Abstract
The National Lung Cancer Screening Trial has recently demonstrated that screening of high-risk populations with the use of low-dose computed tomography (LDCT) reduces lung cancer mortality[1]. Based on this encouraging result, the National Comprehensive Cancer Network guidelines recommended LDCT for selected patients at high risk of lung cancer[2]. This suggests that an increasing number of CT screening examinations will be performed. The LDCT technique is relatively simple but some CT parameters are important and should be accurately defined in order to achieve good diagnostic quality and minimize the delivered dose. In addition, LDCT examinations are not as easy to read as they may initially appear; different approaches and tools are available for nodule detection and measurement. Moreover, the management of positive results can be a complex process and can differ significantly from routine clinical practice. Therefore this paper deals with the LDCT technique, reading methods and interpretation in lung cancer screening, particularly for those radiologists who have little experience of the technique.
Keywords: Computed tomography, low-dose CT, lung cancer, screening
LDCT: technical notes
Even if low-dose computed tomography (LDCT) can be acquired with single-slice spiral computed tomography (CT)[3], currently the use of multidetector row CT (MDCT) scanners is recommended. The rationale for using MDCT scanners is that most of the nodules detected on screening are small and require optimal spatial resolution for computer-aided detection (CAD) and nodule volume assessment[4].
According to the National Lung Cancer Screening Trial (NLST) and International Early Lung Cancer Action Program (I-ELCAP), no less than 4-detector MDCT scanners are recommended for LDCT, to ensure that the chest can be scanned in a single breath-hold and to achieve good spatial resolution[5,6].
Due to a high contrast resolution between air and lung nodules, LDCT enables a low radiation dose while maintaining good diagnostic quality. There is no consensus on which level of dose is considered a low dose and the factors affecting dose in CT are different, such as tube voltage (kVp, kilovolt peak), tube current (mA, milliampere) and tube speed rotation (s, second).
Various kVp and mAs values have been used in MDCT lung cancer screening programs[2–8], with different estimated effective doses. A recent study, aimed at determining the distribution of effective dose associated with a single LDCT examination, concluded that acceptable CT screening can be accomplished at an overall average effective dose of approximately 2 mSv[9]).
A few recent lung cancer screening (LCS) trials have optimized the dose delivered based on the patient’s weight; a comparative CT scan and reconstruction parameters of the largest LCS trials are summarized in Table 1.
Table 1.
CT scanning and reconstruction parameters in some recent LCS trials
| Study | Detectors | Voltage (kVp) | Tube current (mA) | Pitch | Rotation time (s) | Effective dose (mSv) | Slice thickness (mm) | Reconstruction interval (mm) |
|---|---|---|---|---|---|---|---|---|
| I-ELCAP | ≥4 | ≤120 | ≤40 | 1.5 | 0.5 | 1–2 | 1.25 | 1.25 |
| NLST | ≥4 | 120–140a | 40–80a | NA | NA | 1.5 | 1.0–3.2 | 1.0–2.5 |
| UKLS | >16 | 90 (<50 kg) | –b | 0.9–1.1 | NA | <2 | NA | NA |
| 120 (50–80 kg) | ||||||||
| 140 (>80 kg) | ||||||||
| NELSON | 16 | 100 (<60 kg) | 20 | 1.5 | NA | <2 | 1 | 0.7 |
| 120 (60–80 kg) | ||||||||
| 140 (>80 kg) |
NA, not available.
aDepending on the CT scanner and the participant’s body habitus.
bDepending on the CT scanner adjusted to achieve the volume CT dose index given.
Recently, CT manufacturers have introduced iterative reconstruction (e.g. adaptive statistical iterative reconstruction (ASIR), iDose, model-based iterative reconstruction (MBIR), etc.) instead of filtered back projection (FBP). The advantage of iterative algorithms is the noise reduction while spatial resolution, CT number accuracy and linearity are preserved. Unfortunately, no extensive data on the use of iterative reconstruction-based algorithms with LDCT are available and further studies are needed to assess the usefulness of this technique.
The collimation of LDCT should be set with the purpose of achieving thin-section reconstruction images, which allow the use of CAD and an optimal volumetric analysis. Therefore, reconstructed slice thickness, at least equal to or lower than 1.5 mm, should be used[2]. It is advisable to set the section interval lower than the slice thickness in order to reduce partial volume artifacts. This is critical for accurate nodule volume assessment.
The reconstruction kernel can affect the volume measurement of pulmonary nodules from LDCT examinations. In a previous study, the repeatability of volume measurements of pulmonary nodules obtained at 1-mm section thickness combined with a soft kernel was almost twice as good as the reconstruction obtained with a 2-mm section thickness combined with a soft kernel, and almost 4 times better than those obtained at 2-mm section thickness combined with a sharp kernel[10].
LDCT: reading methods and nodule detection
Pulmonary nodules
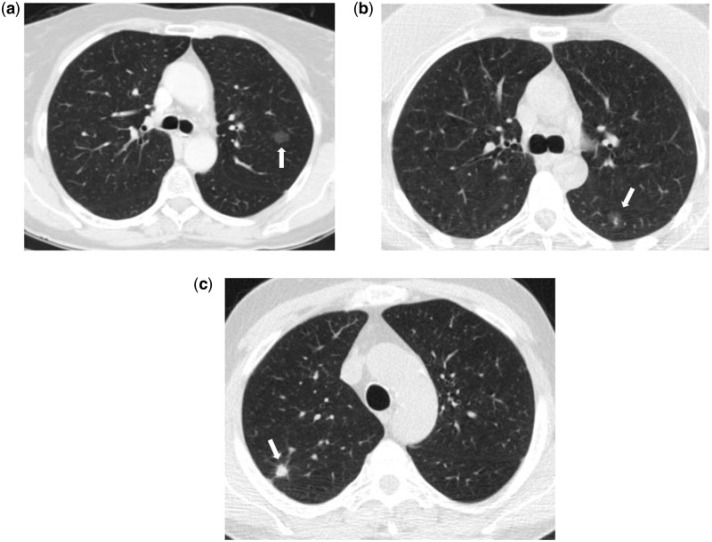
The target of LDCT for screening purposes is the non-calcified pulmonary nodule. Lung nodules can be distinguished in solid nodules and subsolid nodules[11]. Subsolid nodules can be further classified as non-solid nodules and part-solid nodules (Fig. 1). This classification is significant because different nodules require different approaches for their detection, measurement and management.
Figure 1.
(a) Transverse unenhanced low-dose CT scan of a 63-year-old man shows a non-solid nodule in the upper left lobe (arrow). (b) Transverse unenhanced low-dose CT scan of a 66-year-old woman shows a part-solid nodule in the lower left lobe (arrow). (c) Transverse unenhanced low-dose CT scan of a 58-year-old man shows a solid nodule in the upper right lobe (arrow). All 3 nodules were surgically removed and were found to be adenocarcinoma of the lung.
Solid and subsolid nodules have different growth rates and subsolid nodules have a higher probability of malignancy. Li et al.[12] compared malignant and benign nodules in an LCS study and found that the prevalence of malignancy was 59% for non-solid nodules, 48% for part-solid nodules and 11% for solid nodules. In the Early Lung Cancer Action Project[11], 34% (15/44) of subsolid nodules detected at baseline were malignant, whereas malignancy rates for part-solid and non-solid nodules were 63% (10/16) and 18% (5/28), respectively, compared with only 7% for solid nodules.
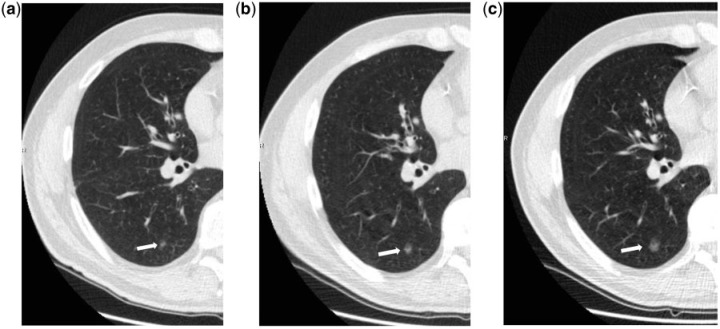
Even if non-solid nodules are more likely to be malignant, their growth rate tends to be considerably slower than solid lesions[13,14] with substantial implication for their follow-up interval and management (Fig. 2). Conversely, solid and part-solid nodules when malignant are more likely to be invasive and faster growing cancers.
Figure 2.
Transverse unenhanced low-dose CT scans show a slow-growing pulmonary nodule of the lower right lobe in a 61-year-old man. In 2012, the nodule was surgically removed and was found to be adenocarcinoma of the lung. The nodule measured 5.5 mm in 2007 (a), 8.5 mm in 2009 (b) and 11 mm in 2012 (c).
Subsolid nodules are mainly atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA). AIS and MIA are terms introduced by the new classification of lung adenocarcinoma[12] (formerly known as bronchioloalveolar carcinoma (BAC)) and 5-year disease-free survival can approach 100% if surgically resected[2].
Nodule detection
False-negative diagnosis due to perceptual error is a common problem in lung cancer diagnosis[15–17]. The performance of radiologists in nodule detection can be influenced by several factors such as CT parameters, reader experience and nodule location[18,19]. If the application of thin-slice MDCT raises the reader’s sensitivity for lung nodule detection[20], the detection rate is also affected by its location. Naidich et al.[18] showed that perihilar lung nodules were detected with a sensitivity of 36.7% versus 73.9% of peripherally located nodules. They also noted that vessel-attached nodules were detected with a significantly low sensitivity (32.5%).
To improve the radiologist’s detection rate, a double reading technique and CAD systems have been proposed for LDCT. Double reading significantly improved the detection rate of pulmonary nodules. Wormanns et al.[21] showed that the average sensitivity for detection of 390 nodules (size 3.9 ± 3.2 mm) for single readers increased from 64% to 79% using double reading. However, a recent study[22] showed that double reading in LCS did not increase the cancer detection rate but led to the detection of 19% more nodules.
The use of a CAD system can be of help for the detection of small pulmonary nodules. Rubin[23] showed that the mean sensitivity for single reading, double reading and reader using CAD was 50%, 63% and 76%, respectively. Armato et al.[16] showed that CAD detected 78% of missed lung nodules and found 93% of nodules undiagnosed due to interpretation error. The accuracy of CAD for nodule detection increases with thinner reconstruction images and with overlapping reconstructed sections[24], even if this causes an increasing number of false-positive results. The use of CAD system seems to be poorly affected by dose level. Lee et al.[25] used 4 different tube currents (32, 16, 8 and 4 mAs) in 25 volunteers and found no significant differences in nodule detection between the scans with 8 mAs and those with 32 mAs. The reader’s experience using CAD systems is also significant. In one study performing receiver operating curve (ROC) analyses of single and combined reader performances involving experienced (6–8 years experience) and inexperienced radiologists (6 months experience), the overall performance of an experienced radiologist with CAD assistance was significantly better than that of an inexperienced radiologist using CAD as second reader[26].
The detection rate of pulmonary nodules can also be improved using maximum intensity projection (MIP). Bastarrika et al.[27] showed that non-overlapping 10-mm-thick axial MIP reconstructions in an LCS study enabled more accurate detection of pulmonary nodules in comparison with 1.25-mm conventional axial images. Jankowski et al.[28] evaluated the diagnostic benefits of MIP and a CAD system for pulmonary nodules detection compared with 1-mm LDCT images. The investigators found that MIP and CAD reduced the number of overlooked nodules compared with LDCT and that the MIP was more sensitive than the CAD. Multiplanar reconstructions images (MPR) were shown to be a sensitive technique in lung nodule detection[29,30].
However, there is no consensus on which technique should be used to read low-dose CT images and further investigations are needed to assess the optimal method to improve the detection rate of pulmonary nodules.
Beyond pulmonary nodules
Chest LDCT can provide additional information, including cardiovascular disease and chronic obstructive pulmonary disease (COPD). Some studies support the idea that coronary artery calcium (CAC) scoring as part of ungated LDCT can be useful to assess the risk of cardiovascular disease[31,32]. In a study to compare CAC and thoracic aorta calcium (TAC) as independent predictors of all-cause mortality and cardiovascular events in an LCS trial, the investigators concluded that CAC is a stronger predictor than TAC for all-cause mortality, cardiovascular and coronary events[33]. LDCT was also shown to be a reliable technique for the quantification and evolution of emphysema[34–36]. The accuracy of COPD diagnosis with LDCT has been recently evaluated[37]; inspiratory and expiratory LDCT scans can identify participants with COPD with a sensitivity and specificity of 63% and 88%, respectively. A comprehensive evaluation of LDCT should not be limited to the lung parenchyma. A recent study shown that extrapulmonary malignancy in an LCS trial is diagnosed with a frequency of 1 case per 200 individuals screened[38].
LDCT: nodule measurement
Size and growth are the most important parameters in the management of pulmonary nodules detected on screening. Therefore, it seems essential to use an accurate and reproducible method for reliable measurement of pulmonary nodules. Manual and volumetric methods are currently used to assess nodule size but no consensus has yet been reached on which method should be used in LCS. The 2 largest randomized screening trials have a different approach; NLST uses manual diameter and the NELSON trial uses volume and diameter[5,8].
Solid nodules
Both manual (the largest or the 2 largest orthogonal dimensions) and volumetric measurement of lung nodules can be used in solid nodules. As reasonable doubts arise on the accuracy of manual measurements, in relation to the significant intra- and interobserver variability[39,40], some authors suggest that volumetric analysis is a more accurate tool[41,42]. In the NELSON trial (in which solid nodules were measured by volumetric software with the exception of pleural-attached nodules), semi-automated measurements were not reproducible in 11% of solid nodules and, thus, may cause errors in the assessment of nodule growth[43].
Various CT parameters can affect the volumetric analysis of lung nodules[44]. Among these, the slice thickness is the most important. Winer-Muram et al.[45] showed an average percentage difference of 20% in volumetric measurements between thin and thick sections (36% for the smallest tumors). Nietert et al.[46] showed that for assessing growth in pulmonary nodules, slice thicknesses ≥2.5 mm are essentially inadequate for 1-mm changes in nodule diameter[46].
Size and shape can also influence the precision and accuracy of volumetric measurements. Lung nodule volume estimation error increases with decreasing nodule size[47–49]. Yankelevitz et al.[49] studied nodule shape and found that volume measurements showed larger measurement error for elongated shapes (0.9–2.8%) than for spherical shapes (0.7–1.43%). Similar findings were reported by Marten et al.[50]. Moreover, juxtapleural and juxtavascular nodules can be segmented less accurately and sometimes they require manual correction, leading to higher variability in nodule measurements.
Different software packages provide different results and different segmentation algorithms, within the same software, which can affect nodule segmentation[51,52].
Subsolid nodules
The measurement of subsolid nodules is more complex than for solid nodules; moreover, the typical slow growth of such nodules makes a precise estimation of growth more difficult at follow-up. Even though some commercially available software provides specific volumetric algorithms for non-solid nodules, the segmentation is often poor and manual correction is often required. To date, there is less compelling data supporting the use of the volumetric approach for subsolid nodules[42,49,53,54]. In addition, a subsolid nodule stable in diameter but with an increasing solid component at follow-up is suspicious for malignancy. In this case, neither manual nor volumetric assessment is appropriate. To address this issue, de Hoop et al.[55] introduced the estimation of nodule mass (calculated by multiplying nodule volume by mean nodule density) as a method for measuring change in non-solid nodules. They demonstrated that mass measurements can enable detection of growth earlier and are subject to less variability than volume or diameter measurements. However, measuring the mass of a nodule requires a manual assessment of the volume of the lesion, which increases both reporting time and observer variability.
Follow-up studies and nodule growth
The same CT scanning and reconstruction parameters should be used at LDCT follow-up studies. When nodule size is determined volumetrically, the same software version and segmentation algorithm is recommended at follow-up examinations to avoid measurement inaccuracy[51,52,56].
The definition of nodule growth in LCS is not widely accepted. In the I-ELCAP study, the definition of nodule growth was related to mean nodule diameter (defined as the mean of the longest diameter and its perpendicular diameter): ≥50% increase in mean diameter for nodules <5 mm, ≥30% for nodules 5–9 mm and ≥20% for nodules larger than 10 mm[6]. The definition of growth in the NLST trial was an increase in nodule diameter ≥10%[5]. In the NELSON study, nodule growth (for solid nodules) was defined as a ≥25% change in volume after at least a 3-month interval[8]. Recently, the National Comprehensive Cancer Network defined nodule growth as an increase in mean diameter of 2 mm or more for nodules ≤15 mm (or in the solid portion of a part-solid nodule) or an increase of 15% in mean diameter for nodules >15 mm[2].
The estimation of nodule growth rate between 2 or more LDCT scans can be calculated by the doubling time (DT). The DT is defined as the time, expressed in days, for a nodule to double its volume. DT can be obtained from the maximum axial diameter:
from the area
or by volumetric analysis
where Dt is the interval between 2 CT scans, D1 is the tumor diameter at the initial CT, D2 is the tumor diameter at the second CT, A1 is the tumor area at the initial CT, A2 is the tumor area at the second CT, V1 is the tumor volume at the initial CT, V2 is the tumor volume at the second CT. The DT calculation based on diameter estimates assumes uniform growth in 3 dimensions. Because nodules do not necessarily grow uniformly in all dimensions, volumetric determinations should theoretically provide more accurate information.
Regardless of diameter or volume assessment, DT is based on an exponential growth model. However, Lindell et al.[57] showed that in a series of 18 screen-detected lung neoplasms, lung cancers are not limited to exponential growth. This has substantial meaning because studies and equations assuming exponential growth may potentially misrepresent an indeterminate nodule or the aggressiveness of a lung cancer.
Malignant nodules are generally rapidly growing lesions. Revel et al.[58] reported that 98% of all cases of solid nodules with a DT of more than 500 days were non-malignant. The time of 500 days is generally accepted as the upper limit of the doubling time for malignant pulmonary lesions[59], even though some lesions grow more slowly, especially for the spectrum of adenocarcinomas. In one LDCT study[60], the DT was 988 ± 470 days for AAH, 567 ± 168 days for BAC, and 384 ± 212 days for mixed subtype of adenocarcinoma with BAC features. This implies that the widely accepted concept that 2-year stability is needed to differentiate malignant from benign nodules can be applied only to solid nodules but not to subsolid nodules that are mainly adenocarcinomas[61].
The doubling times of benign nodules is generally less than 20 days or more than 450 days. However, the NELSON study has reported that significant growth may also occur in benign nodules: 58/68 (85%) nodules with a volume doubling time of <400 days after 3 months were benign[62].
LDCT: nodule management
The pulmonary nodule is a complex challenge in the interpretation of LDCT examinations. There are several nodule management protocols proposed by different LCS trials[5–8,63], but currently it is difficult to determinate the most accurate protocol for LCS because of differing patient populations and study designs. Nodule size and growth rate have to be taken into consideration to assess which is the most appropriate work-up for each nodule. Most importantly, the approach to manage pulmonary nodules is multidisciplinary, with input from radiologists, pulmonologists, surgeons and nuclear medicine physicians. Therefore, we would not suggest a specific protocol for nodule management but rather the evaluation of the main variables that can influence the diagnostic algorithm in LCS.
Nodule size
Nodule size is one of the most important characteristics, as good evidence exists for a strong correlation between size and risk of malignancy[64,65]. In a meta-analysis of 8 large LDCT screening trials, the prevalence of malignancy depended on nodule size, ranging from 0% to 1% for nodules ≤5 mm, from 6% to 28% for those between 5 and 10 mm, and from 64% to 82% for nodules ≥20mm[63].
To reduce the number of false-positive results in LCS trials, there is a broad consensus on considering an LDCT scan negative when lung nodule diameter is smaller than 4 mm[5] or 5 mm[6,63] or when volume is smaller than 50 mm3 (diameter 4.6 mm)[8]. In this case, the participants received a repeat LDCT scan at a 1-year interval. Currently, if a nodule size at baseline or a new nodule at repeat scan is larger than such measurements, it is considered a positive result and further tests, according to nodule size and attenuation, are recommended.
In cases of a new nodule at repeat LDCT scan, size is the discriminant factor for its management. If it is <5 mm (or <4 mm or <50 mm3), follow-up at a 1-year interval is suggested. If it is between 5 mm and 8–10 mm, an LDCT follow-up at 3 or 6 months is generally suggested. If nodule size is larger than 8–10 mm, LDCT follow-up at 1 or 3 months (with or without antibiotics), or positron emission tomography (PET) and/or biopsy should be considered, according to nodule attenuation.
If a nodule was present at the baseline scan and no growth is assessed at annual repeat LDCT scan, follow-up at 1 year is recommended by all screening protocols. The concept of 2-year stability at follow-up CT, as an indicator of the benign nature of a nodule, should be limited to solid nodules[61,66].
Growth rate
The increase in the volume of a nodule over time is used as a method to differentiate benign from malignant nodules. If a nodule has grown, the size and the speed of growth should be considered to define its management. A very rapid growth rate (doubling time less than 1 month) is more suggestive of a benign lesion and, in this case, a course of antibiotics followed by CT 1 month later can be performed. If the nodule doubling time is less than 400 days, short LDCT follow-up (e.g. at 3 months) or PET or biopsy can be performed (according to nodule size); when the doubling time is 400–600 days, a follow-up scan can be obtained at 6 months or 1 year after[8]. If the doubling time is more than 600 days, repeat LDCT scan at 1 year can be suggested.
Subsolid nodules
Formal management guidelines for subsolid nodules have not yet been issued but interim management guidelines have recently been proposed by Godoy and Naidich[66]. As previously stated, non-solid nodules grow slowly but, at the same time, have a high malignancy rate (Fig. 2). This implies that a minimum follow-up of 3 years is required for non-solid nodules to differentiate malignant from benign nodules and that any change in size or the development of a solid component is suspicious for malignancy[66–69]. Close follow-up is justified when a non-solid nodule is ≤10 mm[68–70]. When a non-solid nodule increases in size or if a solid component develops, PET and/or CT-guided biopsy or surgical biopsy should be performed. Many benign diseases, such as inflammatory disease or fibrosis, can also manifest as non-solid nodules.
Role of PET in LCS
PET scan using [18F]fluorodeoxyglucose can discriminate between malignant and benign pulmonary nodules[71,72]. Veronesi et al.[73] found that the diagnostic sensitivity of PET/CT at baseline was 88% for solid nodules >8 mm, but increased to 100% for solid nodules >10 mm. However, in LCS, the value of PET is still unclear. Most screen-detected lung nodules are small and unfortunately the accuracy of PET for the assessment of small lesions is limited by the resolution power of scanners. Therefore, for nodules ≤8 mm PET scan should not be considered for nodule characterization[74,75]. Regardless of nodule size, however, PET has a lower sensitivity for slow-growing lesions, such as BACs and carcinoid tumors[74–77]. As most BACs are depicted as non-solid nodules, the role of PET imaging for assessing non-solid nodules remains to be established.
Invasive procedures and complications
Histologic samples of lung nodules can be obtained by CT-guided biopsy, bronchoscopic biopsy or wedge resection. Invasive procedures in LCS ranged from 10% to 43% according to different study designs[1,7,78–81]. Recently, the number of invasive procedures recommended for suspicious nodules in 4782 high-risk smokers was assessed[82]. The investigators showed that 104 of 124 biopsies (84%) were correctly indicated (true-positive recommendation) for malignancy. The only study reporting on complications resulting from LDCT screening is the NLST. In this study, the frequency of major complications occurring during the diagnostic evaluation was 33 per 10,000 individuals screened by LDCT.
Conclusions
The NLST has demonstrated that the screening of high-risk populations with the use of LDCT reduces lung cancer mortality[1]. Reporting LDCT may appear unproblematic but radiologists involved in LCS should be aware that the interpretation of low-dose CT scans is not as easy as it may initially seem. Several considerations such as different nodule detection, tools and measurement assessments should be taken into account in order to avoid false-negative diagnoses as well as unnecessary follow-up examinations. Moreover, knowledge of the significance of different nodule types is essential, as different nodules require different approaches for their detection, measurement and management.
The management of pulmonary nodules in asymptomatic high-risk populations is a challenge and can differ significantly from clinical practice. Therefore, the experience of radiologists is crucial. Quality control in the interpretation of low-dose CT images and a multidisciplinary approach to manage positive results are decisive. CT scans should be interpreted by radiologists who have undergone specific training.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network (NCCN) Guidelines Version 1.2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 22 November 2011.
- 3.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–597. doi: 10.1016/S0140-6736(03)14188-8. . PMid:12944057. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66:308–313. doi: 10.1136/thx.2010.152066. . PMid:21317179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team. Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. I-ELCAP. http://www.ielcap.org/professionals/docs/ielcap.pdf. Accessed 21 October 2011.
- 7.Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226:756–761. doi: 10.1148/radiol.2263020036. . PMid:12601181. [DOI] [PubMed] [Google Scholar]
- 8.Xu DM, Gietema HA, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;54:177–184. doi: 10.1016/j.lungcan.2006.08.006. . PMid:16989922. [DOI] [PubMed] [Google Scholar]
- 9.Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR. 2011;197:1165–1169. doi: 10.2214/AJR.11.6533. . PMid:22021510. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, de Bock GH, van Klaveren RJ, et al. Volumetric measurement of pulmonary nodules at low-dose chest CT: effect of reconstruction setting on measurement variability. Eur Radiol. 2010;20:1180–1187. doi: 10.1007/s00330-009-1634-9. . PMid:19921204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178:1053–1057. doi: 10.2214/ajr.178.5.1781053. PMid:11959700. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. . PMid:21252716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima S, Sone S, Li F, Maruyama Y, Hasegawa M, Kadoya M. Indeterminate solitary pulmonary nodules revealed at population-based CT screening of the lung: using first follow-up diagnostic CT to differentiate benign and malignant lesions. AJR Am J Roentgenol. 2003;180:1255–1263. doi: 10.2214/ajr.180.5.1801255. PMid:12704034. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245:267–275. doi: 10.1148/radiol.2451061682. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Sone S, Abe H, MacMahon H, Armato 3rd SG, Doi K. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology. 2002;225:673–683. doi: 10.1148/radiol.2253011375. . PMid:12461245. [DOI] [PubMed] [Google Scholar]
- 16.Armato 3rd SG, Li F, Giger ML, MacMahon H, Sone S, Doi K. Lung cancer: performance of automated lung nodule detection applied to cancers missed in a CT screening program. Radiology. 2002;225:685–692. doi: 10.1148/radiol.2253011376. [DOI] [PubMed] [Google Scholar]
- 17.Kakinuma R, Ohmatsu H, Kaneko M, et al. Detection failures in spiral CT screening for lung cancer: analysis of CT findings. Radiology. 1999;212:61–66. doi: 10.1148/radiology.212.1.r99jn1461. PMid:10405721. [DOI] [PubMed] [Google Scholar]
- 18.Naidich DP, Rusinek H, McGuinness G, Leitman B, McCauley DI, Henschke CI. Variables affecting pulmonary nodule detection with computed tomography: evaluation with three-dimensional computer simulation. J Thorac Imaging. 1993;8:291–299. doi: 10.1097/00005382-199323000-00005. . PMid:8246327. [DOI] [PubMed] [Google Scholar]
- 19.Rusinek H, Naidich DP, McGuinness G, et al. Pulmonary nodule detection: low-dose versus conventional CT. Radiology. 1998;209:243–249. doi: 10.1148/radiology.209.1.9769838. PMid:9769838. [DOI] [PubMed] [Google Scholar]
- 20.Fischbach F, Knollmann F, Griesshaber V, Freund T, Akkol E, Felix R. Detection of pulmonary nodules by multislice computed tomography: improved detection rate with reduced slice thickness. Eur Radiol. 2003;13:2378–2383. doi: 10.1007/s00330-003-1915-7. . PMid:12743736. [DOI] [PubMed] [Google Scholar]
- 21.Wormanns D, Ludwig K, Beyer F, Heindel W, Diederich S. Detection of pulmonary nodules at multirow-detector CT: effectiveness of double reading to improve sensitivity at standard-dose and low-dose chest CT. Eur Radiol. 2005;1:14–22. doi: 10.1007/s00330-004-2527-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, van Klaveren RJ, de Bock GH, et al. No benefit for consensus double reading at baseline screening for lung cancer with the use of semiautomated volumetry software. Radiology. 2012;262:320–326. doi: 10.1148/radiol.11102289. . PMid:22106357. [DOI] [PubMed] [Google Scholar]
- 23.Rubin GD, Lyo JK, Paik DS, et al. Pulmonary nodules on multi-detector row CT scans: performance comparison of radiologists and computer-aided detection. Radiology. 2005;234:274–283. doi: 10.1148/radiol.2341040589. . PMid:15537839. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Kim JH, Cho G, Bae KT. Automated detection of pulmonary nodules on CT images: effect of section thickness and reconstruction interval—initial results. Radiology. 2005;236:295–299. doi: 10.1148/radiol.2361041288. . PMid:15955863. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Chung MJ, Yi CA, Lee KS. Ultra-low-dose MDCT of the chest: influence on automated lung nodule detection. Korean J Radiol. 2008;9:95–101. doi: 10.3348/kjr.2008.9.2.95. . PMid:18385555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marten K, Seyfarth T, Auer F, et al. Computer-assisted detection of pulmonary nodules: performance evaluation of an expert knowledge-based detection system in consensus reading with experienced and inexperienced chest radiologists. Eur Radiol. 2004;14:1930–1938. doi: 10.1007/s00330-004-2389-y. PMid:15235812. [DOI] [PubMed] [Google Scholar]
- 27.Bastarrika Alemañ G, Domínguez Echávarri PD, Noguera Tajadura JJ, Arraiza Sarasa M, Zudaire Díaz-Tejeiro B, Zulueta Francés J. Usefulness of maximum intensity projections in low-radiation multislice CT lung cancer screening. Radiologia. 2008;50:231–237. doi: 10.1016/S0033-8338(08)71969-6. [DOI] [PubMed] [Google Scholar]
- 28.Jankowski A, Martinelli T, Timsit JF, et al. Pulmonary nodule detection on MDCT images: evaluation of diagnostic performance using thin axial images, maximum intensity projections, and computer-assisted detection. Eur Radiol. 2007;17:3148–3156. doi: 10.1007/s00330-007-0727-6. . PMid:17763856. [DOI] [PubMed] [Google Scholar]
- 29.Eibel R, Türk TR, Kulinna C, Herrmann K, Reiser MF. Multidetector-row CT of the lungs: Multiplanar reconstructions and maximum intensity projections for the detection of pulmonary nodules. Rofo. 2001;173:815–821. doi: 10.1055/s-2001-16981. . PMid:11582561. [DOI] [PubMed] [Google Scholar]
- 30.Kawel N, Seifert B, Luetolf M, Boehm T. Effect of slab thickness on the CT detection of pulmonary nodules: use of sliding thin-slab maximum intensity projection and volume rendering. AJR Am J Roentgenol. 2009;192:1324–1329. doi: 10.2214/AJR.08.1689. . PMid:19380557. [DOI] [PubMed] [Google Scholar]
- 31.Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190:917–922. doi: 10.2214/AJR.07.2979. . PMid:18356437. [DOI] [PubMed] [Google Scholar]
- 32.Wu MT, Yang P, Huang YL, et al. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: concordance study with dedicated cardiac CT. AJR Am J Roentgenol. 2008;190:923–928. doi: 10.2214/AJR.07.2974. . PMid:18356438. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs PC, Prokop M, van der Graaf Y, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. . PMid:19875116. [DOI] [PubMed] [Google Scholar]
- 34.Bastarrika G, Wisnivesky JP, Pueyo JC, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thorac Imaging. 2009;24:206–211. doi: 10.1097/RTI.0b013e3181a65263. . PMid:19704324. [DOI] [PubMed] [Google Scholar]
- 35.Gierada DS, Pilgram TK, Whiting BR, et al. Comparison of standard- and low-radiation-dose CT for quantification of emphysema. AJR Am J Roentgenol. 2007;188:42–47. doi: 10.2214/AJR.05.1498. . PMid:17179344. [DOI] [PubMed] [Google Scholar]
- 36.Bellomi M, Rampinelli C, Veronesi G, et al. Evolution of emphysema in relation to smoking. Eur Radiol. 2010;20:286–292. doi: 10.1007/s00330-009-1548-6. . PMid:19705126. [DOI] [PubMed] [Google Scholar]
- 37.Mets OM, Buckens CF, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306:1775–1781. doi: 10.1001/jama.2011.1531. . PMid:22028353. [DOI] [PubMed] [Google Scholar]
- 38.Rampinelli C, Preda L, Maniglio M, et al. Extrapulmonary malignancies detected at lung cancer screening. Radiology. 2011;261:293–299. doi: 10.1148/radiol.11102231. . PMid:21828191. [DOI] [PubMed] [Google Scholar]
- 39.Revel MP, Bissery A, Bienvenu M, Aycard L, Lefort C, Frija G. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology. 2004;231:453–458. doi: 10.1148/radiol.2312030167. . PMid:15128990. [DOI] [PubMed] [Google Scholar]
- 40.Marten K, Auer F, Schmidt S, Kohl G, Rummeny EJ, Engelke C. Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur Radiol. 2006;16:781–790. doi: 10.1007/s00330-005-0036-x. . PMid:16331462. [DOI] [PubMed] [Google Scholar]
- 41.Goodman LR, Gulsun M, Washington L, Nagy PG, Piacsek KL. Inherent variability of CT lung nodule measurements in vivo using semiautomated volumetric measurements. AJR Am J Roentgenol. 2006;186:989–994. doi: 10.2214/AJR.04.1821. . PMid:16554568. [DOI] [PubMed] [Google Scholar]
- 42.Wormanns D, Kohl G, Klotz E, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol. 2004;14:86–92. doi: 10.1007/s00330-003-2132-0. . PMid:14615902. [DOI] [PubMed] [Google Scholar]
- 43.Gietema HA, Wang Y, Xu D, et al. Pulmonary nodules detected at lung cancer screening: interobserver variability of semiautomated volume measurements. Radiology. 2006;241:251–256. doi: 10.1148/radiol.2411050860. . PMid:16908677. [DOI] [PubMed] [Google Scholar]
- 44.Gavrielides MA, Kinnard LM, Myers KJ, Petrick N. Noncalcified lung nodules: volumetric assessment with thoracic CT. Radiology. 2009;251:26–37. doi: 10.1148/radiol.2511071897. . PMid:19332844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winer-Muram HT, Jennings SG, Meyer CA, et al. Effect of varying CT section width on volumetric measurement of lung tumors and application of compensatory equations. Radiology. 2003;229:184–194. doi: 10.1148/radiol.2291020859. . PMid:14519875. [DOI] [PubMed] [Google Scholar]
- 46.Nietert PJ, Ravenel JG, Leue WM, et al. Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation. Chest. 2009;135:1580–1587. doi: 10.1378/chest.08-2040. . PMid:19141526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goo JM, Tongdee T, Tongdee R, Yeo K, Hildebolt CF, Bae KT. Volumetric measurement of synthetic lung nodules with multi–detector row CT: effect of various image reconstruction parameters and segmentation thresholds on measurement accuracy. Radiology. 2005;235:850–856. doi: 10.1148/radiol.2353040737. . PMid:15914478. [DOI] [PubMed] [Google Scholar]
- 48.Petrou M, Quint LE, Nan B, Baker LH. Pulmonary nodule volumetric measurement variability as a function of CT slice thickness and nodule morphology. AJR Am J Roentgenol. 2007;188:306–312. doi: 10.2214/AJR.05.1063. . PMid:17242235. [DOI] [PubMed] [Google Scholar]
- 49.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217:251–256. doi: 10.1148/radiology.217.1.r00oc33251. PMid:11012453. [DOI] [PubMed] [Google Scholar]
- 50.Marten K, Funke M, Engelke C. Flat panel detector-based volumetric CT: prototype evaluation with volumetry of small artificial nodules in a pulmonary phantom. J Thorac Imaging. 2004;19:156–163. doi: 10.1097/01.rti.0000131591.12777.a8. . PMid:15273611. [DOI] [PubMed] [Google Scholar]
- 51.de Hoop B, Gietema H, van Ginneken B, Zanen P, Groenewegen G, Prokop M. A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: what is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol. 2009;19:800–808. doi: 10.1007/s00330-008-1229-x. . PMid:19018537. [DOI] [PubMed] [Google Scholar]
- 52.Ashraf H, de Hoop B, Shaker SB, et al. Lung nodule volumetry: segmentation algorithms within the same software package cannot be used interchangeably. Eur Radiol. 2010;20:1878–1885. doi: 10.1007/s00330-010-1749-z. . PMid:20306082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko JP, Rusinek H, Jacobs EL, et al. Small pulmonary nodules: volume measurement at chest CT—phantom study. Radiology. 2003;228:864–870. doi: 10.1148/radiol.2283020059. . PMid:12954901. [DOI] [PubMed] [Google Scholar]
- 54.Revel MP, Lefort C, Bissery A, et al. Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology. 2004;231:459–466. doi: 10.1148/radiol.2312030241. . PMid:15128991. [DOI] [PubMed] [Google Scholar]
- 55.de Hoop B, Gietema H, van de Vorst S, Murphy K, van Klaveren RJ, Prokop M. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology. 2010;255:199–206. doi: 10.1148/radiol.09090571. . PMid:20123896. [DOI] [PubMed] [Google Scholar]
- 56.Rinaldi MF, Bartalena T, Braccaioli L, et al. Three-dimensional analysis of pulmonary nodules: variability of semiautomated volume measurements between different versions of the same software. Radiol Med. 2010;115:403–412. doi: 10.1007/s11547-010-0511-6. . PMid:20082224. [DOI] [PubMed] [Google Scholar]
- 57.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Mandrekar JN. 5-year lung cancer screening experience: growth curves of 18 lung cancers compared to histologic type, CT attenuation, stage, survival, and size. Chest. 2009;136:1586–1595. doi: 10.1378/chest.09-0915. . PMid:19581354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol. 2006;187:135–142. doi: 10.2214/AJR.05.1228. . PMid:16794167. [DOI] [PubMed] [Google Scholar]
- 59.Winer-Muram HT, Jennings SG, Tarver RD, et al. Volumetric growth rate of stage I lung cancer prior to treatment: serial CT scanning. Radiology. 2002;223:798–805. doi: 10.1148/radiol.2233011026. . PMid:12034952. [DOI] [PubMed] [Google Scholar]
- 60.Takashima S, Sone S, Li F, Maruyama Y, Hasegawa M, Kadoya M. Indeterminate solitary pulmonary nodules revealed at population-based CT screening of the lung: using first follow-up diagnostic CT to differentiate benign and malignant lesions. AJR Am J Roentgenol. 2003;180:1255–1263. doi: 10.2214/ajr.180.5.1801255. PMid:12704034. [DOI] [PubMed] [Google Scholar]
- 61.Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol. 1997;168:325–328. doi: 10.2214/ajr.168.2.9016198. PMid:9016198. [DOI] [PubMed] [Google Scholar]
- 62.Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology. 2009;250:264–272. doi: 10.1148/radiol.2493070847. . PMid:18984780. [DOI] [PubMed] [Google Scholar]
- 63.Veronesi G, Bellomi M, Scanagatta P, et al. Difficulties encountered managing nodules detected during a computed tomography lung cancer screening program. J Thorac Cardiovasc Surg. 2008;136:611–617. doi: 10.1016/j.jtcvs.2008.02.082. . PMid:18805261. [DOI] [PubMed] [Google Scholar]
- 64.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):94S–107S. doi: 10.1378/chest.07-1352. . PMid:17873163. [DOI] [PubMed] [Google Scholar]
- 65.Bellomi M, Veronesi G, Rampinelli C, Ferretti S, De Fiori E, Maisonneuve P. Evolution of lung nodules < or =5 mm detected with low-dose CT in asymptomatic smokers. Br J Radiol. 2007;80:708–712. doi: 10.1259/bjr/46019726. . PMid:17928499. [DOI] [PubMed] [Google Scholar]
- 66.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–622. doi: 10.1148/radiol.2533090179. . PMid:19952025. [DOI] [PubMed] [Google Scholar]
- 67.Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol. 2000;174:763–768. doi: 10.2214/ajr.174.3.1740763. PMid:10701622. [DOI] [PubMed] [Google Scholar]
- 68.Kakinuma R, Ohmatsu H, Kaneko M, et al. Progression of focal pure ground-glass opacity detected by low-dose helical computed tomography screening for lung cancer. J Comput Assist Tomogr. 2004;28:17–23. doi: 10.1097/00004728-200401000-00003. . PMid:14716227. [DOI] [PubMed] [Google Scholar]
- 69.Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242:555–562. doi: 10.1148/radiol.2422052090. . PMid:17255425. [DOI] [PubMed] [Google Scholar]
- 70.Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg. 2002;73:386–392. doi: 10.1016/S0003-4975(01)03410-5. . PMid:11845847. [DOI] [PubMed] [Google Scholar]
- 71.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–924. doi: 10.1001/jama.285.7.914. . PMid:11180735. [DOI] [PubMed] [Google Scholar]
- 72.Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med. 2007;48:214–220. PMid:17268017. [PubMed] [Google Scholar]
- 73.Veronesi G, Bellomi M, Veronesi U, et al. Role of positron emission tomography scanning in the management of lung nodules detected at baseline computed tomography screening. Ann Thorac Surg. 2007;84:959–965. doi: 10.1016/j.athoracsur.2007.04.058. . PMid:17720408. [DOI] [PubMed] [Google Scholar]
- 74.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004;45:19–27. doi: 10.1016/j.lungcan.2004.01.009. . PMid:15196730. [DOI] [PubMed] [Google Scholar]
- 75.Tsunezuka Y, Shimizu Y, Tanaka N, Takayanagi T, Kawano M. Positron emission tomography in relation to Noguchi’s classification for diagnosis of peripheral non-small cell lung cancer 2 cm or less in size. World J Surg. 2007;31:314–317. doi: 10.1007/s00268-006-0475-9. . PMid:17219276. [DOI] [PubMed] [Google Scholar]
- 76.Higashi K, Ueda Y, Seki H, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39:1016–1020. PMid:9627336. [PubMed] [Google Scholar]
- 77.Kim BT, Kim Y, Lee KS, et al. Localized form of bronchioloalveolar carcinoma: FDG PET findings. AJR Am J Roentgenol. 1998;170:935–939. doi: 10.2214/ajr.170.4.9530038. PMid:9530038. [DOI] [PubMed] [Google Scholar]
- 78.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. . PMid:10408484. [DOI] [PubMed] [Google Scholar]
- 79.Diederich S, Thomas M, Semik M, et al. Screening for early lung cancer with low dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol. 2004;14:691–702. doi: 10.1007/s00330-003-2200-5. PMid:14727146. [DOI] [PubMed] [Google Scholar]
- 80.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer. 2005;47:9–15. doi: 10.1016/j.lungcan.2004.06.007. . PMid:15603850. [DOI] [PubMed] [Google Scholar]
- 81.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol. 2002;20:911–920. doi: 10.1200/JCO.20.4.911. . PMid:11844811. [DOI] [PubMed] [Google Scholar]
- 82.Wagnetz U, Menezes RJ, Boerner S, et al. CT screening for lung cancer: implication of lung biopsy recommendations. AJR Am J Roentgenol. 2012;198:351–358. doi: 10.2214/AJR.11.6726. . PMid:22268177. [DOI] [PubMed] [Google Scholar]