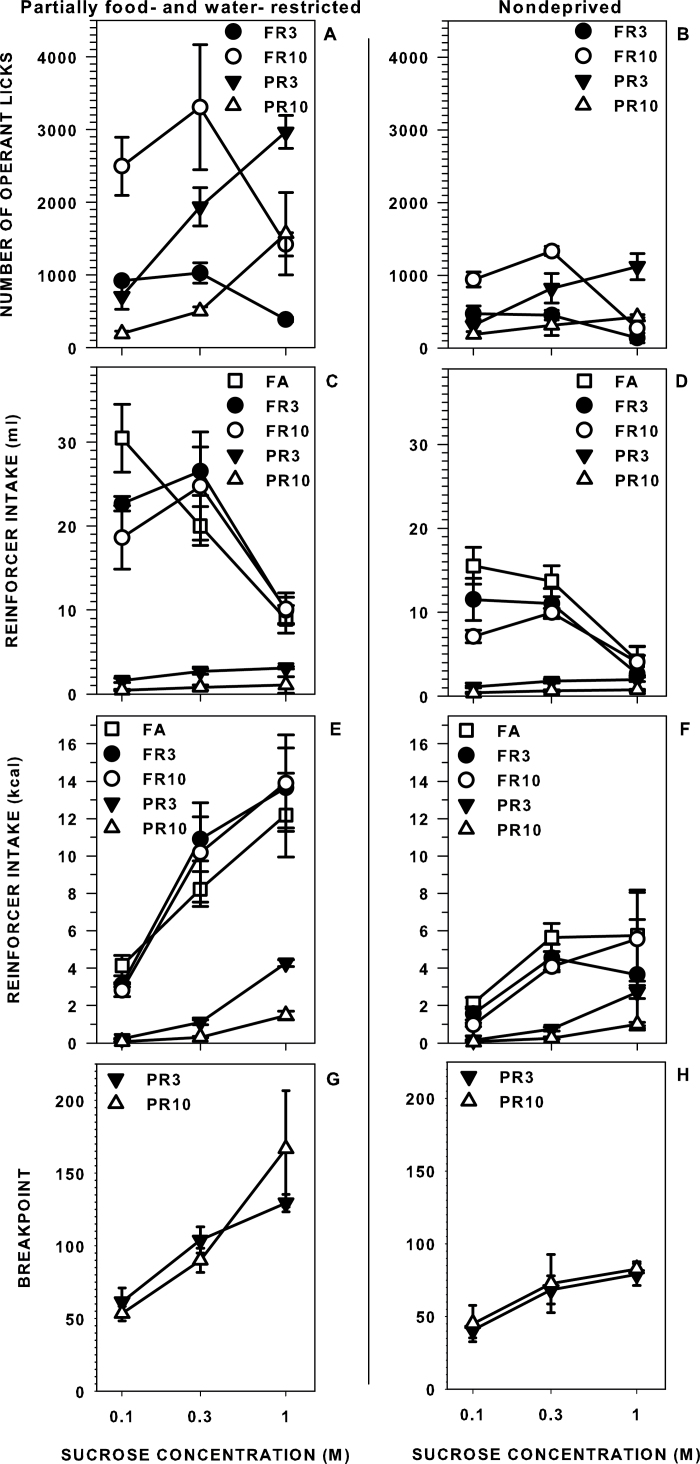
Significant effects of sucrose concentration on median operant responses and intakes (milliliter and kilocalories) were revealed across all of the reinforcement schedules independent of the feeding status of the animals when tested (i.e., partially food- and water-restricted or nondeprived; Table 1; Figure 1). When tested on FR schedules, the rats operantly licked in a nonmonotonic or decreasing monotonic fashion, whereas when tested on PR schedules, the rats operantly responded in an increasing monotonic manner. This pattern was also seen in terms of intake volume, although caloric intake increased in a monotonic fashion across all schedules when the rats were tested while partially food- and water-restricted. Particularly striking is the large disparity between intake of the rats during the FR schedules and during the PR schedules in lieu of small differences seen in the number of operant responses performed under FR and PR schedules. For example, the rats when partially food- and water-restricted and tested on a FR10 responded, on average, 1421 times, earning 10.2mL of 1.0M sucrose, whereas when on a PR3, they responded 1566 times for 10 times less sucrose. In other words, when rats were required to work on a PR schedule for sucrose when either partially food- and water-restricted or nondeprived, they performed just as hard as they did on an FR schedule but received substantially less fluid reinforcement for their efforts.
Table 1.
χ2 output and P-values of Friedman analysis of median total responses of rats given FA to or required to respond operantly on FR or PR schedules of reinforcement for a concentration series of sucrose solutions
| FA | FR3 | FR10 | PR3 | PR10 | |
|---|---|---|---|---|---|
| Partially food- and water-restricted | |||||
| Operant responses | NA | χ2(2) = 12.25 | χ2(2) = 10.75 | χ2(2) = 13 | χ2(2) = 16 |
| P = 0.002 | P = 0.005 | P = 0.002 | P < 0.001 | ||
| Intake (mL) | χ2(2) = 13 | χ2(2) = 12.25 | χ2(2) = 10.75 | χ2(2) = 14.25 | χ2(2) = 12.25 |
| P = 0.002 | P = 0.002 | P = 0.005 | P = 0.001 | P = 0.002 | |
| Intake (kcal) | χ2(2) = 9.25 | χ2(2) = 13 | χ2(2) = 7.75 | χ2(2) = 16 | χ2(2) = 16 |
| P = 0.01 | P = 0.002 | P = 0.021 | P < 0.001 | P < 0.001 | |
| Breakpoint | NA | NA | NA | χ2(2) = 14.25 | χ2(2) = 12.25 |
| P = 0.001 | P = 0.002 | ||||
| Nondeprived | |||||
| Operant responses | NA | χ2(2) = 9.25 | χ2(2) = 14.25 | χ2(2) = 13 | χ2(2) = 7.75 |
| P = 0.01 | P = 0.001 | P = 0.002 | P = 0.021 | ||
| Intake (mL) | χ2(2) = 12 | χ2(2) = 9.25 | χ2(2) = 10.75 | χ2(2) = 14.25 | χ2(2) = 9 |
| P = 0.002 | P = 0.01 | P = 0.005 | P = 0.001 | P = 0.011 | |
| Intake (kcal) | χ2(2) = 6.25 | χ2(2) = 9.25 | χ2(2) = 12 | χ2(2) = 16 | χ2(2) = 16 |
| P = 0.044 | P = 0.01 | P = 0.002 | P < 0.001 | P < 0.001 | |
| Breakpoint | NA | NA | NA | χ2(2) = 14 | χ2(2) = 10.129 |
| P = 0.001 | P = 0.006 | ||||
Figure 1.
Median (± seminterquartile range) operant licks (A and B), intakes (volume: C and D; calories: E and F), and breakpoints (G and H) of rats licking for a series of sucrose concentrations while either partially food- and water-restricted (A, C, E, and G) or nondeprived (B, D, F, and H) when given FA and when required to operantly perform across various FR and PR schedules of reinforcement.
PR breakpoint, which is the number of operant licks that a rat makes for its last reinforcer access, also increased monotonically across concentration. Breakpoint during PR3 performance was higher when the rats were tested while partially food- and water-restricted compared with when nondeprived at all concentrations (P < 0.05) and, breakpoint during PR10 performance was higher in restricted conditions at 1.0M (P < 0.05). Furthermore, although rats emitted, on average, 2–4 times more total responses during sessions when tested on a PR3 compared with when tested with a PR10, the breakpoint did not differ between these 2 schedules at any concentration or in either state tested (P >0.05). For example, rats responded for 0.3M sucrose, on average, 1938 times on a PR3 schedule and 504 times on a PR10 schedule across an entire session, but rats stopped responding when the ratio requirement for access to a single reinforcer was, on average, 104 on a PR3 and 90 on a PR10. This suggests that breakpoint was dependent on the number of operant responses emitted prior to a single sucrose reinforcer rather than the total number operant responses produced within a session or the total number of reinforcers earned (similar to Stafford and Branch 1998).
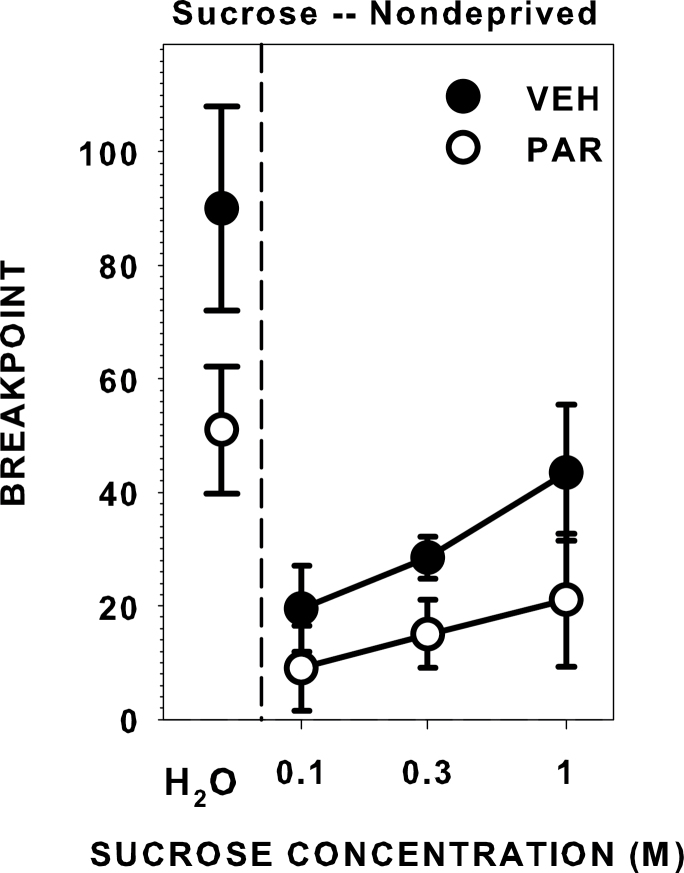
Effects of vehicle and paroxetine injection on PR3 breakpoint for water and sucrose
During PR3 sessions after vehicle injections, the nondeprived rats increased breakpoint as a monotonic function of sucrose concentration (χ2(2) = 12.698, P = 0.002; Figure 2). The breakpoints after vehicle administration were lower than that seen during testing across schedules, and we assume this difference is due to the slight stress resulting from the injection and/or to the possibility that the overall motivational state was lower because the nondeprived sessions were no longer flanked by sessions in which the rats were tested in a partially food- and water-restricted state. Regardless, when injected with paroxetine, the breakpoint curve was blunted and responses did not statistically differ across concentrations (χ2(2) = 1.355, P = 0.508; Figure 2). Furthermore, breakpoint responding was lower when the rats were injected with paroxetine compared with vehicle during testing with water (z = −2.524, P = 0.012), 0.3M sucrose (z = −2.38, P = 0.017), and 1.0M sucrose (z = −2.524, P = 0.012), but not 0.1M sucrose (z = −1.474, P = 0.141). This demonstrates that, when injected with paroxetine, water-deprived rats did not work as hard for water, and nondeprived rats did not work as hard for high sucrose concentrations, suggesting that systemic increases in serotonergic signaling as a result of reuptake blockade decreases the appetitive reward value of these stimuli in certain physiological states.
Figure 2.
Median (± seminterquartile range) breakpoints of rats performing on a PR3 schedule of reinforcement for water while water-deprived and a series of sucrose concentrations while nondeprived after injection with either vehicle (10% DMSO, 1mL/kg, ip) or paroxetine (5mg/kg, ip). Paroxetine decreased breakpoint responding to water, 0.3M sucrose, and 1.0M sucrose.
Experiment 2
Concentration-dependent performance for quinine across reinforcement schedules
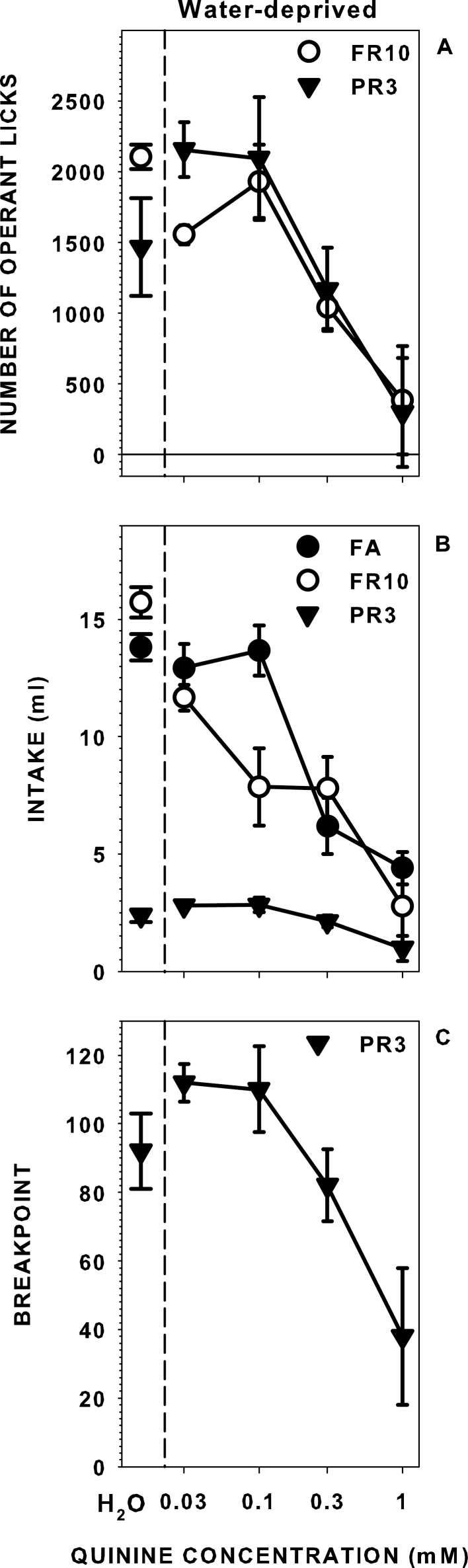
Significant effects of quinine concentration on median operant responses and intakes were revealed across all of the schedules and for breakpoint during PR3 testing (Table 2; Figure 3). When water deprived and tested with quinine, rats responded in a relatively monotonic fashion across concentrations, with operant licks and intake decreasing as concentration increased. As in Experiment 1, rats required to work on the PR schedule for quinine emitted a similar number of responses as when on the FR schedule despite receiving substantially less fluid reinforcement. For example, the rats operantly licked, on average, 1931 times for 7.9mL of 0.1mM quinine when on a FR10 schedule and licked 2093 times for 2.8mL when on a PR3.
Table 2.
χ2 output and P-values of Friedman analysis of median total responses of water-deprived rats given FA to or required to operantly respond on a FR or PR schedule of reinforcement for a concentration series of quinine solutions
| FA | FR10 | PR3 | |
|---|---|---|---|
| Operant responses | NA | χ2(2) = 25.943 | χ2(2) = 12 |
| P < 0.001 | χ = 0.017 | ||
| Intake (mL) | χ2(2) = 22.171 | χ2(2) = 25.943 | χ2(2) = 12 |
| P < 0.001 | P < 0.001 | P = 0.017 | |
| Breakpoint | NA | NA | χ2(2) = 12.058 |
| P = 0.017 |
Figure 3.
Median (± seminterquartile range) operant licks (A), intakes (B), and breakpoints (C) of rats licking for water and a series of quinine concentrations while water-deprived when given FA or required to operantly perform across a FR and a PR schedule of reinforcement.
Effects of vehicle and paroxetine injection on PR3 breakpoint for water and quinine
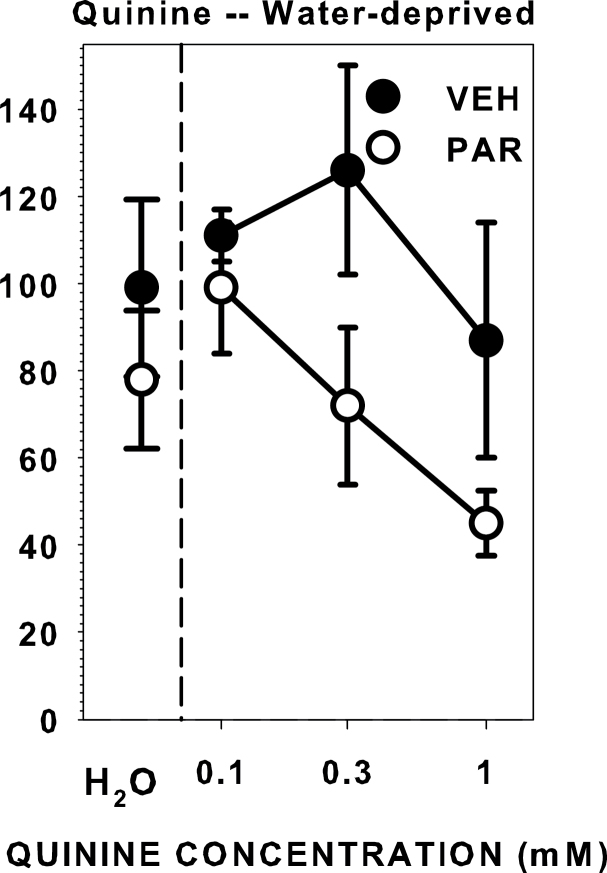
During PR3 sessions after vehicle injections, the water-deprived rats showed decreasing relatively monotonic breakpoints (χ2(3) = 14.652, P = 0.002; Figure 4) similar to those seen when the rats were not injected. When injected with paroxetine, the breakpoint curve remained monotonic and concentration-dependent (χ2(3) = 16.739, P = 0.001), but was blunted (Figure 4). Breakpoints were lower when the rats were injected with paroxetine compared with vehicle during testing with water (z = −2.371, P = 0.018) and all quinine concentrations (0.1 mM: z = −2.366, P = 0.018; 0.3 mM: z = −2.371, P = 0.018; 1.0 mM: z = −2.117, P = 0.034). This replicates the effect of paroxetine on PR3 responding for water seen in Experiment 1, despite that the mean breakpoint for water after vehicle injection was higher in Experiment 2 compared with Experiment 1. We suspect that this latter result is related to the different training histories of the rats (i.e., the rats in Experiment 2 were fully water-deprived every other day, whereas the rats in Experiment 1 were only partially restricted). The results of Experiment 2 demonstrate that water-deprived rats injected with paroxetine will also not work as hard for water when adulterated with quinine compared with when injected with vehicle.
Figure 4.
Median (± seminterquartile range) breakpoints of rats performing on a PR3 schedule of reinforcement for water and a concentration series of quinine while water-deprived after injection with either vehicle (10% DMSO, 1mL/kg, ip) or paroxetine (5mg/kg, ip). Paroxetine decreased breakpoint responding to water and all quinine concentrations.
Discussion
With these studies we have replicated the feasibility of using licking as an operant for fluid reinforcers during measures of PR breakpoint, which quantifies appetitive behavior while obviating satiation. We also extended the application of the PR task to examine licking responses of rats to a concentration array of an aversive, as well as a preferred, taste stimulus. Despite different training and testing parameters, the breakpoints that we obtained using partially food- and water-restricted rats resemble those seen in food-restricted rats operantly licking (Sclafani and Ackroff 2003) or lever pressing (Reilly 1999) for sucrose on various PR schedules. The breakpoints of lever pressing for quinine by water-deprived rats that Reilly (1999) reported were lower than those measured here possibly because of the difference in response topography (e.g., lever press vs. a lick operant response) and/or because the rats in the Reilly (1999) experiment had experience with the task in which other potentially less aversive tastants served as reinforcers. Also, we used a concentration array of quinine that increased in half-log rather than full-log steps. The breakpoints for sucrose of nondeprived rats in our study were lower than those seen in the studies of Sclafani and Ackroff (2003), but data from the latter experiment were collected during 23-h PR tests, whereas ours were derived from shorter duration (20–60min) tests. Thus, we were able to show that in short-term tests, nondeprived rats will respond to sucrose in a concentration-dependent fashion, allowing discrete tests of the effects of a drug in conditions where taste is a primary factor guiding appetitive behavior. We also found that water serves as a more potent reinforcer for a water-deprived animal than does high concentrations of sucrose for a nondeprived animal, an outcome consistent with the results from brief-access taste tests (e.g., Mathes and Spector 2011) and analyses of ingestive microstructure (e.g., Spector et al. 1998; Spector and St John 1998), but measured more directly in this report. These findings highlight the elasticity of taste reinforcer value in the context of physiological drive state.
It should be noted that PR breakpoints seem sensitive to the training and testing schedule of the animal. For example, we observed that when rats were tested on alternating days in a partially food- and water-restricted state, their breakpoints for sucrose on the days when they were tested while nondeprived were higher than when the animals were tested every day while nondeprived. Although we cannot rule out the role of potential stress from the vehicle injection in this comparison, we also observed that, when water-deprived and injected with vehicle, the rats in Experiment 1 had lower breakpoints for water than those seen from rats in Experiment 2 when injected with vehicle. Again, these rats had quite different training regimens (e.g., partial food and water restriction every other day as opposed to total water deprivation every other day). These findings suggest that taste reinforcer value, as assessed by breakpoint, is not only sensitive to the current physiological state of the animal but also appears to be sensitive to the pattern of variation in the deprivation conditions across prior training and testing sessions. Thus, there is no absolute breakpoint for a given taste reinforcer. Rather, the reinforcing efficacy of a taste stimulus in a PR task is relative to the physiological state and training history of the animal and must be accordingly considered in experimental designs and interpretation of results within and across studies.
We also demonstrated that rats injected with the SSRI paroxetine had lower PR breakpoints for high concentrations of sucrose when they were tested while nondeprived compared with when they were injected with vehicle. In addition, water-deprived rats had lower breakpoints for water and quinine when injected with paroxetine compared with vehicle. Apparently, a general systemic increase in serotonergic activity decreases the reward value of both preferred and avoided fluids under different motivational states. These findings are consistent with those from a brief-access taste test (Mathes and Spector 2011) in which paroxetine decreased the number of trials that water-deprived rats initiated when quinine was the stimulus and that nondeprived rats initiated when sucrose was the stimulus. In this study, we also found that paroxetine decreased water breakpoints in water-deprived rats, whereas only a trend toward an effect on the number of water trials taken was seen during the brief-access test. The difference may be due in part to the fact that the PR task is a more selective assessment of appetitive behavior. Our results are also in agreement with other studies showing that increased serotonergic signaling induced through various pharmacological means decreases lever pressing for food pellets by food-deprived rats tested on FR and PR schedules (e.g., De Vry et al. 2003; Ho et al. 2003).
In these studies, we extended the analysis of the effect of serotonergic activity on the reward value of fluid reinforcers by using multiple concentrations of sucrose and quinine. We found that paroxetine administration lowered breakpoints to quinine, but given that under the drug condition, breakpoint remained concentration-dependent coupled with the observation that the SSRI lowered breakpoints to water suggests that the effect was due to a decrease in the effectiveness of the physiological drive state established by the water restriction schedule. On the other hand, paroxetine administration severely blunted the concentration-breakpoint relationship when sucrose was tested in nondeprived rats. The fact that the sucrose concentration-response function in nondeprived rats was relatively flat under conditions of systemic paroxetine administration in a task that precludes any appreciable accumulation of postingestive load suggests that the SSRI blunts taste-guided appetitive responsiveness, as seen using other tests (Mathes and Spector 2011), and we demonstrated here that it does so across multiple concentrations. Although only sucrose and quinine were tested here, these findings suggest that paroxetine has a selective effect on the reinforcing value of preferred, but not avoided, taste stimuli. In the case of the latter, the decreases in breakpoints to quinine appear to be largely driven by the decreased effectiveness of the physiological drive state established through water deprivation and not a direct effect on the reward value of the aversive taste per se.
Although the presence and activity of serotonin in the taste bud suggests that these peripheral structures may mediate effects of paroxetine on taste-guided appetitive behavior, it is just as possible that a central mechanism, such as those impinging on general reward circuits, underlies these results. For example, systemically injected paroxetine increases extracellular levels of serotonin (as well as norepinephrine) in the frontal cortex (Dekeyne et al. 2002). Indeed, we would propose that the decreased water intake that we report here and that we and others have previously observed (e.g., Castro et al. 2002; Mathes and Spector 2011) is under the control of central mechanisms because systemic injection of serotonin, which, unlike paroxetine (Cummings and Gjedde 1993; Uhr et al. 2003), has limited access to the brain, increases water intake (Fletcher and Burton 1984; Montgomery and Burton 1986; Higgins et al. 1992). Consistent with a central hypothesis, ablation of serotonergic pathways via injection of 5,7-dihydroxytryptamine into the dorsal and median raphe nuclei, which decreases serotonin levels throughout the brain, increases the “value” of sucrose as measured via steady-state operant responding on a variable interval schedule (Wogar et al. 1991). Also, the sucrose intake-decreasing effect of d-fenfluramine, a general serotonin receptor agonist and reuptake inhibitor, is blocked by metergoline, a central serotonin receptor antagonist, but not xylamidine, a serotonin receptor antagonist that does not readily cross the blood–brain barrier (Borsini et al. 1985). Furthermore, 8-OH-DPAT, an agonist of the serotonin-1A receptor subtype, injected bilaterally into the paraventricular nucleus of the hypothalamus decreases intake of 1.8% NaCl solution in Na+-deprived rats, though it does not affect the free intake of sucrose (Villa et al. 2007). Effects of the injection of drugs that modulate serotonin activity into other hypothalamic areas, brain reward circuits, or central relays of the gustatory system (e.g., nucleus of the solitary tract, parabrachial nucleus) on the mechanisms underlying the effect of serotonin on sucrose and quinine taste-guided behavior remain to be studied.
Conclusion
In conclusion, we reconfirm here the efficacy and value of using licking as an operant in tests assessing the chemospecific and concentration-dependent value of fluid reinforcers by demonstrating that the SSRI paroxetine decreases PR breakpoint to water, sucrose, and quinine across different physiological states. These findings invite further investigations with the goal of understanding the role of serotonin in taste-guided behavior and the extent to which peripheral and/or central systems underlie the effects.
Funding
This work was supported in part by the National Institute on Deafness and Other Communication Disorders at the National Institute of Health [grant number 1F32DC010517 to C.M.M.].
Acknowledgements
We gratefully recognize the technical contributions of Ginger Blonde. Portions of the data from Experiment 1 were previously presented at the 2010 Annual Meeting of the Society for Neuroscience in San Diego, CA.
References
- Borsini F, Bendotti C, Samanin R. 1985. Salbutamol, d-amphetamine and d-fenfluramine reduce sucrose intake in freely fed rats by acting on different neurochemical mechanisms. Int J Obes. 9(4):277–283 [PubMed] [Google Scholar]
- Blonde GD, Garcea M, Spector AC. 2006. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci. 120(3):580–589 [DOI] [PubMed] [Google Scholar]
- Brennan K, Roberts DC, Anisman H, Merali Z. 2001. Individual differences in sucrose consumption in the rat: motivational and neurochemical correlates of hedonia. Psychopharmacology. 157(3):269–276 [DOI] [PubMed] [Google Scholar]
- Craig W. 1918. Appetites and aversions as constituents of instincts. Biol Bull. 34 91–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L, Maldonado I, Campos I, Varjão B, Angelo AL, Athanazio RA, Barbetta MC, Ramos AC, Fregoneze JB, De Castro e Silva E. 2002. Central administration of mCPP, a serotonin 5-HT(2B/2C) agonist, decreases water intake in rats. Pharmacol Biochem Behav. 72(4):891–898 [DOI] [PubMed] [Google Scholar]
- Cumming P, Gjedde A. 1993. Kinetics of the uptake of [3H]paroxetine in the rat brain. Synapse. 15(2):124–129 [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Gobert A, Auclair A, Girardon S, Millan MJ. 2002. Differential modulation of efficiency in a food-rewarded “differential reinforcement of low-rate” 72-s schedule in rats by norepinephrine and serotonin reuptake inhibitors. Psychopharmacology. 162(2):156–167 [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Daschke A, Jentzsch KR. 2003. Effects of serotonin 5-HT(1/2) receptor agonists in a limited-access operant food intake paradigm in the rat. Eur Neuropsychopharmacol. 13(5):337–345 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Burton MJ. 1984. Effects of manipulations of peripheral serotonin on feeding and drinking in the rat. Pharmacol Biochem Behav. 20(6):835–840 [DOI] [PubMed] [Google Scholar]
- Garfield AS, Heisler LK. 2009. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 587(Pt 1):49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Blundell JE. 2000. Separate systems for serotonin and leptin in appetite control. Ann Med. 32(3):222–232 [DOI] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. 2007. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 67(1):27–55 [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ. 2011. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev. 35(6):1419–1449 [DOI] [PubMed] [Google Scholar]
- Heath TP, Melichar JK, Nutt DJ, Donaldson LF. 2006. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 26(49):12664–12671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. 1992. Effect of drugs influencing 5-HT function on ethanol drinking and feeding behaviour in rats: studies using a drinkometer system. Neurosci Biobehav Rev. 16(4):535–552 [DOI] [PubMed] [Google Scholar]
- Ho MY, Body S, Kheramin S, Bradshaw CM, Szabadi E. 2003. Effects of 8-OH-DPAT and WAY-100635 on performance on a time-constrained progressive-ratio schedule. Psychopharmacology. 167(2):137–144 [DOI] [PubMed] [Google Scholar]
- Hodos W. 1961. Progressive ratio as a measure of reward strength. Science. 134(3483):943–944 [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. 2009. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 29(44):13909–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse SH. 1967. Licking behavior of rats in relation to saccharin concentration and shifts in fixed-ratio reinforcement. J Comp Physiol Psychol. 64(3):478–484 [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. 2004. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 286(4):R649–R658 [DOI] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. 2010. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 97(1):84–91 [DOI] [PubMed] [Google Scholar]
- Mathes CM, Spector AC. 2011. The selective serotonin reuptake inhibitor paroxetine does not alter consummatory concentration-dependent licking of prototypical taste stimuli by rats. Chem Senses. 36(6):515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Saharov T, Hunt GE, Topple AN. 1999. Beer consumption in rats: the influence of ethanol content, food deprivation, and cocaine. Alcohol. 17(1):47–56 [DOI] [PubMed] [Google Scholar]
- Montgomery AM, Burton MJ. 1986. Effects of peripheral 5-HT on consumption of flavoured solutions. Psychopharmacology. 88(2):262–266 [DOI] [PubMed] [Google Scholar]
- Reilly S. 1999. Reinforcement value of gustatory stimuli determined by progressive ratio performance. Pharmacol Biochem Behav. 63(2):301–311 [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 66(1):1–11 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2003. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 79 663–670 [DOI] [PubMed] [Google Scholar]
- Spector AC, Andrews-Labenski J, Letterio FC. 1990. A new gustometer for psychophysical taste testing in the rat. Physiol Behav. 47(4):795–803 [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. 1998. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 112(3):678–694 [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ. 1998. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol. 274(6 Pt 2):R1687–R1703 [DOI] [PubMed] [Google Scholar]
- Stafford D, Branch MN. 1998. Effects of step size and break-point criterion on progressive-ratio performance. J Exp Anal Behav. 70(2):123–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. 2007. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 27(40):10840–10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr M, Grauer MT, Holsboer F. 2003. Differential enhancement of antidepressant penetration into the brain in mice with abcb1ab (mdr1ab) P-glycoprotein gene disruption. Biol Psychiatry. 54(8):840–846 [DOI] [PubMed] [Google Scholar]
- Villa PS, Camargo GM, Camargo LA, Saad WA. 2007. Activation of paraventricular nucleus of hypothalamus 5-HT1A receptor on sodium intake. Regul Pept. 140(3):142–147 [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. 1991. Evidence for an involvement of 5-hydroxytryptaminergic neurones in the maintenance of operant behaviour by positive reinforcement. Psychopharmacology. 105(1):119–124 [DOI] [PubMed] [Google Scholar]