Abstract
Aim: To investigate the correlation between [18F]fluorodeoxyglucose (FDG) uptake in a primary tumor and pathologic N stages, and to further analyze the possible risk factors contributing to the regional lymph node metastasis. Patients and methods: Eighty patients with non-small cell lung cancer (NSCLC) who underwent positron emission tomography/computed tomography were enrolled in the study. The FDG uptake in the primary tumor was compared for the different N staging groups and further correlation was performed. The degree of FDG uptake in the primary tumor and other possible variables related to the incidence of lymph node metastasis were examined by univariate and logistic multivariate analysis. FDG uptake was quantitated using the maximum standardized uptake value (SUVmax). Results: Statistically significant differences were found in the SUVmax of the primary tumors among different N staging groups (F = 4.124, P = 0.023), and the correlation between them was also statistically significant (r = 0.438, P = 0.000). Univariate analysis showed that blood tumor markers, primary tumor size, histologic grade, and SUVmax of the primary tumor were significantly associated with lymph node involvement. Logistic multivariate analysis showed that blood tumor makers and SUVmax of primary tumor might be considered as significant predictive factors for lymph node metastasis in patients with NSCLC. Conclusion: Our results show that there is a significant relationship between the SUVmax of the primary tumor and the pathologic N stage of NSCLC. FDG uptake by the primary tumor may be an independent predictor of regional lymph node metastasis in patients with NSCLC.
Keywords: Non-small cell lung cancer, FDG, PET/CT, standardized uptake value, staging, lymph node metastasis, risk factor
Introduction
Lung cancer is the leading cause of cancer-related death in the world, and non-small cell lung cancer (NSCLC) accounts for 75–80% of cases[1]. The molecular and quantitative imaging technique, positron emission tomography (PET)/computed tomography (CT) has proven especially useful in lung cancer diagnosis and staging[2], and the positron-emitting radiopharmaceutical [18F]fluorodeoxyglucose (FDG) is the most widely used agent for oncologic PET/CT imaging[3,4]. Studies have shown that FDG uptake reflects the metabolic activity of patients with primary NSCLC, and as such appears to have a prognostic role, especially after surgical resection[5–8]. The standardized uptake value (SUV) is a quantified index of FDG uptake. Patients with NSCLC who had a lower SUV had better therapeutic and prognostic outcomes[9]. Given that TNM stage is the most significant prognostic factor of NSCLC, especially the nodal stage (N stage)[10], we investigated the correlation between FDG uptake of the primary tumor and pathologic N stage.
Over the past decade, FDG-PET/CT has demonstrated more accurate non-invasive nodal staging than traditional imaging, such as CT, in lung cancer; however, the sensitivity of PET/CT remains relatively low[11–14], which has resulted in many nodal micro-metastasis going undetected. Moreover, non-specific inflammatory and granulomatous diseases can lead to FDG uptake and false-positive results[15–17]. Mediastinoscopy was long considered the gold standard of N staging of lung cancer before surgery; however, this was also a traumatic procedure, which was restricted by accessibility to the region and depended on the presence of N2/N3 disease[18]. In order to provide more information for clinical use to predict lymph node metastasis of NSCLC, we analyzed the possible risk factors for regional lymph node metastasis and determined whether the quantitative degree of FDG uptake by the primary tumor is an independent predictor for nodal metastasis in NSCLC. This approach might also represent a new and non-invasive way to evaluate and predict the nodal status of patients with NSCLC.
Materials and methods
Patient enrollment
Our institutional review board approved this study and written informed consent was obtained from all patients. Eighty consecutive patients with NSCLC (52 men and 28 women; mean age, 58 years; range, 35–84 years) who were candidates for surgical resection from October 2006 to March 2009 were enrolled in the study. Then they all underwent curative surgical resection after FDG-PET/CT scan. Seventy patients underwent a whole-body scan and 10 patients had a thoracic scan only. All the patients received no chemotherapy or radiation prior to surgery. The median time between the PET/CT scan and surgery was 6.5 days. Seventy-one patients were positive for a blood tumor marker (TM) prior to surgery. The TMs measured were carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), carbohydrate antigen 125 (CA125), cytokeratin 19 fragment (CK19/cyfra21-1), and squamous cell carcinoma (SCC) antigen. The corresponding diagnostic threshold for each was 5.0 ng/ml, 18.0 ng/ml, 35.0 U/ml, 3.3 ng/ml, and 1.5 ng/ml, respectively.
PET/CT image acquisition
All patients fasted for at least 6 h before the PET/CT examination. After confirmation of a normal blood glucose level (≤144 mg/dl or 8 mmol/l) in a peripheral fingertip blood sample, the patients were administered an intravenous injection of FDG at 3.70–4.44 MBq/kg and were then rested for approximately 60 min before undergoing the body scan, as recommended for tumor PET imaging[2,19]. Image acquisition was performed with an integrated PET/CT device (GE Discovery ST 16). The whole-body scan was performed from the head to the root of the thigh, and the thoracic scan was performed from the super clavicle to the adrenal gland. The CT scan was performed with section thickness of 3.75 mm using a standardized protocol that involved 120 kV, 150 mA, and 0.8 s per tube rotation. No intravenous contrast material was administered. Immediately after CT, PET was performed in an identical transverse field of view with a section thickness of 3.27 mm, and using 3 min per table position and three-dimensional acquisitions. Patients were allowed normal shallow respiration during the image acquisition procedure. Coregistered images were displayed using software (Xeleris), which allowed image integration and analysis.
PET/CT image analysis and surgical staging
Two chest radiologists with PET/CT diagnostic experience prospectively evaluated the integrated PET/CT images. For determination of the SUV, a cylindrical region of interest (ROI) was manually placed over the tumor site on the hottest trans-axial slice. The maximum SUV (SUVmax) within the ROI was used as the reference measurement. The primary tumor was classified according to location: central or peripheral; additional classification included 3 types based on the CT density findings: solid, mixed gross glass opacity (mGGO) or pure gross glass opacity (pGGO).
Lung resection, including lobectomy and pneumonectomy with mediastinal lymph node dissection, was performed on all patients. The dissected lymph nodes were identified as to station, according to the lymph node map proposed by Mountain et al.[20]. The pathologist then evaluated the nodes as numbered in the surgical field. Final pathologic N stage was assigned on the basis of image analysis using the American Joint Committee on Cancer (AJCC) staging system (7th edition)[21].
Statistical analysis
The distribution (normal or non-normal) of quantitative data was assessed using the Kolmogorov–Smirnov) test. Normal distribution data was presented as 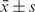 and compared by t test. Abnormal distribution data was presented as M ± Q and compared by Mann–Whitney U test. Comparisons between multiple different groups were performed with the ANOVA or Kruskal–Wallis test. When significant differences were found, further evaluation of the correlation was tested with the Spearman rank non-parametric correlation test. The possible risk factors for regional lymph node metastasis were analyzed by logistic univariate and multivariate analysis. Odds ratio (OR) and its 95% confidence interval (CI) were used to estimate the correlation strength between the risk factors and nodal metastasis. The threshold of primary tumor SUVmax was chosen by the receiver operating curve (ROC) curve. Statistically significant differences were determined with P < 0.05. All analyses were performed using the Stata statistical software (version 11.0, StataCorp, College Station, TX).
and compared by t test. Abnormal distribution data was presented as M ± Q and compared by Mann–Whitney U test. Comparisons between multiple different groups were performed with the ANOVA or Kruskal–Wallis test. When significant differences were found, further evaluation of the correlation was tested with the Spearman rank non-parametric correlation test. The possible risk factors for regional lymph node metastasis were analyzed by logistic univariate and multivariate analysis. Odds ratio (OR) and its 95% confidence interval (CI) were used to estimate the correlation strength between the risk factors and nodal metastasis. The threshold of primary tumor SUVmax was chosen by the receiver operating curve (ROC) curve. Statistically significant differences were determined with P < 0.05. All analyses were performed using the Stata statistical software (version 11.0, StataCorp, College Station, TX).
Results
Pathological staging and PET/CT results
Among the 80 NSCLC cases, 49 were N0 stage, 11 were N1 stage, and 20 were N2 stage, according to the pathologic results. The SUVmax of the primary tumor in patients with N0, N1, N2 and N1,2 (N1 and N2) staging were 5.75 ± 4.76, 8.07 ± 5.05, 8.66 ± 3.43 and 8.45 ± 4.01, respectively.
Correlation between FDG uptake of the primary tumor, N staging and regional nodal metastasis
As shown in Table 1, a statistically significant difference was found in the SUVmax of the primary tumor among N staging groups (F = 4.124, P = 0.023), and the correlation between SUVmax and N stage showed a statistically significant difference (r = 0.438, P = 0.000). Statistically significant differences were also found in SUVmax between N0 and N1,2 groups (t = −2.623, P = 0.010), and the correlation between SUVmax and nodal status (r = 0.416, P = 0.000).
Table 1.
Correlations between SUVmax of the primary lung tumor, N stage and lymph node metastasis
| Stage (group) | n | SUVmax of the primary tumor | ANOVA/t test |
Spearman rank test |
||
|---|---|---|---|---|---|---|
| F (t) | P | r | P | |||
| N stage | ||||||
| N0 | 49 | 5.75 ± 4.76 | 3.462 | 0.036* | 0.439 | 0.000 |
| N1 | 11 | 8.07 ± 5.05 | ||||
| N2 | 20 | 8.66 ± 3.43 | ||||
| LNM | ||||||
| N0 | 49 | 5.75 ± 4.76 | −2.623 | 0.010# | 0.416 | 0.000 |
| N1,2 | 31 | 8.45 ± 4.01 | ||||
LNM, lymph node metastasis. P < 0.05 indicates significant difference.
*ANOVA.
#t test.
Risk factors for regional lymph nodal metastasis
We also evaluated the possible risk factors that might affect nodal metastasis. We showed that the levels of blood TMs, primary tumor size, histologic differentiation, and SUVmax of the primary tumor were significantly associated with lymph node involvement by univariate analysis (Table 2). Multivariate logistic analysis indicated that the SUVmax of the primary tumor and levels of blood TM had significant predictive roles for lymph nodal metastasis in patients with NSCLC (Table 2). The detailed predictive roles combining the SUVmax of the primary tumor and blood TM are shown in Table 3.
Table 2.
Univariate and multivariate analyses of potential risk factors for LNM
| Variables | LNM |
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| + | − | OR (95%CI) | P | adjusted OR (95%CI)a | P | |
| Age | ||||||
| ≤60 years | 12 | 25 | Ref | Ref | ||
| >60 years | 19 | 24 | 1.65 (0.66–4.12) | 0.282 | 1.20 (0.33–4.32) | 0.782 |
| Gender | ||||||
| Female | 10 | 18 | Ref | Ref | ||
| Male | 21 | 31 | 1.22 (0.47–3.16) | 0.683 | 0.53 (0.14–2.06) | 0.366 |
| Smoking | ||||||
| No | 13 | 26 | Ref | Ref | ||
| Yes | 18 | 23 | 1.57 (0.63–3.88) | 0.333 | 1.39 (0.25–7.62) | 0.704 |
| Blood TM | ||||||
| Normal | 6 | 27 | Ref | Ref | ||
| Increased | 22 | 17 | 5.82 (1.96–17.28) | 0.001 | 5.63 (1.39–22.80) | 0.016 |
| Location | ||||||
| Peripheral | 27 | 46 | Ref | Ref | ||
| Central | 4 | 3 | 3.28 (0.87–12.34) | 0.068 | 1.26 (0.22–7.07) | 0.789 |
| Primary tumor size | ||||||
| ≤3.0 cm | 10 | 33 | Ref | Ref | ||
| 3.1–5.0 cm | 13 | 12 | 3.58 (1.24–10.28) | 0.018 | 1.38 (0.28–6.94) | 0.693 |
| >5.0 cm | 8 | 4 | 6.60 (1.64–26.59) | 0.008 | 1.41 (0.21–9.73) | 0.724 |
| Pathologic type | ||||||
| Adenocarcinoma | 20 | 36 | Ref | Ref | ||
| Non-adenocarcinoma | 11 | 13 | 1.52 (0.58–4.02) | 0.395 | 0.76 (0.17–3.33) | 0.716 |
| Histologic differentiation | ||||||
| High | 2 | 11 | Ref | Ref | ||
| Moderate | 13 | 23 | 3.11 (0.60–16.24) | 0.179 | 5.58 (0.45–68.90) | 0.179 |
| Poor | 16 | 15 | 5.87 (1.11–30.95) | 0.037 | 10.65 (0.82–138.21) | 0.071 |
| CT density | ||||||
| Solid | 29 | 38 | Ref | Ref | ||
| mGGO and pGGO | 2 | 11 | 0.24 (0.05–1.16) | 0.076 | 1.81 (0.22–14.70) | 0.579 |
| SUVmax of primary tumor | ||||||
| <5.4 | 5 | 29 | Ref | Ref | ||
| ≥5.4 | 26 | 20 | 7.54 (2.48–22.97) | 0.001 | 6.39 (1.28–31.89) | 0.024 |
LNM, lymph node metastasis; OR, odds ratio; 95% CI, 95% confidence interval. P < 0.05 indicates significant difference.
aAdjusted for age, gender, blood TM, primary tumor size, histologic differentiation and SUVmax of the primary tumor.
Table 3.
Predictive significance of combined SUVmax of the primary tumor and blood TM for LNM in patients with NSCLC
| SUVmax of primary tumor | Blood TM | LNM |
OR (95%CI) | P | |
|---|---|---|---|---|---|
| + | − | ||||
| − | − | 1 | 19 | Ref | |
| + | − | 5 | 8 | 11.88 (1.20–118.50) | 0.035 |
| − | + | 3 | 7 | 8.14 (0.72–91.89) | 0.090 |
| + | + | 19 | 10 | 36.10 (4.20–310.45) | 0.001 |
LNM, lymph node metastasis. OR, odds ratio; 95% CI, 95% confidence interval. P < 0.05 indicates statistically significant difference. SUVmax of the primary tumor: −, <5.4; +, ≥5.4. Blood TM: −, normal; +, increased.
Discussion
In this study, we evaluated the relationship between primary NSCLC FDG uptake and N stage. In addition, we determined whether tumor FDG uptake could predict the N status of patients with lung cancer. Our study demonstrated a correlation between primary NSCLC FDG uptake quantified by SUVmax and pathologic N stage. The SUVmax of the primary tumor showed a statistically significant difference between the group of patients without lymphatic involvement (N0) and the patients with tumor cells detected in the lymph nodes (N1,2); moreover, correlation between the SUVmax of the primary tumor and nodal status was also found. Thus, the greater the tumor FDG uptake, the higher the malignant grade. FDG was mainly taken up by tumor cells because cancer tissue consumes a large amount of glucose as an energy source[22]. Some previous studies have also shown that tumor FDG uptake could be affected by cell differentiation, proliferative rate potential, microvessel density, and hypoxia, which are all consistent with the biological behavior of malignant cells[23–25]. The detailed mechanism, however, needs to be further determined. Nonetheless, the findings from our study demonstrate that FDG uptake can reflect the potential metastasis of NSCLC.
As a quantified index of FDG uptake, SUV can be determined by either SUVmean or SUVmax. The reasons we choose to use SUVmax in this study were as follows: (1) the metabolic activity of glucose in tumor tissue is heterogeneous; thus, the most active area of metabolism measured as SUVmax could largely represent the malignant behavior of the tumor. (2) SUVmean can be easily affected by necrotic and cystic areas. (3) SUVmax is less variable and subjective, but more reproductive, than SUVmean[26]. (4) SUVmax is more commonly used in current clinical practice.
Several studies have shown that FDG-PET/CT was superior to CT for mediastinal nodal staging; however, the sensitivity of PET/CT remained low[11–14]. The lack of sufficient FDG uptake in lymph nodes with micro-metastasis can result in a false-negative interpretation[27]. In order to predict nodal metastasis in at-risk patients with NSCLC, we assessed the possible risk factors of nodal metastasis and analyzes the contributions of each of the clinical, pathologic and imaging features. By univariate analysis, we demonstrated that primary tumor size, blood TM, histologic differentiation and SUVmax of the primary tumor were risk factors (OR > 1, P < 0.05) for lymph node involvement. In other words, patients with larger tumor size, higher blood TM level, poorer histologic differentiation, or higher SUVmax of the primary tumor may be more likely to experience nodal metastasis. Our results are consistent with other reports[28–30]. Furthermore, we analyzed these factors by logistic multivariate analysis to assess the joint effects and interactions of the variables on lymph node involvement. Our results show that only blood TM and SUVmax of the primary tumor retained statistical significance, whereas tumor size and histologic differentiation had no significant difference on multivariate analysis; we believe that their effects were substituted by the enrolled index.
A TM found in the blood or other body fluid can either be the product of the cancer cells themselves or produced by the body in response to the cancer or other concomitant conditions. The blood TMs selected in our research represent those commonly used in clinical practice for lung cancer screening, diagnosis, and detection. Our results suggest that the levels of blood TM were also associated with regional nodal metastases and might be used as important predictive factors.
Through univariate and multivariate analyses, we found that SUVmax of the primary tumor showed statistical significance, suggesting that the FDG uptake in the primary tumor was an independent predictor for N status of patients with NSCLC. In this study, the degree of SUVmax was classified in 2 levels according to the threshold of 5.4 (the cutoff value of ROC curves of primary tumor SUVmax for lymph nodal metastases). The probability of nodal metastasis for the 2 levels was 14.7% (5/34) and 56.5% (26/46), respectively. When the SUVmax of the primary tumor exceeded 5.4, the risk of nodal metastasis increased. We further utilized the SUVmax of the primary tumor and blood TM jointly to predict lymph node metastasis in NSCLC. The results showed that whenever the SUVmax of the primary tumor and blood TM were both positive, the risk of nodal metastasis increased over 30-fold (36.10) compared with patients with both factors negative. This result may provide a non-invasive way to predict the high-risk population for lymph node metastasis in patients with NSCLC.
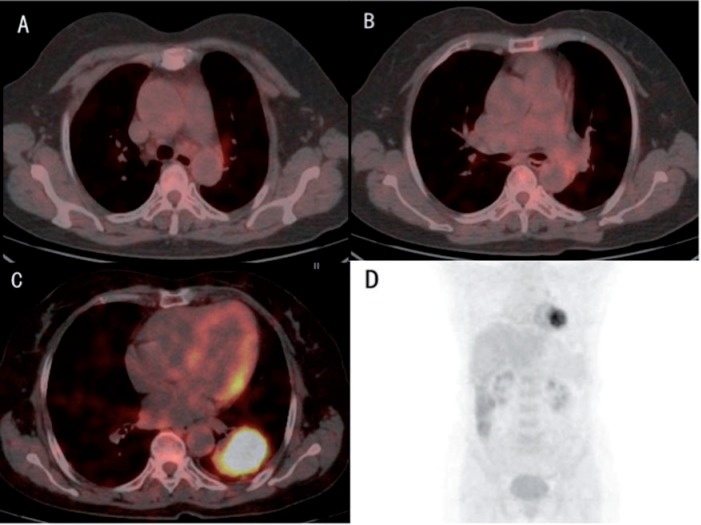
Figure 1.
A 67-year-old woman, with blood CEA of 14.95 ng/ml, was diagnosed with poorly differentiated adenocarcinoma in the inferior lobe of the left lung. Three different slices of integrated FDG-PET/CT images (A–C) and maximum intensity projection showed an SUVmax of the primary tumor of 9.4. Although there was no obvious mediastinal lymph nodal metastasis on imaging, the high SUVmax of the primary tumor and CEA level indicated that the patient might be at risk for lymph nodal metastasis. Pathologic results after surgery showed micro-metastases in subaortic and subcarinal lymph nodes (N2).
Primary tumor FDG uptake has high affinity for cells undergoing nodal metastasis, although it cannot substitute for the benefit of immediate imaging of the mediastinum. This suggests that evaluation of FDG uptake may be a useful tool to help plan the appropriate surgical treatment. Studies have reported that lymph node metastasis was found in about 20% of clinical stage IA (T1N0M0) pulmonary adenocarcinomas, and that a higher local recurrence rate after limited resection, such as a wedge resection or a segmentectomy, was even observed in those with pathologically confirmed negative surgical margins[31,32]. These findings might be due to micro-metastasis in lymph nodes or the spread of tumor cells into lymphatic vessels beyond the primary tumor, even at the N0 stage. Therefore, we should maintain a positive attitude toward lobectomy or major lung resection with systematic dissection of hilar or mediastinal lymph nodes for patients with NSCLC presenting with higher FDG uptake in the primary tumor, even when the preoperative nodal imaging is negative. On the other hand, limited resection might be performed successfully in stage IA patients whose primary cancer showed lower FDG uptake.
This study had several limitations. First, to ensure thoroughness of surgical staging, all the patients with NSCLC underwent curative surgical resection, which resulted in no N3 patients being included in our study. Second, despite the fact that the area of GGO on thin-section CT images and tumor location have been reported as predictors of lymph node metastasis[28,33,34], the population of patients in our study with GGO, especially with pGGO (only one case), and central location of the tumor was relatively small, which may have influenced our statistical results. Thus, future studies with large sample populations are needed to confirm our findings.
Collectively, our results show that there are significant relationships between the SUVmax of the primary tumor and pathologic N stage of NSCLC. FDG uptake by the primary tumor may be an independent predictor of regional lymph node metastasis in patients with NSCLC. SUVmax is readily amenable to clinical use due to its non-invasive nature and convenience.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Science and Technology Major Projects (2009ZX09501-026), the Doctoral Fund of Educational Ministry of China (20101106110017) and the Beijing Hope Run Special Fund (Grant LC2007A02).
References
- 1.Devaraj A, Cook GJ, Hansell DM. PET/CT in non-small cell lung cancer staging-promises and problems. Clin Radiol. 2007;62:97–108. doi: 10.1016/j.crad.2006.09.015. . PMid:17207691. [DOI] [PubMed] [Google Scholar]
- 2.Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nuclear Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–422. doi: 10.1148/radiol.2382041977. . PMid:16436809. [DOI] [PubMed] [Google Scholar]
- 4.Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol. 2009;193:1203–1211. doi: 10.2214/AJR.09.3193. . PMid:19843732. [DOI] [PubMed] [Google Scholar]
- 5.Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. . PMid:15310769. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka T, Nomori H, Watanabe K, et al. Prognostic significance of [(18)F]fluorodeoxyglucose uptake on positron emission tomography in patients with pathologic stage I lung adenocarcinoma. Cancer. 2006;107:2468–2473. doi: 10.1002/cncr.22268. . PMid:17036361. [DOI] [PubMed] [Google Scholar]
- 7.Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. PMid:11801701. [PubMed] [Google Scholar]
- 8.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. . PMid:18166834. [DOI] [PubMed] [Google Scholar]
- 9.Rankin S. PET/CT for staging and monitoring non small cell lung cancer. Cancer Imaging. 2008;8(A):S27–31. doi: 10.1102/1470-7330.2008.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganeshalingam S, Koh DM. Nodal staging. Cancer Imaging. 2009;9:104–111. doi: 10.1102/1470-7330.2009.0017. PMid:20080453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BT, Lee KS, Shim SS, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT–a prospective study. Radiology. 2006;241:501–509. doi: 10.1148/radiol.2412051173. . PMid:16966480. [DOI] [PubMed] [Google Scholar]
- 12.Kim YK, Lee KS, Kim BT, et al. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007;109:1068–1077. doi: 10.1002/cncr.22518. . PMid:17311309. [DOI] [PubMed] [Google Scholar]
- 13.Liu BJ, Dong JC, Xu CQ, et al. Accuracy of 18F-FDG PET/CT for lymph node staging in non-small-cell lung cancers. Chin Med J (Engl) 2009;122:1749–1754. [PubMed] [Google Scholar]
- 14.Al-Sarraf N, Gately K, Lucey J, et al. Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: analysis of 1,145 lymph nodes. Lung Cancer. 2008;60:62–68. doi: 10.1016/j.lungcan.2007.08.036. . PMid:17920724. [DOI] [PubMed] [Google Scholar]
- 15.Mattar EH. Integrated PET/CT in imaging of non-small cell lung cancer. J Egypt Natl Cancer Inst. 2007;19:263–274. [PubMed] [Google Scholar]
- 16.Kumar R, Halanaik D, Malhotra A. Clinical applications of positron emission tomography-computed tomography in oncology. Indian J Cancer. 2010;47:100–119. doi: 10.4103/0019-509X.62997. . PMid:20448371. [DOI] [PubMed] [Google Scholar]
- 17.Saif MW, Tzannou I, Makrilia N, Syrigos K. Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med. 2010;83:53–65. PMid:20589185. [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg. 2003;238:180–188. doi: 10.1097/01.SLA.0000081086.37779.1a. PMid:12894010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nuclear Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 20.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. . PMid:9187199. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. Heidelberg: Springer; 2010. pp. 253–270. [Google Scholar]
- 22.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–648. PMid:2013803. [PubMed] [Google Scholar]
- 23.Buck AK, Glatting G, Reske SN. Quantification of 18F-FDG uptake in non-small cell lung cancer: a feasible prognostic marker? J Nucl Med. 2004;45:1274–1276. PMid:15299047. [PubMed] [Google Scholar]
- 24.Guo J, Higashi K, Ueda Y, et al. Microvessel density: correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med. 2006;47:419–425. PMid:16513610. [PubMed] [Google Scholar]
- 25.Nguyen XC, Lee WW, Chung JH, et al. FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol. 2007;62:214–219. doi: 10.1016/j.ejrad.2006.12.008. . PMid:17239556. [DOI] [PubMed] [Google Scholar]
- 26.Lee JR, Madsen MT, Bushnel D, Menda Y. A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun. 2000;21:685–690. doi: 10.1097/00006231-200007000-00013. . PMid:10994673. [DOI] [PubMed] [Google Scholar]
- 27.Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1109. doi: 10.1148/radiol.2363041310. . PMid:16014441. [DOI] [PubMed] [Google Scholar]
- 28.Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:177–181. doi: 10.1016/j.athoracsur.2007.03.081. . PMid:17588407. [DOI] [PubMed] [Google Scholar]
- 29.Higashi K, Ito K, Hiramatsu Y, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med. 2005;46:267–273. PMid:15695786. [PubMed] [Google Scholar]
- 30.Maeda R, Isowa N, Onuma H, et al. The maximum standardized 18F-fluorodeoxyglucose uptake on positron emission tomography predicts lymph node metastasis and invasiveness in clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2009;9:79–82. doi: 10.1510/icvts.2008.201251. . PMid:19366724. [DOI] [PubMed] [Google Scholar]
- 31.Asamura H, Nakayama H, Kondo H, et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg. 1996;111:1125–1134. doi: 10.1016/S0022-5223(96)70213-1. . PMid:8642812. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622; discussion 622–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–1639. doi: 10.1016/S0003-4975(02)03895-X. . PMid:12440622. [DOI] [PubMed] [Google Scholar]
- 34.Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. J Thorac Cardiovasc Surg. 2002;124:278–284. doi: 10.1067/mtc.2002.122298. . PMid:12167787. [DOI] [PubMed] [Google Scholar]